Optimization and Characterization of Chitosan Films for Transdermal Delivery of Ondansetron
Abstract
:1. Introduction
2. Results and Discussion
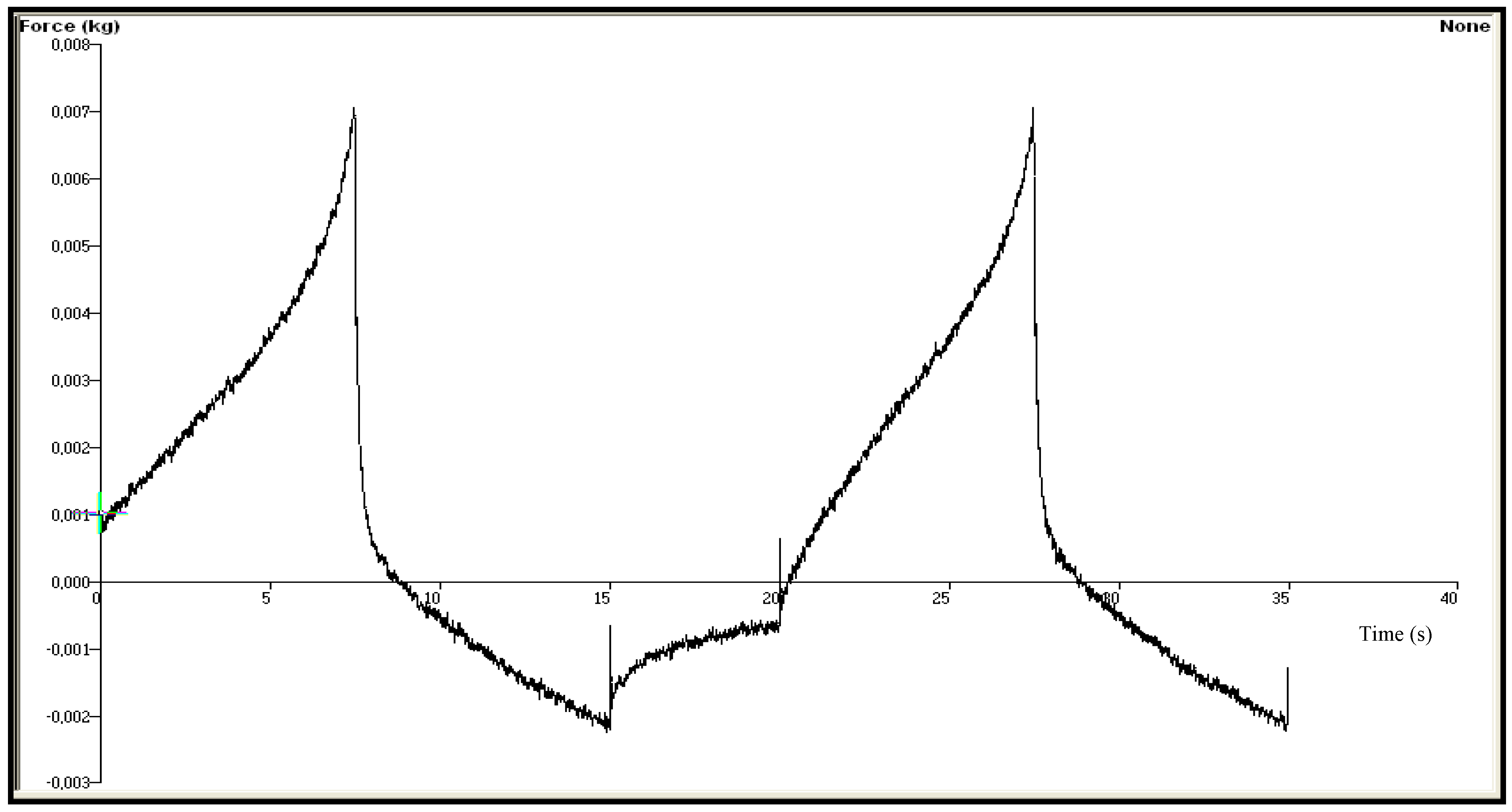
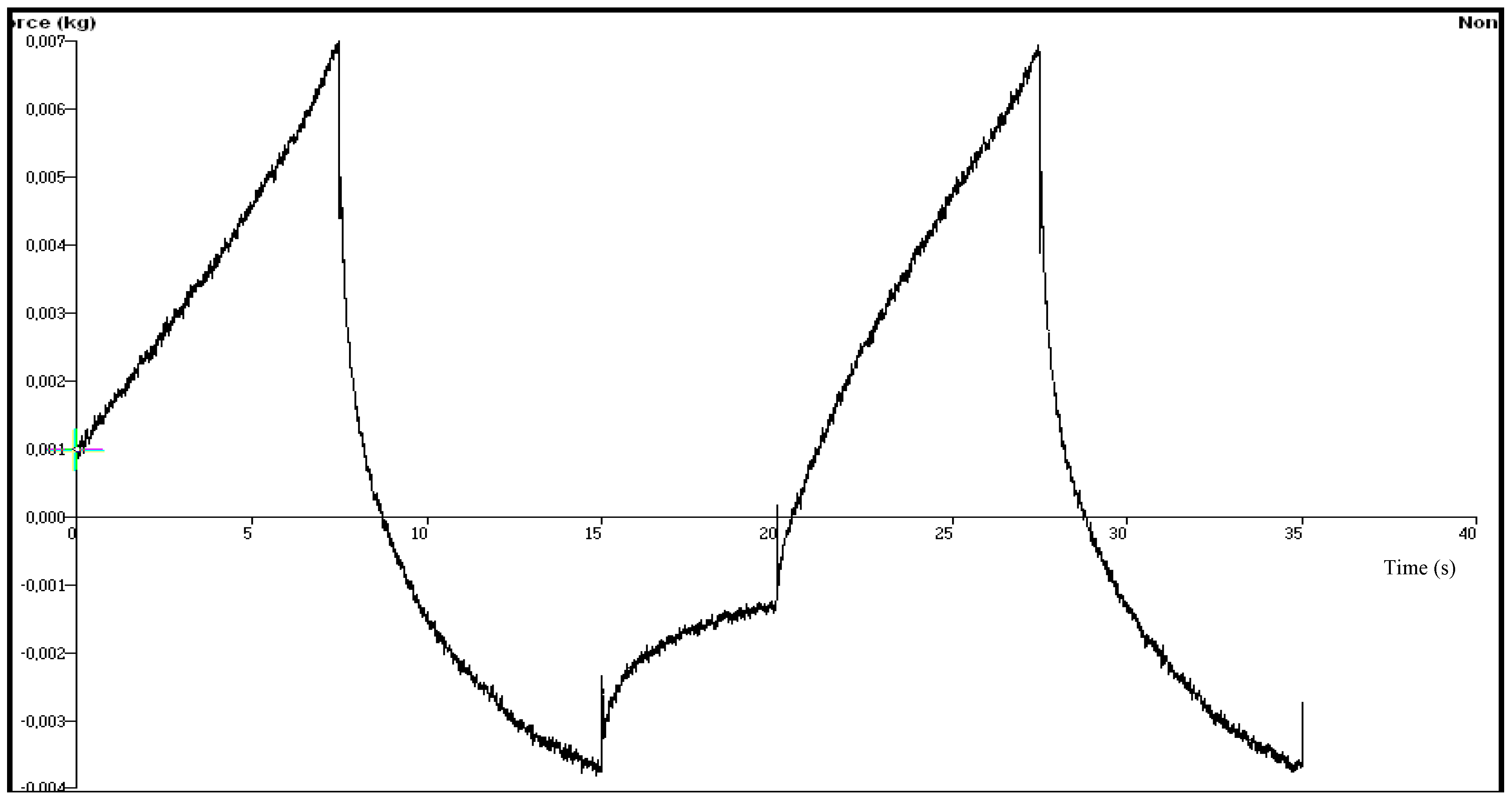
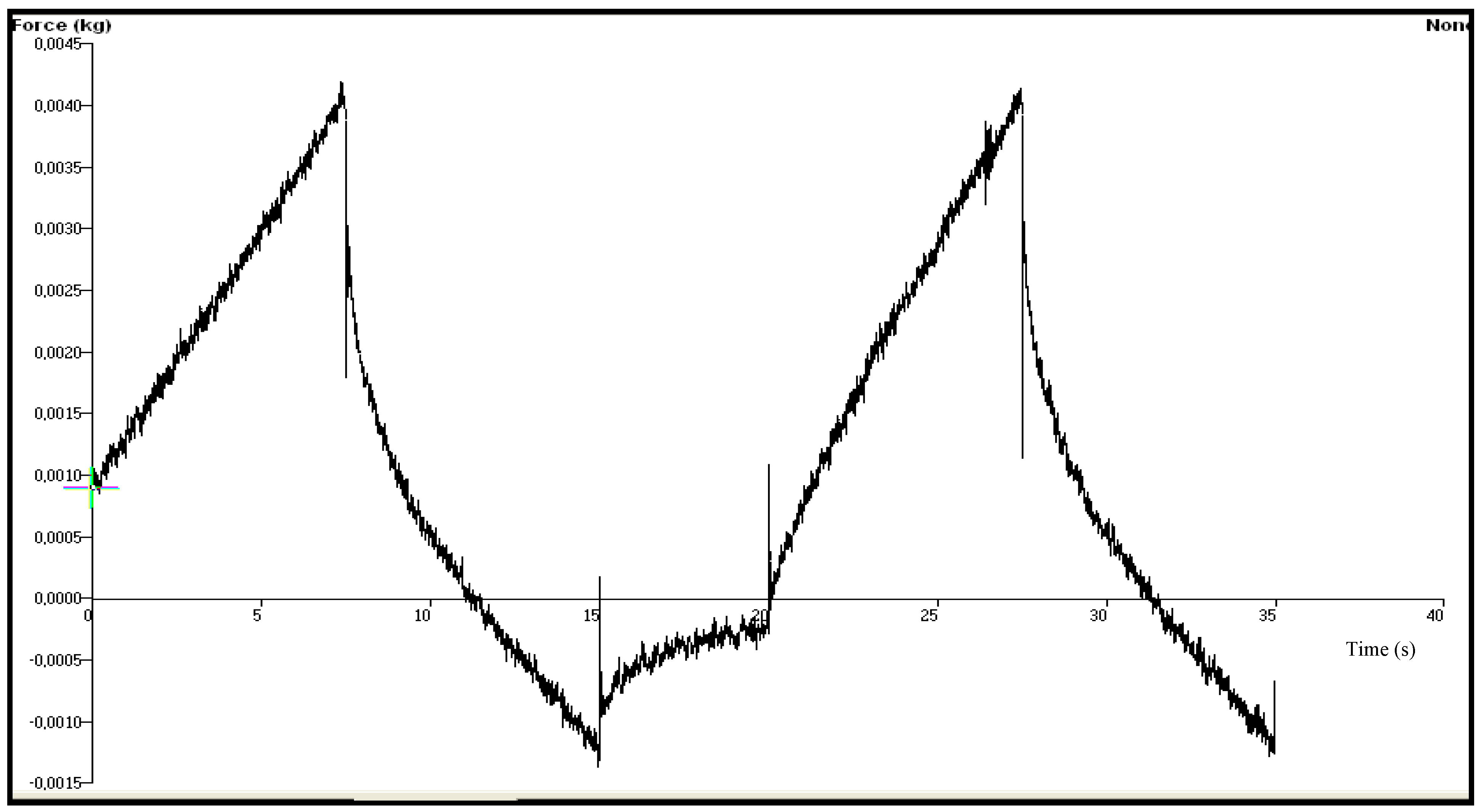
| Chitosan Gels | Hardness (N) | Elasticity | Cohesiveness | Adhesiveness (µJ) | Compressiblity (N.mm) |
|---|---|---|---|---|---|
| G-LIMO | 0.059 ± 0.006 | 0.904 ± 0.078 | 0.956 ± 0.046 | 123.789 ± 10.091 | 0.023 ± 0.004 |
| G-NERO | 0.040 ± 0.002 | 0.956 ± 0.098 | 0.909 ± 0.078 | 120.345 ± 8.963 | 0.022 ± 0.003 |
| G-EUCO | 0.069 ± 0.005 | 0.978 ± 0.092 | 0.933 ± 0.089 | 117.357 ± 9.089 | 0.030 ± 0.007 |
| Transdermal Film | Thickness (mm) | Weight (mg.cm−2) | OND Content (%) |
|---|---|---|---|
| T-CONT | 0.10 ± 0.03 | 33.57 ± 1.62 | 97.13 ± 0.23 |
| T-LIMO | 0.12 ± 0.02 | 35.23 ±1.54 | 96.92 ± 0.41 |
| T-NERO | 0.10 ± 0.02 | 34.56 ± 1.23 | 97.15 ± 1.56 |
| T-EUCO | 0.15 ± 0.04 | 36.45 ± 1.45 | 97.89 ± 0.63 |

| Transdermal Film | Flux (Jss) (μg·cm−2·h−1) | ERFlux | Q24 (μg·cm−2) |
|---|---|---|---|
| T-CONT | 2.81 ± 2.56 | 1.00 | 81.93 |
| T-LIMO | 2.64 ± 2.45 | 0.94 | 59.1 |
| T-NERO | 2.38 ± 3.64 | 0.85 | 47.77 |
| T-EUCO | 9.07 ± 4.34 | 3.23 | 163.59 |
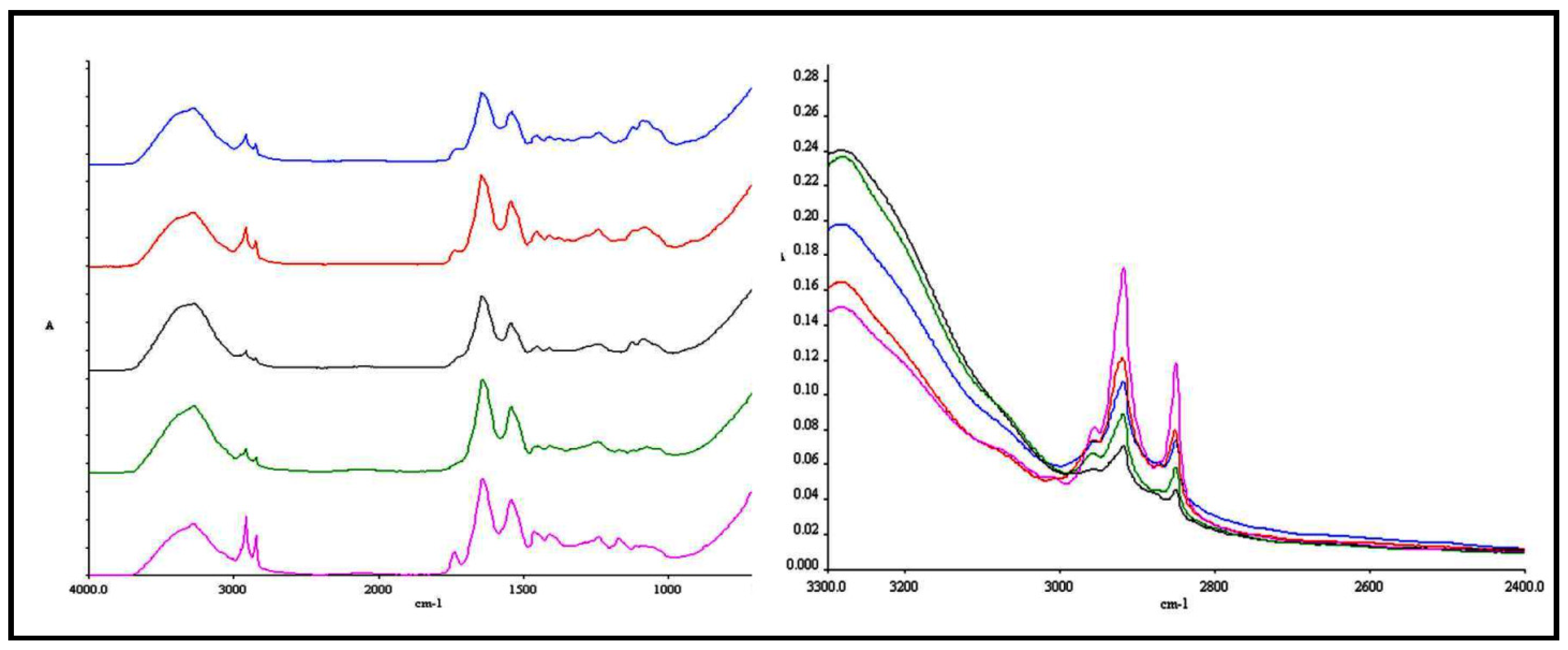
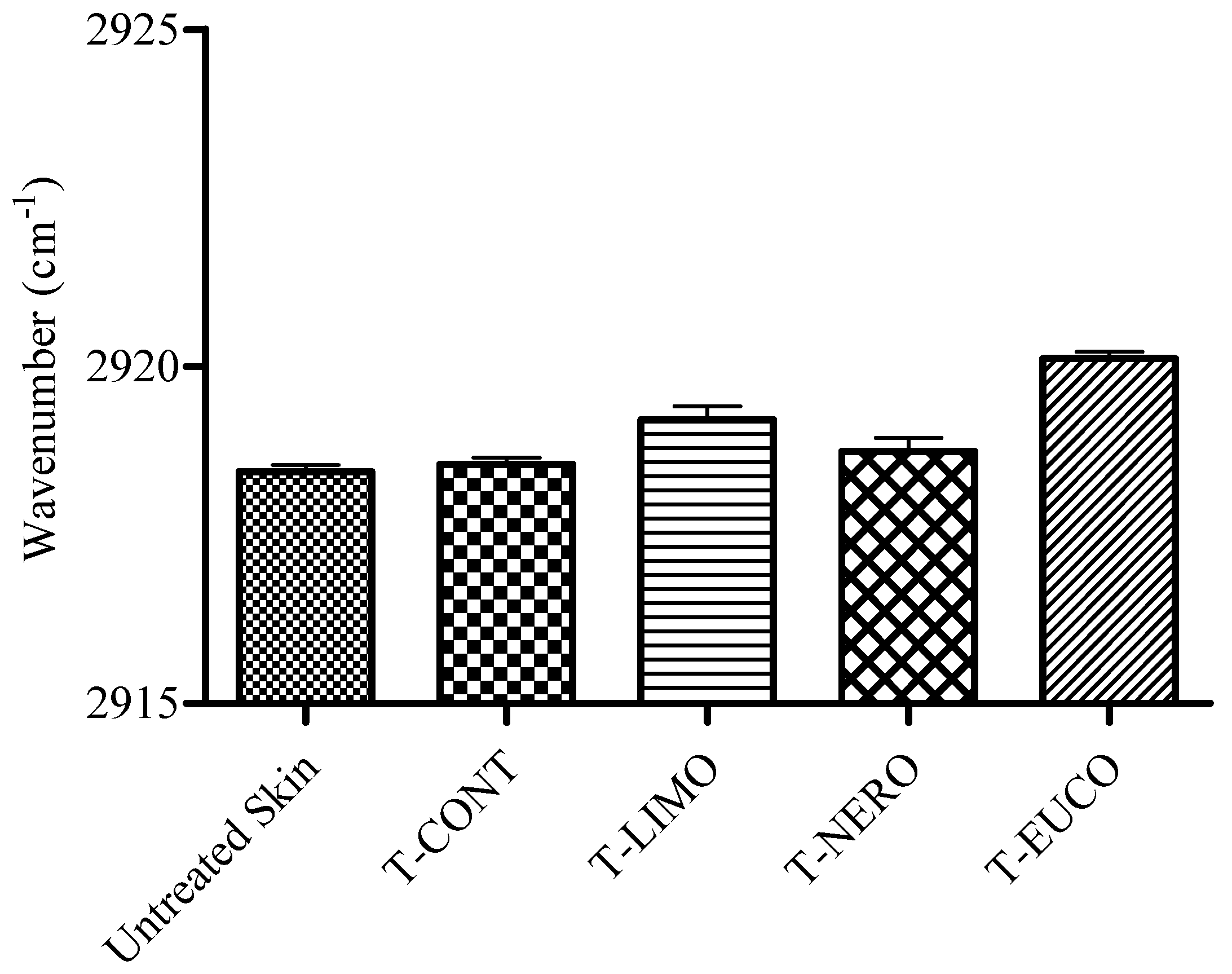
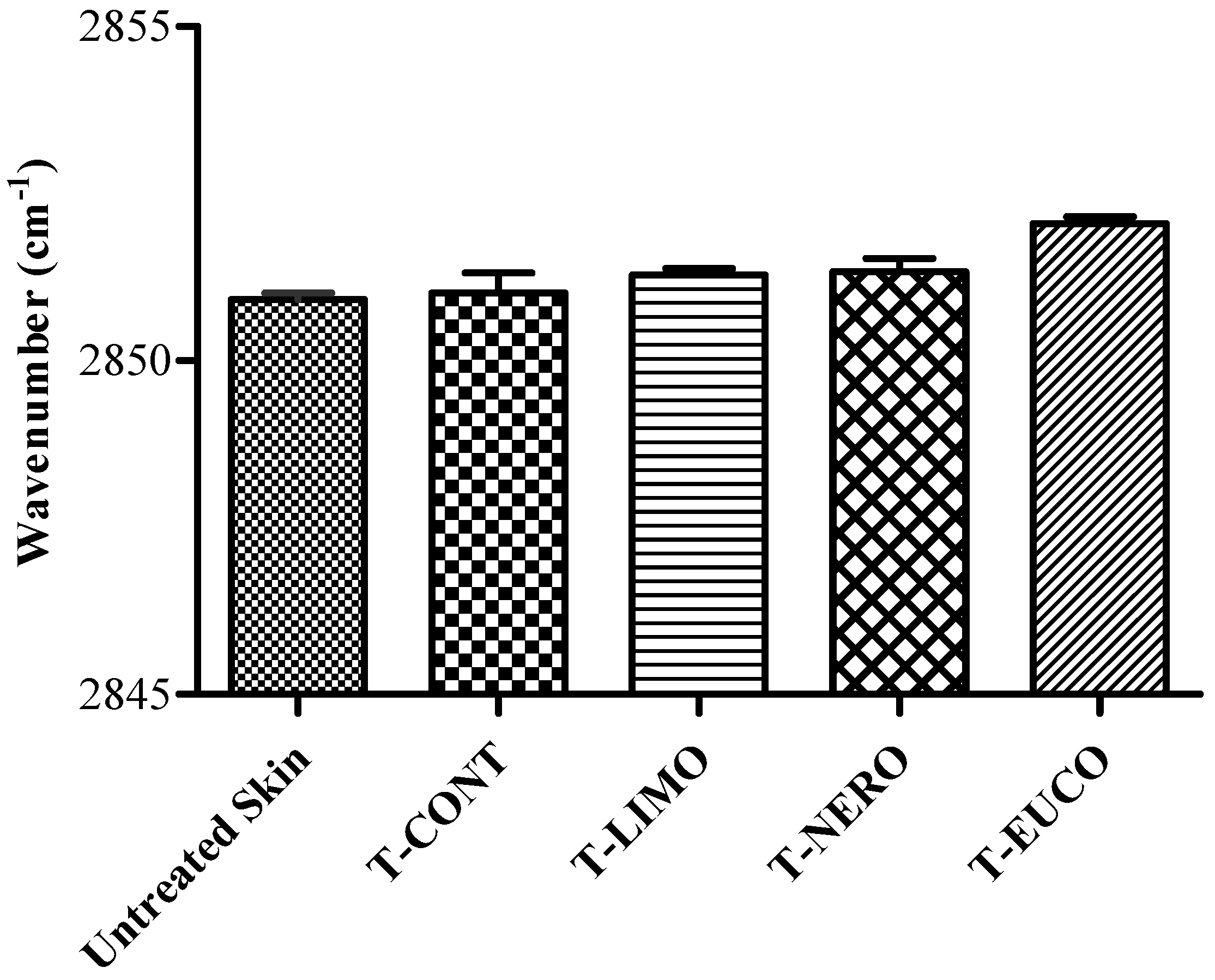
3. Experimental
3.1. Materials
3.2. Preparation of Chitosan Gel Formulations
| Component | G-CONT | G-LIMO | G-NERO | G-EUCO |
|---|---|---|---|---|
| Chitosan | 3 | 3 | 3 | 3 |
| Lactic Acid | 3 | 3 | 3 | 3 |
| Limonene | --- | 1 | --- | --- |
| Nerolidol | --- | --- | 1 | --- |
| Eucalyptol | --- | --- | --- | 1 |
| OND | 0.5 | 0.5 | 0.5 | 0.5 |
| Transcutol® P | 5 | 5 | 5 | 5 |
| Tween 80 | 1 | 1 | 1 | 1 |
| Deionized Water | qs. 100 | qs. 100 | qs. 100 | qs. 100 |
3.3. Mechanical Properties of the Chitosan Gels-Texture Profile Analysis (TPA)
3.4. Formulation of OND Transdermal Films
3.5. Physicochemical Characteristics of OND Transdermal Films
3.5.1. Organoleptic Examination
3.5.2. Film Thickness
3.5.3. Uniformity of Weight
3.5.4. Uniformity of Drug Content
3.6. In vitro Bioadhesion Test
3.7. In Vitro Skin Permeation Study
3.7.1. Skin Preparation
3.7.2. Permeation Experiments
3.7.3. HPLC Analysis
3.7.4. Permeation Data Analysis
3.8. ATR-FTIR Spectroscopy
3.9. Statistical Data Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Tanner, T.; Marks, R. Delivering drugs by the transdermal route: Review and comment. Skin Res. Technol. 2008, 14, 249–260. [Google Scholar] [CrossRef]
- Guy, R.H. Transdermal Drug Delivery. In Drug Delivery, Handbook of Experimental Pharmacology; Schäfer-Korting, M., Ed.; Springer Verlag: Berlin, Germany, 2010; pp. 11–13. [Google Scholar]
- Valenta, C.; Auner, B.G. The use of polymers for dermal and transdermal delivery. Eur. J. Pharm. Biopharm. 2004, 58, 279–289. [Google Scholar] [CrossRef]
- Güngör, S.; Erdal, M.S.; Özsoy, Y. Plasticizers in Transdermal Delivery Systems. In Recent Advances in Plasticizers; Luqman, M., Ed.; Intech: Rijeka, Crotia, 2012; pp. 91–112. [Google Scholar]
- Williams, A.C.; Barry, B.W. Penetration enhancers. Adv. Drug Deliv. Rev. 2012, 64, 128–137. [Google Scholar] [CrossRef]
- Güngör, S.; Bektaş, A.; Alp, F.I.; Uydeş-Doğan, B.S.; Özdemir, O.; Araman, A.; Özsoy, Y. Matrix type transdermal patches of verapamil hydrochloride: In vitro permeation studies through excised rat skin and pharmacodynamic evaluation in rats. Pharm. Dev. Technol. 2008, 13, 283–289. [Google Scholar] [CrossRef]
- Aktar, B.; Erdal, M.S.; Sairli, O.; Güngör, S.; Özsoy, Y. Transdermal films of metoclopramide hydrochloride with terpenes as penetration enhancers: Design, characterization, in vitro evaluation and ATR-FTIR spectroscopic investigation on excised pig skin. Asian Chem. Lett. 2011, 16, 67–78. [Google Scholar]
- Ravi Kumar, M.N.V. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- He, W.; Guo, X.; Xiao, L.; Feng, M. Study on the mechanisms of chitosan and its derivatives used as trasdermal penetration enhancers. Int. J. Pharm. 2009, 382, 234–243. [Google Scholar] [CrossRef]
- Uztan, A.H.; Aksu, B.; Boyacıoğlu, H.; Güneri, T.; Özer, Ö. Enhanced topical delivery of terbinafine hydrochloride with chitosan hydrogels. AAPS PharmSciTech 2009, 10, 1024–1031. [Google Scholar] [CrossRef]
- Mi, F.L.; Wu, Y.B.; Shyu, S.S.; Shyong, J.Y.; Huang, R.N.; Tsai, Y.H.; Hao, J.Y. Control of wound infections using a bilayer chitosan wound dressing with sustainable antibiotic delivery. J. Biomed. Mater. Res. 2002, 59, 438–449. [Google Scholar] [CrossRef]
- Thein-Han, W.W.; Stevens, W.F. Transdermal delivery controlled by a chitosan membrane. Drug Dev. Ind. Pharm. 2004, 30, 397–404. [Google Scholar] [CrossRef]
- Kählig, H.; Hasanovic, A.; Biruss, B.; Höller, S.; Grim, J.; Valenta, C. Chitosan-glycolic acid: A possible matrix for progesterone delivery into skin. Drug Dev. Ind. Pharm. 2009, 35, 997–1002. [Google Scholar] [CrossRef]
- Li, X.; Kong, X.; Zhang, Z.; Nan, K.; Li, L.; Wang, X.; Chen, H. Cytotoxicity and biocompatibility evaluation of N,O-carboxymethyl chitosan/oxidized alginate hydrogel for drug delivery application. Int. J. Biol. Macromol. 2012, 50, 1299–1305. [Google Scholar] [CrossRef]
- Ammar, H.O.; Ghorab, M.; El-Nahhas, S.A.; Kamel, R. Polymeric matrix system for prolonged delivery of tramadol hydro chloride, Part I: Physicochemical evaluation. AAPS PharmSciTech 2009, 10, 7–20. [Google Scholar] [CrossRef]
- He, W.; Guo, X.; Zhang, M. Transdermal permeation enhancement of N-trimethyl chitosan for testosterone. Int. J. Pharm. 2008, 356, 82–87. [Google Scholar] [CrossRef]
- Baber, N.; Palmer, J.L.; Frazer, N.M.; Pritchard, J.F. Clinical pharmacology of ondansetron in postoperative nausea and vomiting. Eur. J. Anaesthesiol. 1992, 6, 11–18. [Google Scholar]
- Mashru, R.C.; Sutariya, V.B.; Sankalia, M.G.; Sankalia, J.M. Effect of pH on in vitro permeation of ondansetron hydrochloride across porcine buccal mucosa. Pharm. Dev. Technol. 2005, 10, 241–247. [Google Scholar] [CrossRef]
- Al Abood, R.M.; Talegaonkar, S.; Tariq, M.; Ahmad, F.J. Microemulsion as a tool for the transdermal delivery of ondansetron for the treatment of chemotherapy induced nausea and vomiting. Colloids. Surf. B 2013, 101, 143–151. [Google Scholar] [CrossRef]
- Gwak, H.S.; Oh, I.S.; Chun, I.K. Transdermal delivery of ondansetron hydrochloride: Effects of vehicles and penetration enhancers. Drug. Dev. Ind. Pharm. 2004, 30, 187–194. [Google Scholar] [CrossRef]
- Krishnaiah, Y.S.R.; Raju, V.; Kumar, S.M.; Rama, B.; Raghumurthy, V.; Ramanamurthy, K.V. Studies on optimizing in vitro transdermal permeation of ondansetron hydrochloride using nerodilol, Carvone, And limonene as penetration enhancers. Pharm. Dev. Technol. 2008, 13, 177–185. [Google Scholar] [CrossRef]
- Krishnaiah, Y.S.R.; Rama, B.; Raghumurthy, V.; Ramanamurthy, K.V.; Satyanarayana, V. Effect of PEG 6000 on the in vitro and in vivo transdermal permeation of ondansetron hydrochloride from EVA1802 membranes. Pharm. Dev. Technol. 2009, 14, 50–61. [Google Scholar]
- Gwak, H.S.; Oh, I.S.; Chun, I.K. In vitro percutaneous absorption of ondansetron hydrochloride from pressure-sensitive adhesive matrices through hairless mouse skin. Arch. Pharm. Res. 2003, 26, 644–648. [Google Scholar] [CrossRef]
- Dimas, D.A.; Dallas, P.P.; Rekkas, D.M. Ion pair formation as a possible mechanism for the enhancement effect of lauric acid on the transdermal permeation of ondansetron. Pharm. Dev. Technol. 2004, 9, 311–320. [Google Scholar] [CrossRef]
- Swain, K.; Pattnaik, S.; Sahu, S.C.; Pattnaik, K.K.; Mallicks, S. Drug in adhesive type transdermal matrix systems of ondansetron hydrochloride: Optimization of permeation pattern using response surface methodology. J. Drug. Target. 2010, 18, 106–114. [Google Scholar] [CrossRef]
- Bansal, K.; Rawat, M.K.; Jain, A.; Rajput, A.; Chaturvedi, T.P.; Singh, S. Development of satranidazole mucoadhesive gel for the treatment of periodontitis. AAPS PharmSciTech 2009, 10, 716–723. [Google Scholar] [CrossRef]
- Lau, M.H.; Tang, J.; Paulson, A.T. Texture profile and turbidity of gellan/gelatin mixed gels. Food Res. Int. 2000, 33, 665–671. [Google Scholar] [CrossRef]
- Cevher, E.; Sensoy, D.; Taha, M.A. Effect of thiolated polymers to textural and mucoadhesive properties of vaginal gel formulations prepared with polycarbophil and chitosan. AAPS PharmSciTech 2008, 9, 953–965. [Google Scholar] [CrossRef]
- Baloğlu, E.; Karavana, S.Y.; Hyusein, I.Y.; Köse, T. Design and formulation of mebeverine HCl semisolid formulations for intraorally administration. AAPS PharmSciTech 2010, 11, 181–188. [Google Scholar] [CrossRef]
- Cevher, E.; Taha, M.A.M.; Orlu, M.; Araman, A. Evaluation of mechanical and mucoadhesive properties of clomiphene citrate gel formulations containing carbomers and their thiolated derivatives. Drug Deliv. 2008, 15, 57–67. [Google Scholar] [CrossRef]
- Jones, D.S.; Woolfson, A.D.; Djokic, J.; Coulter, W.A. Development and mechanical characterization of bioadhesive semi-solid, polymeric systems containing tetracycline for the treatment of periodontal diseases. Pharm. Res. 1996, 13, 1734–1738. [Google Scholar] [CrossRef]
- Jones, D.S.; Woolfson, A.D.; Brown, A.F.; O'Neill, M.J. Mucoadhesive, syringeable drug delivery systems for controlled application of metronidazole to the periodontal pocket: In vitro release kinetics, syringeability, mechanical and mucoadhesive properties. J. Control. Release 1997, 49, 71–79. [Google Scholar] [CrossRef]
- Gutschke, E.; Bracht, S.; Nagel, S.; Weitschies, W. Adhesion testing of transdermal matrix patches with a probe tack test—In vitro and in vivo evaluation. Eur. J. Pharm. Biopharm. 2010, 75, 399–404. [Google Scholar] [CrossRef]
- Wokovich, A.M.; Prodduturi, S.; Doub, W.H.; Hussain, A.S.; Buhse, L.F. Transdermal drug delivery system (TDDS) adhesion as a critical safety, efficacy and quality attribute. Eur. J. Pharm. Biopharm. 2006, 64, 1–8. [Google Scholar] [CrossRef]
- McCarron, P.A.; Donnelly, R.F.; Zawislak, A.; Woolfson, A.D.; Price, J.H.; McClelland, R. Evaluation of a water-soluble bioadhesive patch for photodynamic therapy of vulval lesions. Int. J. Pharm. 2005, 293, 11–33. [Google Scholar] [CrossRef]
- Abdul Rasool, B.K.; Aziz, U.S.; Sarheed, O.; Abdul Rasool, A.A. Design and evaluation of bioadhesive film for transdermal delivery of propanolol hydrochloride. Acta Pharm. 2011, 61, 271–282. [Google Scholar]
- Arvanitoyannis, I.; Kolokuris, I.; Nakayama, A.; Yamamoto, N.; Aiba, S. Physico-chemical studies of chitosan-poly(vinyl alcohol) blends plasticized with sorbitol and sucrose. Carbohydr. Polym. 1997, 34, 9–19. [Google Scholar] [CrossRef]
- El-Kattan, A.F.; Asbill, C.S.; Kim, N.; Michniak, B.B. The effects of terpene enhancers on the percutaneous permeation of drugs with different lipophilicities. Int. J. Pharm. 2001, 215, 229–240. [Google Scholar] [CrossRef]
- Aqil, M.; Ahad, A.; Sultana, Y.; Ali, A. Status of terpenes as skin penetration enhancers. Drug Discov. Today 2007, 12, 1061–1067. [Google Scholar] [CrossRef]
- Lim, P.F.C.; Liu, X.Y.; Chan, S.Y. A review on terpenes as skin penetration enhaancers in transdermal drug delivery. J. Essential Oil Res. 2009, 21, 423–428. [Google Scholar] [CrossRef]
- Anjos, J.L.V.D.; Neto, D.D.S.; Alonso, A. Effects of 1,8-cineole on the dynamics of lipids and proteins of stratum corneum. Int. J. Pharm. 2007, 345, 81–87. [Google Scholar] [CrossRef]
- Barbero, A.M.; Frasch, H.F. Pig and guinea pig skin as surrogates for human in vitro penetration studies: A quantitative review. Toxicol. In Vitro 2009, 23, 1–13. [Google Scholar] [CrossRef]
- Nokhodchi, A.; Sharabiani, K.; Rashidi, M.R.; Ghafourian, T. The effect of terpene concentrations on the skin penetration of diclofenac sodium. Int. J. Pharm. 2007, 335, 97–105. [Google Scholar] [CrossRef]
- Narishetty, S.T.K.; Panchagnula, R. Transdermal delivery of zidovudine: Effect of terpenes and their mechanism of action. J. Control. Release 2004, 95, 367–379. [Google Scholar] [CrossRef]
- Narishetty, S.T.K.; Panchagnula, R. Effect of L-menthol and 1,8-cineole on phase behavior and molecular organization of SC lipids and skin permeation of zidovudine. J. Control. Release 2005, 102, 59–70. [Google Scholar] [CrossRef]
- Babita, K.; Kumar, V.; Rana, V.; Jain, S.; Tiwary, A.K. Thermotropic and spectroscopic behavior of skin: relationship with percutaneous permeation enhancement. Curr. Drug Del. 2006, 3, 95–113. [Google Scholar] [CrossRef]
- Jones, D.S.; Irwin, C.R.; Woolfson, A.D.; Djokic, J.; Adams, V. Physicochemical characterization and preliminary in vivo efficiacy of bioadhesive, semisolid formulations containing flurbiprofen for the treatment of gingivitis. J. Pharm. Sci. 1999, 88, 592–598. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Can, A.S.; Erdal, M.S.; Güngör, S.; Özsoy, Y. Optimization and Characterization of Chitosan Films for Transdermal Delivery of Ondansetron. Molecules 2013, 18, 5455-5471. https://doi.org/10.3390/molecules18055455
Can AS, Erdal MS, Güngör S, Özsoy Y. Optimization and Characterization of Chitosan Films for Transdermal Delivery of Ondansetron. Molecules. 2013; 18(5):5455-5471. https://doi.org/10.3390/molecules18055455
Chicago/Turabian StyleCan, Aslı Sedef, Meryem Sedef Erdal, Sevgi Güngör, and Yıldız Özsoy. 2013. "Optimization and Characterization of Chitosan Films for Transdermal Delivery of Ondansetron" Molecules 18, no. 5: 5455-5471. https://doi.org/10.3390/molecules18055455




