Synthesis of δ-Oxo-1,1-bis(triflyl)alkanes and Their Acidities
Abstract
:1. Introduction

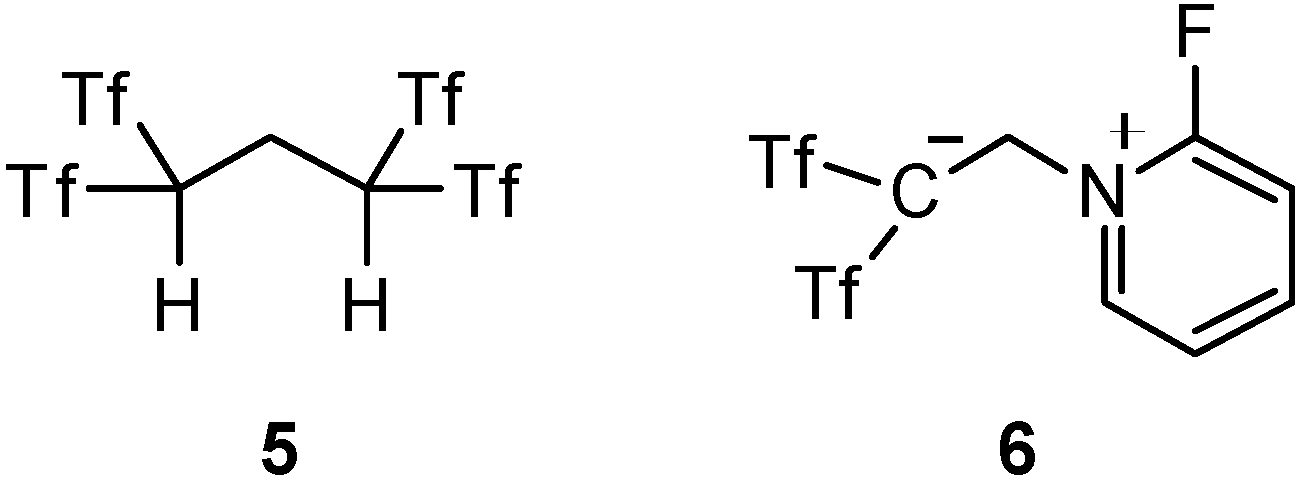
2. Results and Discussion
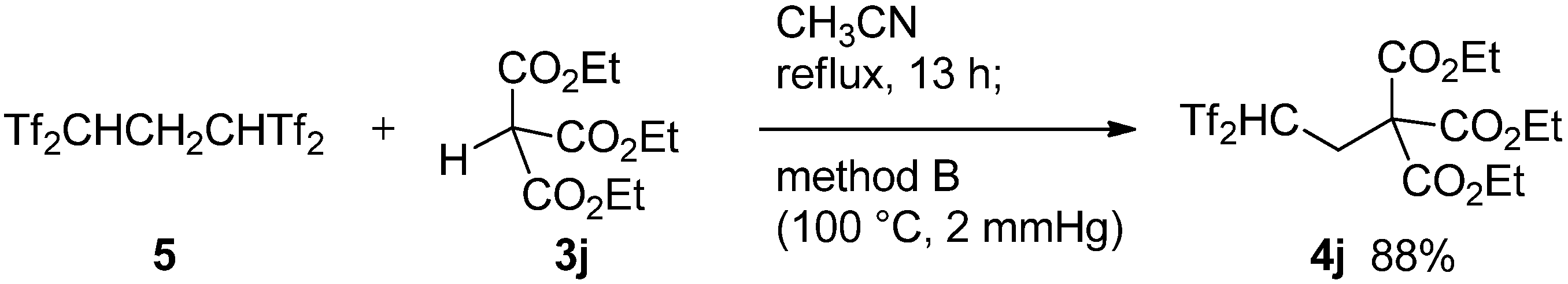
2.1. Improved Synthesis of δ-Oxo-1,1-bis(triflyl)alkanes
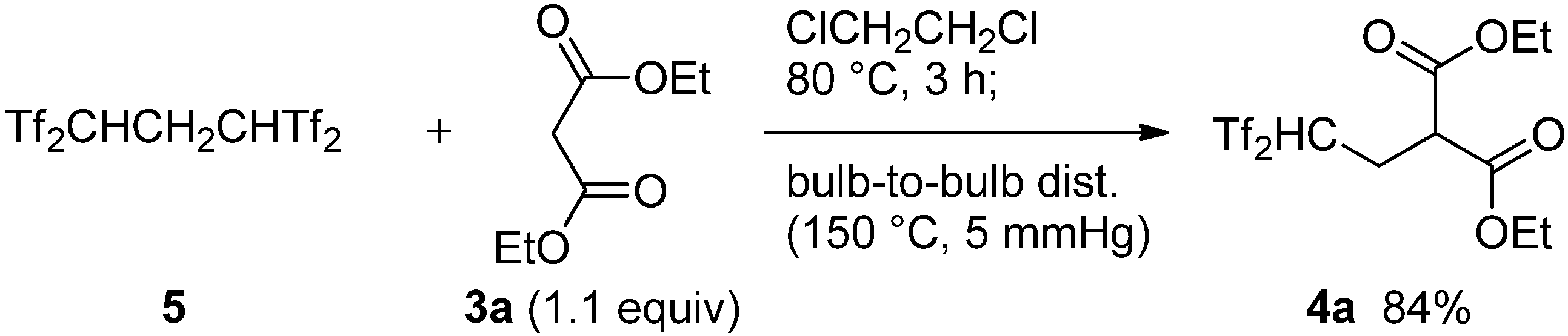

| Entry | 3 | Temp. (°C) | Time (h) | Method a | 4 | Yield b (%) | |
|---|---|---|---|---|---|---|---|
| 1 c | 3b | CH2(CO2CH3)2 | 80 | 8 | A | 4b | 84 |
| 2 c | 3c | CH2(CO2Bn)2 | 80 | 8 | B | 4c | 98 |
| 3 c | 3d | CH2(CO2CH3)P(O)(OCH3)2 | 80 | 5.5 | A | 4d | 57 |
| 4 | 3e | CH2(COt-Bu)CO2CH3 | 40 | 2 | A | 4e | 93 |
| 5 | 3f | CH2(COt-Bu)CO2Et | 40 | 5 | A | 4f | 82 |
| 6 | 3g | CH2(COPh)CO2Et | 40 | 4.5 | B | 4g | 86 |
| 7 | 3h | CH2(COt-Bu)2 | Rt | 4 | A | 4h | 73 |
| 8 | 3i | CH2(COi-Pr)2 | Rt | 2.5 | A | 4i | 80 |
2.2. Gas-Phase and Solution-Phase Acidities of Carbon Acids
3. Experimental
3.1. General
3.2. General Procedure for Bis(triflyl)ethylation Reaction of Enolizable Carbonyls
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Leito, I.; Raamat, E.; Kütt, A.; Saame, J.; Kipper, K.; Koppel, I.A.; Koppel, I.; Zhang, M.; Mishima, M.; Yagupolskii, L.M.; et al. Revision of the gas-phase acidity scale below 300 kcal mol−1. J. Phys. Chem. A 2009, 113, 8421–8424. [Google Scholar] [CrossRef]
- Koppel, I.A.; Taft, R.W.; Anvia, F.; Zhu, S.-Z.; Hu, L.-Q.; Sung, K.-S.; DesMarteau, D.D.; Yagupolskii, L.M.; Yagupolskii, Y.L.; Ignat’ev, N.V.; et al. The gas-phase acidities of very strong neutral Brønsted acids. J. Am. Chem. Soc. 1994, 116, 3047–3057. [Google Scholar] [CrossRef]
- Bordwell, F.G. Equilibrium acidities in dimethyl sulfoxide solution. Acc. Chem. Res. 1988, 21, 456–463. [Google Scholar] [CrossRef]
- Ishihara, K.; Hasegawa, A.; Yamamoto, H. Polystyrene-bound tetrafluorophenylbis(triflyl)methane as an organic-solvent-swellable and strong Brønsted acid catalyst. Angew. Chem. Int. Ed. 2001, 40, 4077–4079. [Google Scholar] [CrossRef]
- Hasegawa, A.; Ishikawa, T.; Ishihara, K.; Yamamoto, H. Facile Synthesis of aryl- and alkyl-bis(trifluoromethylsulfonyl)methanes. Bull. Chem. Soc. Jpn. 2005, 78, 1401–1410. [Google Scholar] [CrossRef]
- Hasegawa, A.; Naganawa, Y.; Fushimi, M.; Ishihara, K.; Yamamoto, H. Design of Brønsted acid-assisted chiral Brønsted acid catalyst bearing a bis(triflyl)methyl group for a Mannich-type reaction. Org. Lett. 2006, 8, 3175–3178. [Google Scholar] [CrossRef]
- Yanai, H.; Takahashi, A.; Taguchi, T. 1,4-Addition of silicon dienoates to α,β-unsaturated aldehydes catalyzed by in situ-generated silicon Lewis acid. Chem. Commun. 2010, 46, 8728–8730. [Google Scholar] [CrossRef]
- Yanai, H.; Yoshino, Y.; Takahashi, A.; Taguchi, T. Carbon acid induced Mukaiyama aldol type reaction of sterically hindered ketones. J. Org. Chem. 2010, 75, 5375–5378. [Google Scholar] [CrossRef]
- Takahashi, A.; Yanai, H.; Zhang, M.; Sonoda, T.; Mishima, M.; Taguchi, T. Highly effective vinylogous Mukaiyama-Michael reaction catalyzed by silyl methide species generated from 1,1,3,3-tetrakis(trifluoromethanesulfonyl)propane. J. Org. Chem. 2010, 75, 1259–1265. [Google Scholar]
- Takahashi, A.; Yanai, H.; Taguchi, T. Tetrakis(trifluoromethanesulfonyl)propane: Highly effective Brønsted acid catalyst for vinylogous Mukaiyama–Michael reaction of α,β-enones with silyloxyfurans. Chem. Commun. 2008, 2385–2387. [Google Scholar] [CrossRef]
- Yanai, H.; Ogura, H.; Fukaya, H.; Kotani, A.; Kusu, F.; Taguchi, T. An effective method to introduce carbon acid functionality: 2,2-Bis(trifluoromethanesulfonyl)ethylation reaction of arenes. Chem. Eur. J. 2011, 17, 11747–11751. [Google Scholar]
- Yanai, H.; Taguchi, T. Organic acid induced olefination reaction of lactone. Chem. Commun. 2012, 48, 8967–8969. [Google Scholar] [CrossRef]
- Yanai, H.; Ishii, N.; Matsumoto, T.; Taguchi, T. Organic acid catalysis in reactions of lactones with silicon enolates. Asian J. Org. Chem. 2013, 2, 989–996. [Google Scholar] [CrossRef]
- Yanai, H.; Egawa, S.; Taguchi, T. Reductive alkylation of bis(triflyl)methane through self-promoting formation of easily isolable 1,1-bis(triflyl)alkenes. Tetrahedron Lett. 2013, 54, 2160–2163. [Google Scholar] [CrossRef]
- Koshar, R.J.; Barber, L.L. 1,1,3,3-Tetrakis(perfluoroalkylsulfonyl)propanes. U.S. Patent 4053519, 11 October 1977. [Google Scholar]
- Yanai, H.; Fujita, M.; Taguchi, T. A regioselective synthesis of poly-substituted aryl triflones through self-promoting three component reaction. Chem. Commun. 2011, 47, 7245–7247. [Google Scholar] [CrossRef]
- Yanai, H.; Yoshino, T.; Fujita, M.; Fukaya, H.; Kotani, A.; Kusu, F.; Taguchi, T. Synthesis, characterization, and applications of zwitterions containing a carbanion moiety. Angew. Chem. Int. Ed. 2013, 52, 1560–1563. [Google Scholar]
- Yanai, H.; Takahashi, Y.; Fukaya, H.; Dobashi, Y.; Matsumoto, T. 2-(Pyridinium-1-yl)-1,1-bis(triflyl)ethanides: Structural behaviour and availability as bis(triflyl)ethylating reagents. Chem. Commun. 2013, 49, 10091–10093. [Google Scholar] [CrossRef]
- Lias, S.G.; Bartmess, J.E.; Liebman, J.F.; Holmes, J.L.; Levin, R.D.; Mallard, G.W. Gas-phase ion and neutral thermochemistry. J. Phys. Chem. Ref. Data 1988, 17 (Suppl. 1), 1–861. [Google Scholar] [CrossRef]
- Zhang, M.; Badal, M.M.R.; Koppel, I.A.; Mishima, M. Gas-phase acidities of α- and α, α-SO2CF3-substituted toluenes varying resonance demand in the electron-rich system. Bull. Chem. Soc. Jpn. 2013, 86, 813–820. [Google Scholar] [CrossRef]
- Takamura, K.; Fuse, T.; Arai, K.; Kusu, F. A review of a new voltammetric method for determining acids. J. Electroanal. Chem. 1999, 468, 53–63. [Google Scholar] [CrossRef]
- Kim, H.-S.; Chung, T.D.; Kim, H. Voltammetric determination of the pKa of various acids in polar aprotic solvents using 1,4-benzoquinone. J. Electroanal. Chem. 2001, 498, 209–215. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yanai, H.; Fujita, M.; Takahashi, A.; Zhang, M.; Mishima, M.; Kotani, A.; Matsumoto, T.; Taguchi, T. Synthesis of δ-Oxo-1,1-bis(triflyl)alkanes and Their Acidities. Molecules 2013, 18, 15531-15540. https://doi.org/10.3390/molecules181215531
Yanai H, Fujita M, Takahashi A, Zhang M, Mishima M, Kotani A, Matsumoto T, Taguchi T. Synthesis of δ-Oxo-1,1-bis(triflyl)alkanes and Their Acidities. Molecules. 2013; 18(12):15531-15540. https://doi.org/10.3390/molecules181215531
Chicago/Turabian StyleYanai, Hikaru, Masaya Fujita, Arata Takahashi, Min Zhang, Masaaki Mishima, Akira Kotani, Takashi Matsumoto, and Takeo Taguchi. 2013. "Synthesis of δ-Oxo-1,1-bis(triflyl)alkanes and Their Acidities" Molecules 18, no. 12: 15531-15540. https://doi.org/10.3390/molecules181215531





