Synthesis of 3,4-Dibenzyltetrahydrofuran Lignans (9,9′-Epoxylignanes)
Abstract
:1. Introduction
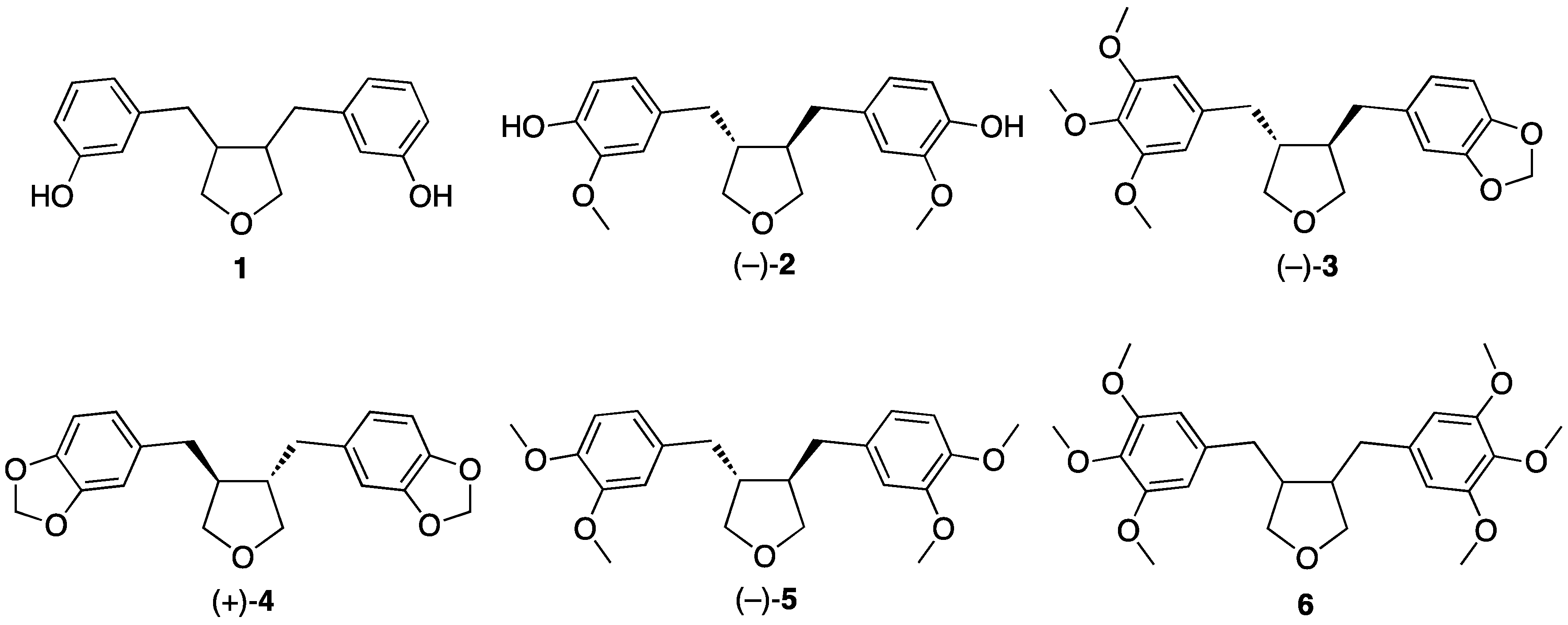
2. Discussion on the Synthesis of Tetrahydrofuran Lignans
2.1. Building the Lignan Skeleton to a Furan or a Tetrahydrofuran
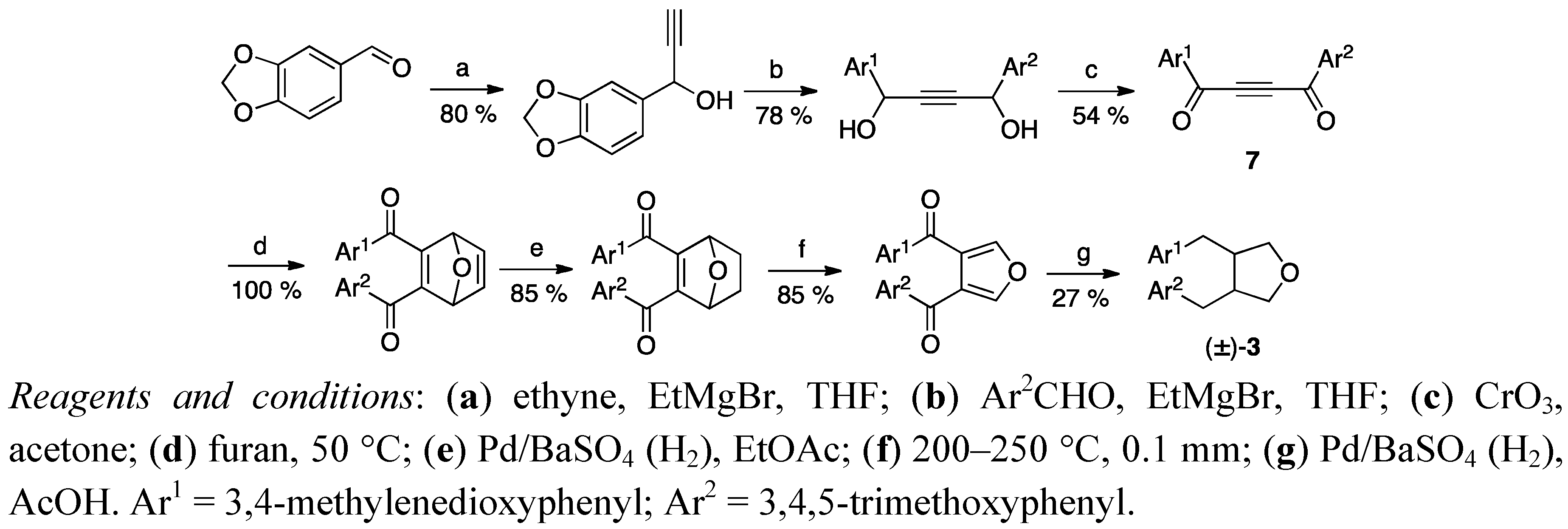
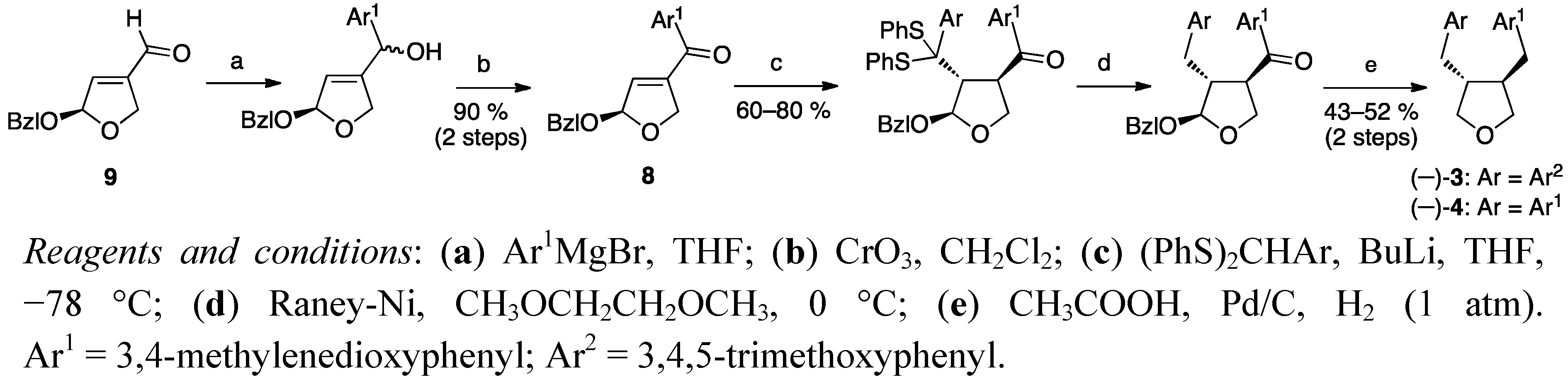
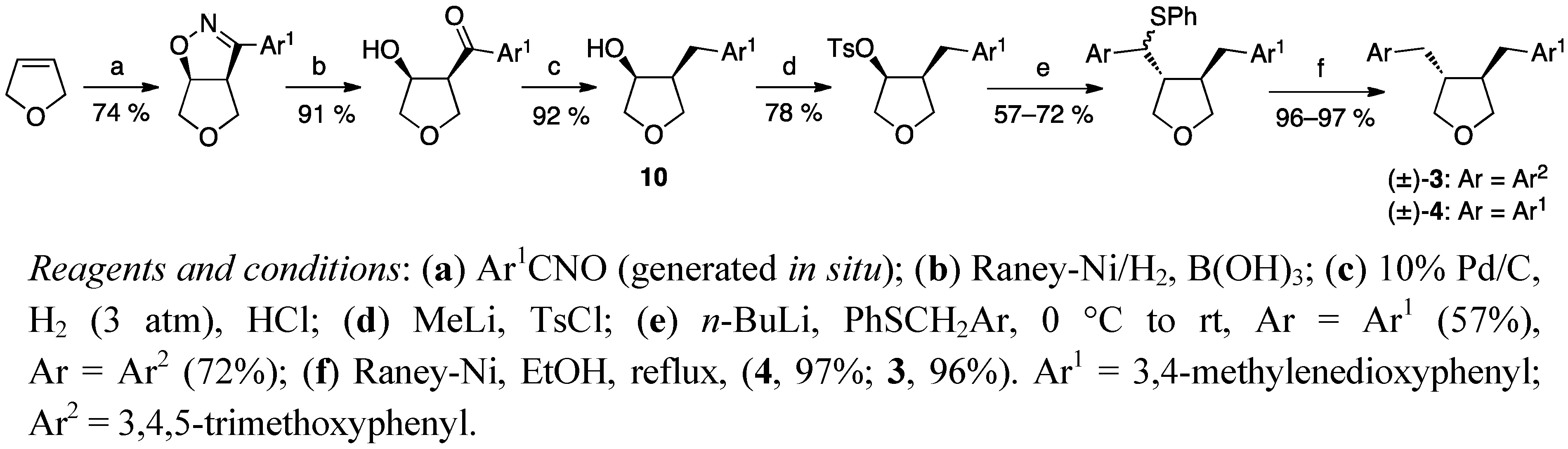
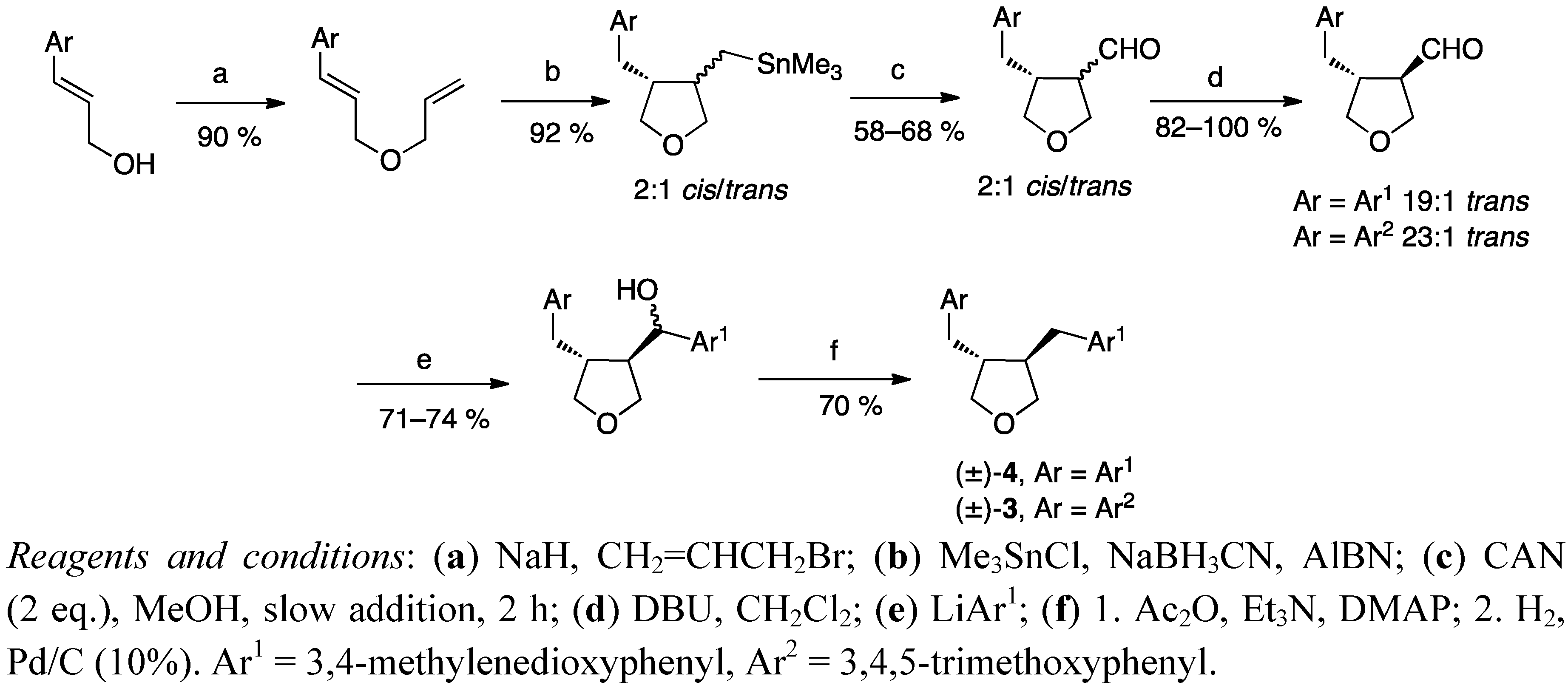
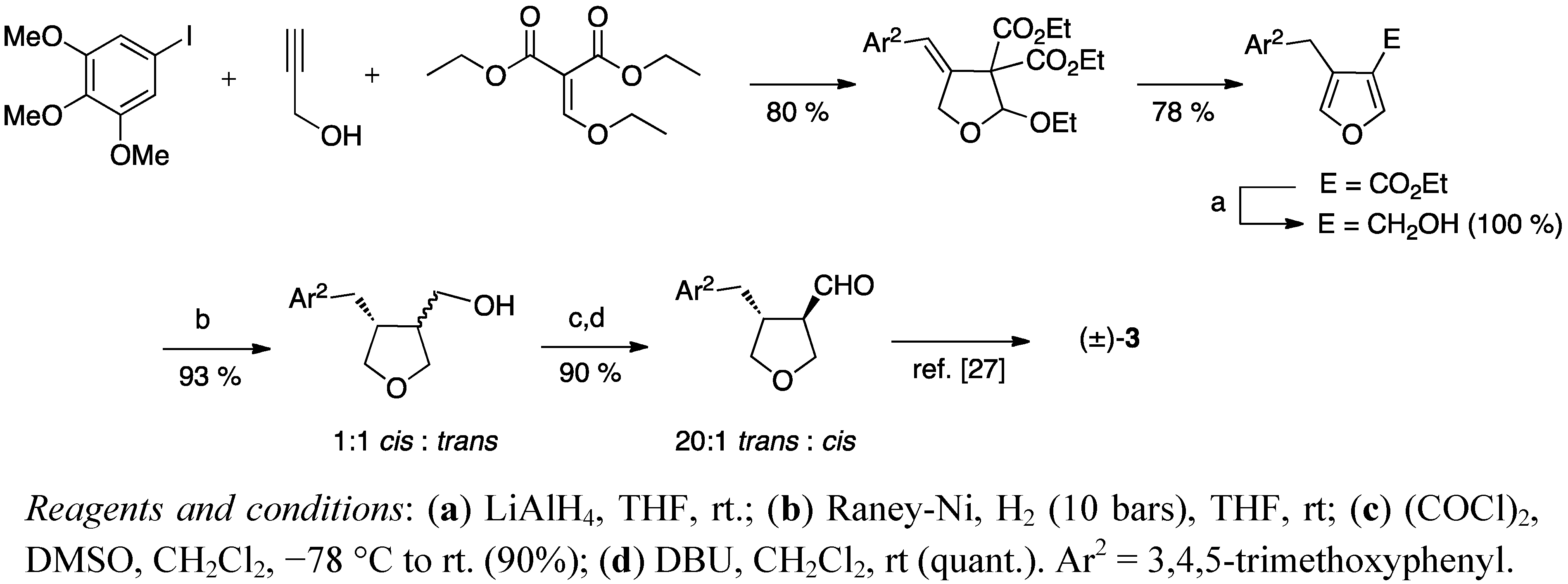
2.2. Cyclisation of a 1,4-Butanediol to a Tetrahydrofuran

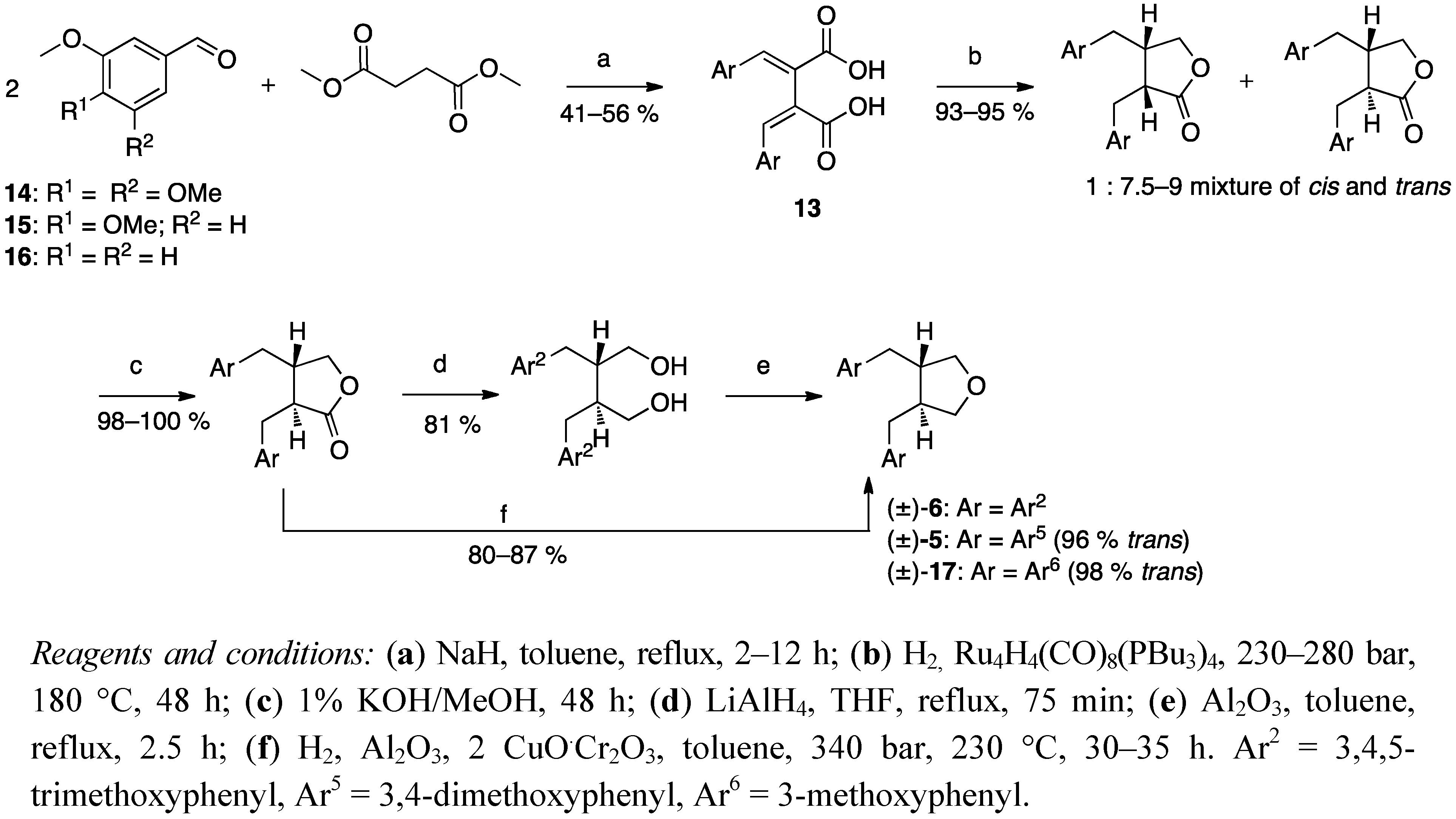
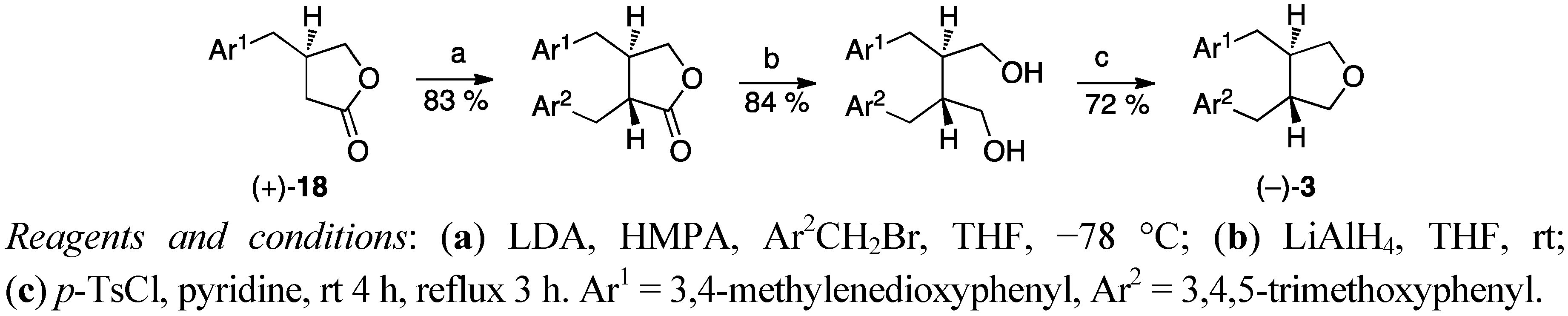
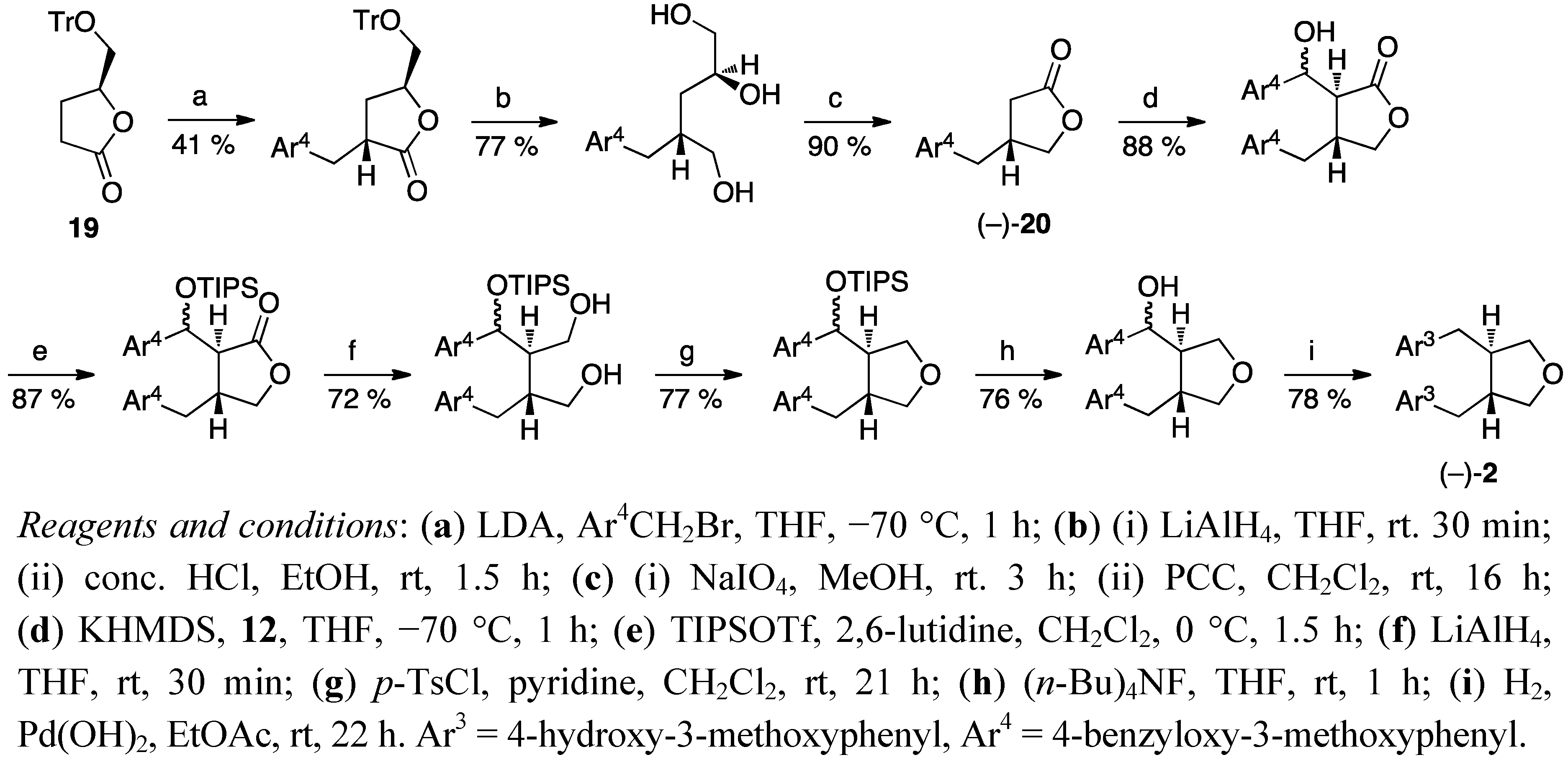
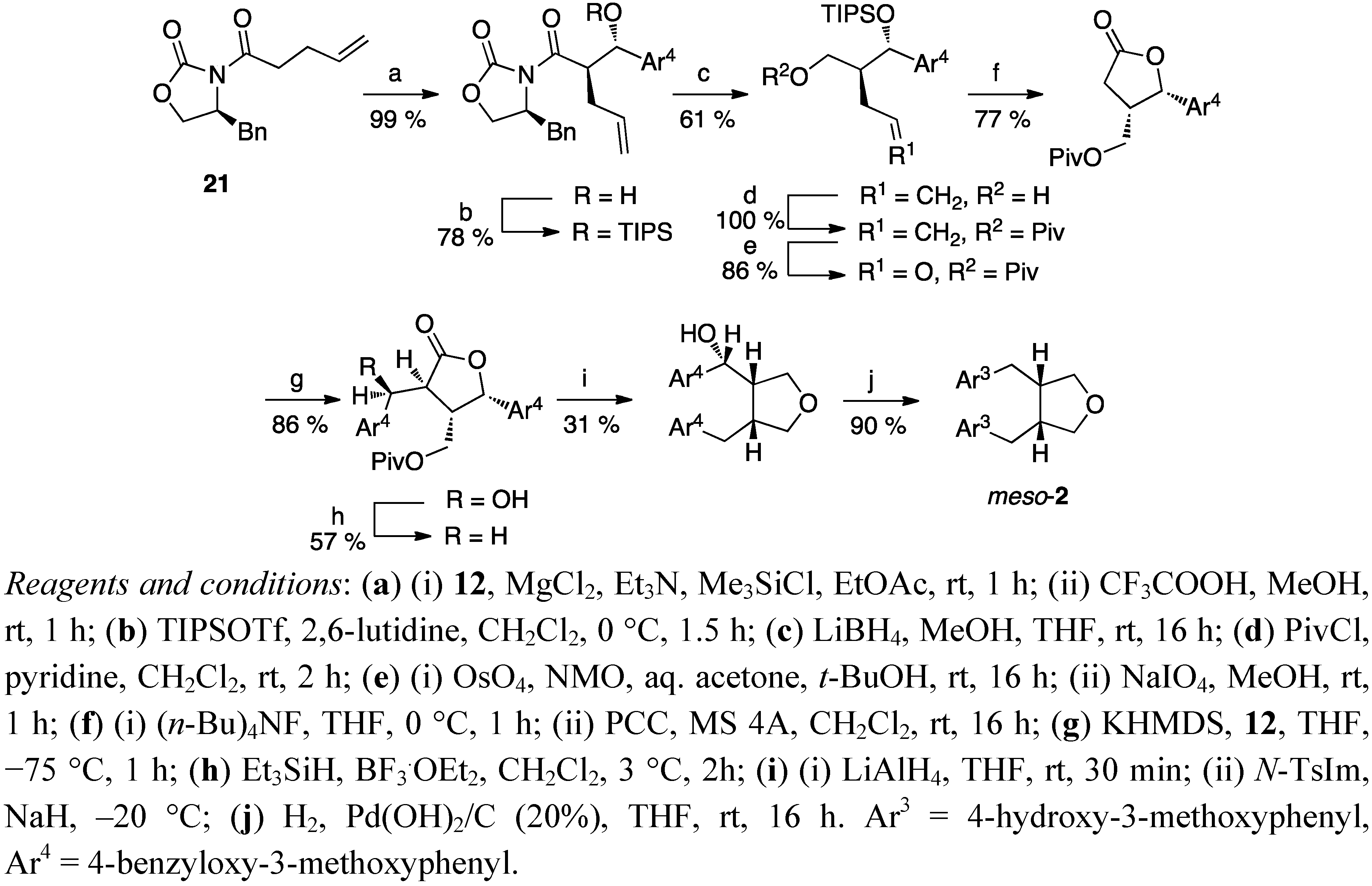
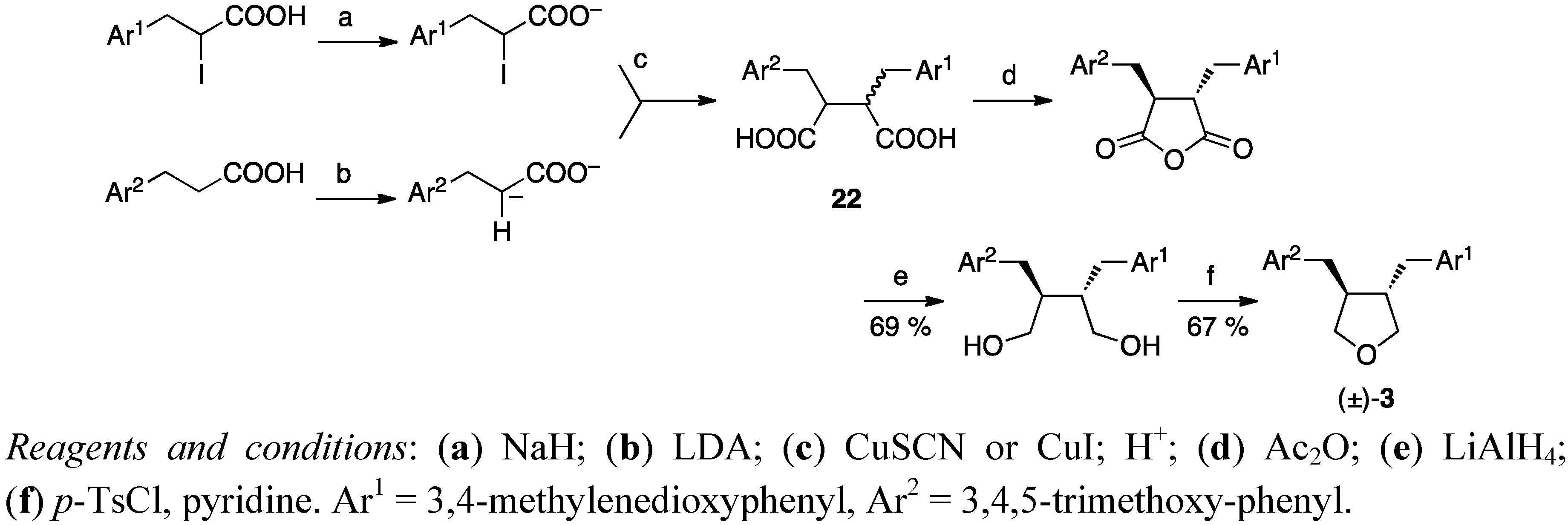
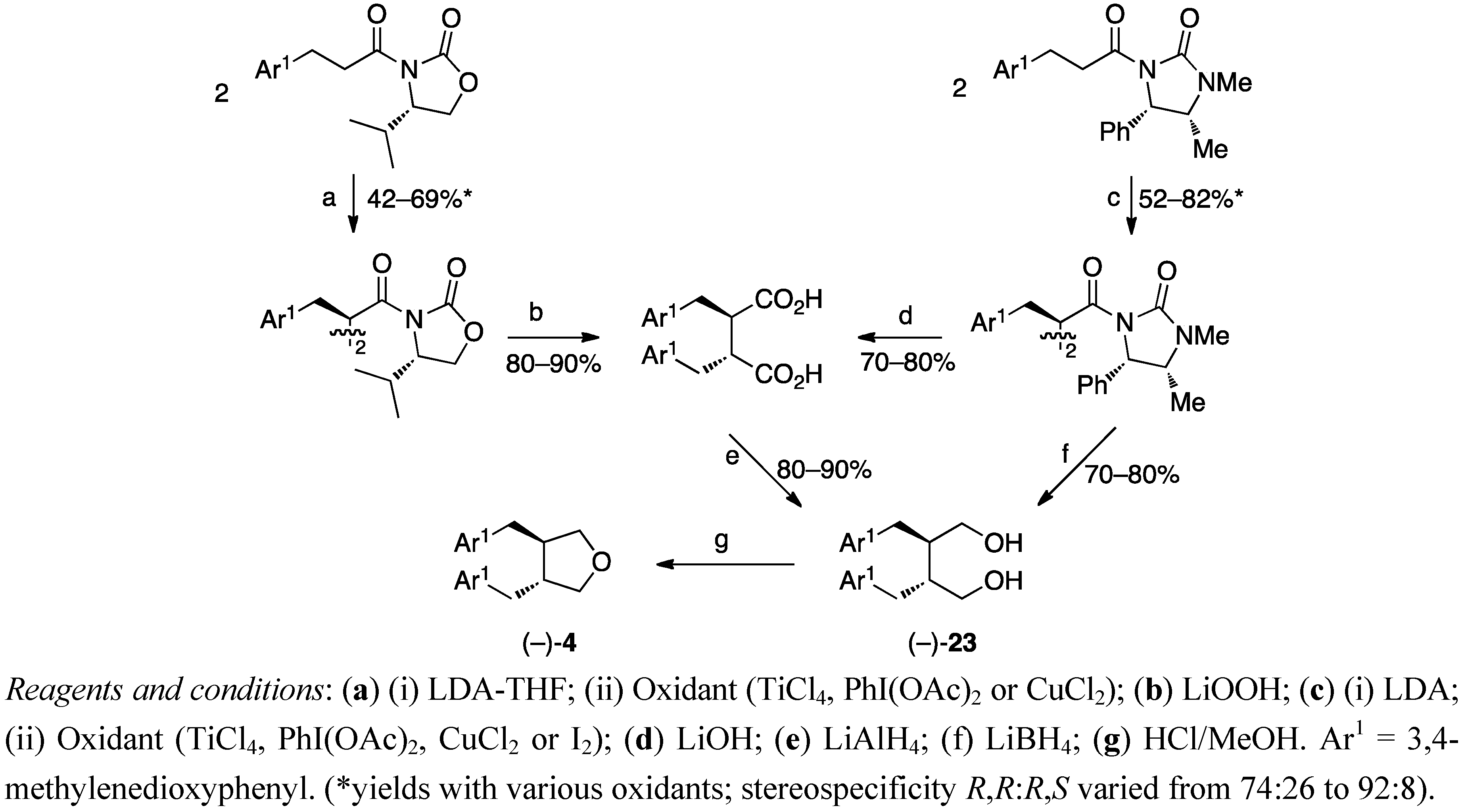


3. Feasible Experimental Approaches
4. Conclusions
Conflicts of Interest
References and Notes
- Benassayag, C.; Perrot-Applanat, M.; Ferre, F. Phytoestrogens as modulators of steroid action in target cells. J. Chromatogr. B 2002, 777, 233–248. [Google Scholar] [CrossRef]
- Stitch, S.R.; Toumba, J.K.; Groen, M.B.; Funke, C.W.; Leemhuis, J.; Vink, J.; Woods, G.F. Excretion, isolation and structure of new phenolic constituent of female urine. Nature 1980, 287, 738–740. [Google Scholar] [CrossRef]
- Setchell, K.D.R.; Lawson, A.M.; Mitchell, F.L.; Adlercreutz, H.; Kirk, D.N.; Axelson, M. Lignans in man and in animal species. Nature 1980, 287, 740–742. [Google Scholar] [CrossRef]
- Struijs, K.; Vincken, J.-P.; Gruppen, H. Bacterial conversion of secoisolariciresinol and anhydrosecoisolariciresinol. J. Appl. Microbiol. 2009, 107, 308–317. [Google Scholar] [CrossRef]
- Niemeyer, H.B.; Honig, D.M.; Kulling, S.E.; Metzler, M. Studies on the metabolism of the plant lignans secoisolariciresinol and matairesinol. J. Agric. Food Chem. 2003, 51, 6317–6325. [Google Scholar] [CrossRef]
- Heinonen, S.; Nurmi, T.; Liukkonen, K.; Poutanen, K.; Wähälä, K.; Deyama, T.; Nishibe, S.; Adlercreutz, H. In vitro metabolism of plant lignans: new presursors of mammalian lignans enterolactone and enterodiol. J. Agric. Food Chem. 2001, 49, 3178–3186. [Google Scholar] [CrossRef]
- Smeds, A.I.; Saarinen, N.M.; Eklund, P.C.; Sjöholm, R.E.; Mäkelä, S.I. New lignan metabolites in rat urine. J. Chromatogr. B 2005, 816, 87–97. [Google Scholar] [CrossRef]
- Matsuura, S.; Iinuma, M. Lignan from calyses of Diospyros kaki. Phytochemistry 1985, 24, 626–628. [Google Scholar] [CrossRef]
- Yamauchi, S.; Hayashi, Y.; Nakashima, Y.; Kirikihira, T.; Yamada, K.; Masuda, T. Effect of benzylic oxygen on the antioxidant activity of phenolic lignans. J. Nat. Prod. 2005, 68, 1459–1470. [Google Scholar] [CrossRef]
- Akiyama, K.; Maruyama, M.; Yamauchi, S.; Nakashima, Y.; Nakato, T.; Tago, R.; Sugahara, T.; Kishida, T.; Koba, Y. Antimicrobiological activity of lignan: Effect of benzylic oxygen and stereochemistry of 2,3-dibenzyl-4-butanolide and 3,4-dibenzyltetrahydrofuran lignans on activity. Biosci. Biotechnol. Biochem. 2007, 71, 1745–1751. [Google Scholar] [CrossRef]
- Wukirsari, T.; Nishiwaki, H.; Hasebe, A.; Shuto, Y.; Yamauchi, S. First discovery of insecticidal activity of 9,9’-epoxylignane and dihydroguaiaretic acid against houseflies and the structure–activity relationship. J. Agric. Food Chem. 2013, 61, 4318–4325. [Google Scholar] [CrossRef]
- Cole, J.R.; Bianchi, E.; Trumbull, E.R. Antitumor agents form Bursera microphylla (Burseraceae) II: Isolation of a new lignan: burseran. J. Pharm. Sci. 1969, 58, 175–176. [Google Scholar] [CrossRef]
- Adlercreutz, H. Lignans and human health. Crit. Rev. Clin. Lab. Sci. 2007, 44, 483–525. [Google Scholar] [CrossRef]
- Schöttner, M.; Ganßer, D.; Spiteller, G. Lignans from the roots of Urtica dioica and their metabolites bind to human sex hormone binding globulin (SHBG). Planta Med. 1997, 63, 529–532. [Google Scholar] [CrossRef]
- Schöttner, M.; Spiteller, G.; Ganßer, D. Lignans interfering with 5α-dihydrotestosterone binding to human sex hormone-binding globulin. J. Nat. Prod. 1998, 61, 119–121. [Google Scholar] [CrossRef]
- Raffaelli, B.; Hoikkala, A.; Leppälä, E.; Wähälä, K. Enterolignans. J. Chromatogr. B 2002, 777, 29–43. [Google Scholar] [CrossRef]
- Wähälä, K.; Rasku, S.; Parikka, K. Deuterated phytoestrogen flavonoids and isoflavonoids for quantitation. J Chromatogr. B 2002, 777, 111–122. [Google Scholar] [CrossRef]
- Fang, J.-M.; Hsu, K.-C.; Cheng, Y.-S. Lignans from leaves of Calocedrus formosana. Phytochemistry 1980, 28, 3553–3555. [Google Scholar] [CrossRef]
- Pan, H.; Lundgren, L.N. Phenolic extractives from root bark of Picea abies. Phytochemistry 1995, 39, 1423–1428. [Google Scholar] [CrossRef]
- Grougnet, R.; Magiatis, P.; Fokialakis, N.; Mitaku, S.; Skaltsounis, A.-L.; Tillequin, F.; Sévenet, T.; Litaudon, M. Koniamborine, the first pyrano[3,2-b]indole alkaloid and other secondary metabolites from Boronella koniambiensis. J. Nat. Prod. 2005, 68, 1083–1086. [Google Scholar] [CrossRef]
- Nimgirawath, S.; Ritchie, E.; Taylor, W.C. The chemical constituents of Australian Flindersia species. XXII: Some extractives of F. brassii. Aust. J. Chem. 1977, 30, 451–453. [Google Scholar] [CrossRef]
- Fuzzatti, N.; Dyatmiko, W.; Rahman, A.; Achmad, F.; Hostettmann, K. Triterpenoids, lignans and a benzofuran derivative from the bark of Aglaia elaeagnoidea. Phytochemistry 1996, 42, 1395–1398. [Google Scholar] [CrossRef]
- Trumbull, E.R; Cole, J.R. Antitumor agents from Bursera microphylla (Burseraceae) III: Synthesis of Burseran. J. Pharm. Sci. 1969, 58, 176–178. [Google Scholar] [CrossRef]
- Rehnberg, N.; Magnusson, G. General conjugate-addition method for the synthesis of enantiomerically pure lignans. Total synthesis of (−)- and (+)-burseran, (−)-dehydroxycubebin, (−)-trichostin, (−)-cubebin, (−)-5′′-methoxyhinokinin, and (−)-hinokinin. J. Org. Chem. 1990, 55, 4340–4349. [Google Scholar] [CrossRef]
- Sibi, M.P.; Gaboury, J.A. Synthesis of lignan natural products (±)-dehydroxycubebin and (±)-burseran by intermolecular nitrile oxide cycloaddition. Synlett 1992, 83–84. [Google Scholar] [CrossRef]
- Gaboury, J.A.; Sibi, M.P. Enantiocontrolled synthesis of burseran, brassilignan, dehydroxycubebin, and other tetrahydrofuran lignans in both enantiomeric forms. Application of intermolecular nitrile oxide cycloadditions and lipase mediated kinetic resolution. J. Org. Chem. 1993, 58, 2173–2180. [Google Scholar] [CrossRef]
- Hanessian, S.; Léger, R. Total synthesis of the lignan antiobiotic burseran and a novel approach to the podophyllotoxin skeleton. Synlett 1992, 402–404. [Google Scholar] [CrossRef]
- Garçon, S.; Vassiliou, S.; Cavicchioli, M.; Hartmann, B.; Monteiro, N.; Balme, G. An effective one-pot synthesis of 3-benzylfurans and their potential utility as versatile precursors of 3,4-dibenzyltetrahydrofuran lignans. Formal synthesis of (±)-burseran. J. Org. Chem. 2001, 66, 4069–4073. [Google Scholar] [CrossRef]
- Haworth, R.D.; Woodcock, D. The constituents of natural phenolic resins. Part XV. The stereochemical relationship of lariciresinol and pinoresinol. J. Chem. Soc. 1939, 1054–1057. [Google Scholar] [CrossRef]
- Haworth, R.D.; Wilson, L. The constituents of natural phenolic resins. Part XXII. Reduction of some lactonic lignans with lithium aluminium hydride. J. Chem. Soc. 1950, 71–72. [Google Scholar] [CrossRef]
- Eich, E.; Pertz, H.; Kaloga, M.; Schulz, J.; Fesen, M.R.; Mazumder, A.; Pommier, Y. (−)-Arctigenin as a lead structure for inhibitors of human immunodeficiency virus type-1 integrase. J. Med. Chem. 1996, 39, 86–95. [Google Scholar] [CrossRef]
- Xia, Y.-M.; You, J.; Wang, Q. Total synthesis of (±)-divanillyltetrahydrofuran ferulate. J. Chem. Sci. 2010, 122, 433–436. [Google Scholar] [CrossRef]
- Coran, S.A.; Bambagiotti-Alberti, M.; Melani, F.; Giannellini, V.; Vincieri, F.F.; Mulinacci, N.; Sala, R.; Moriggi, E. Synthetic butanolide and tetrahydrofuran lignans with platelet activating factor antagonist activity. Eur. J. Med. Chem. 1991, 26, 643–650. [Google Scholar] [CrossRef]
- Bambagiotti-Alberti, M.; Coran, S.A.; Vincieri, F.F.; Mulinacci, N.; Pieraccini, G.M.L. New synthetic route to butanolide lignans by a ruthenium complex catalyzed hydrogenation of the corresponding Stobbe’s fulgenic acid. Heterocycles 1988, 27, 2185–2196. [Google Scholar] [CrossRef]
- Tomioka, K.; Koga, K. Stereoselective total synthesis of optically active trans- and cis-burseran. Determination of the stereochemisty of natural antitumor burseran. Heterocycles 1979, 12, 1523–1528. [Google Scholar] [CrossRef]
- Tomioka, K.; Ishiguro, T.; Koga, K. Stereoselective reactions. X. Total synthesis of optically pure antitumor lignan, burseran. Chem. Pharm. Bull. 1985, 33, 4333–4337. [Google Scholar] [CrossRef]
- Tomioka, K.; Mizuguchi, H.; Koga, K. Stereoselective reactions. V. Design of the asymmetric synthesis of lignan lactones. Synthesis of optically active podorhizon and deoxypodorhizon by 1,3-asymmetric induction. Chem. Pharm. Bull. 1982, 30, 4304–4313. [Google Scholar] [CrossRef]
- Belletire, J.L.; Fry, D.F.; Fremont, S.L. The role of dianion coupling in the synthesis of dibenzylbutane lignans. J. Nat. Prod. 1992, 55, 184–193. [Google Scholar] [CrossRef]
- Anjaneyulu, A.S.R.; Ramaiah, P.A.; Row, L.R.; Venkateswarlu, R.; Pelter, A.; Ward, R.S. New lignans from the heartwood of Cleistanthus collinus. Tetrahedron 1981, 37, 3641–3652. [Google Scholar] [CrossRef]
- Ward, R.S.; Hughes, D.D. Oxidative cyclisation of 3,4-dibenzyltetrahydrofurans using ruthenium tetra(trifluoroacetate). Tetrahedron 2001, 57, 2057–2064. [Google Scholar] [CrossRef]
- Kise, N.; Ueda, T.; Kumada, K.; Terao, Y.; Ueda, N. Oxidative homocoupling of chiral 3-arylpropanoic acid derivatives. Application to asymmetric synthesis of lignans. J. Org. Chem. 2000, 65, 464–468. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, Y.; Li, Y.; Yu, W.; Hou, Z.J. An efficient method for the synthesis of lignans. Tetrahedron 2006, 62, 6107–6112. [Google Scholar] [CrossRef]
- Raffaelli, B.; Leppälä, E.; Chappuis, C.; Wähälä, K. New potential mammalian lignan metabolites of environmental phytoestrogens. Environ. Chem. Lett. 2006, 4, 1–9. [Google Scholar] [CrossRef]
- Raffaelli, B.; Wähälä, K.; Hase, T. Asymmetric synthesis, stereochemistry and rearrangement reactions of naturally occurring 7’-hydroxylignano-9,9’-lactones. Org. Biomol. Chem. 2006, 4, 331–341. [Google Scholar] [CrossRef]
- General Procedure. Lignanodiols were synthesised by the tandem Michael addition–alkylation method followed by Raney nickel desulfurisation and debenzylation to give lignanolactones which were further reduced to dibenzylbutanediols with LiAlH4, as shown in scheme 11 [40]. The lignanodiol (20–23 mg/ 0.051–0.085 mmol) and conc. HCl (0.3–0.45 mL) were placed into a microwave reactor pressure proof vessel and sealed. The reaction was carried out in a CEM Discover System. Microwave irradiation of 80 W was used to reach 40 °C. The reaction was held at this temperature with stirring and cooling for 30 min. After allowing the mixture to cool to rt, 0.5 mL of H2O was added and the mixture was stirred for 10 min and extracted with EtOAc. The organic phase was washed with water and brine, dried over anhydrous Na2SO4, filtered and concentrated. The crude product was purified with column chromatography using EtOAc and CH2Cl2 as eluents as such or appropriate mixtures to obtain the tetrahydrofuran lignan products as white solids (13–20 mg) in 68%–95% yields. The purified compounds were characterised with 1D-NMR (1H and 13C), 2D-NMR (COSY, HMBC and HSQC) and EI-MS spectra.
- Gedye, R.; Smith, F.; Westaway, K.; Ali, H.; Baldisera, L.; Laberge, L.; Rousell, J. The use of microwave ovens for rapid organic synthesis. Tetrahedron Lett. 1986, 27, 279–282. [Google Scholar]
- Giguere, R.J.; Bray, T.L.; Duncan, S.M.; Majetich, G. Application of commercial microwave ovens to organic synthesis. Tetrahedron Lett. 1986, 27, 4945–4948. [Google Scholar]
- Mondière, A.; Pousse, G.; Bouyssi, D.; Balme, G. Efficient rhodium-catalyzed conjugate addition of arylboronic acids to unsaturated furano esters for the highly stereoselective synthesis of four natural trisubstituted furanolignan. Eur. J. Org. Chem. 2009, 25, 4225–4229. [Google Scholar]
- Mont, N.; Mehta, V.P.; Appukkuttan, P.; Beryozkina, T.; Toppet, S.; Van Hecke, K.; Van Meervelt, L.; Voet, A.; DeMaeyer, M.; Van der Eycken, E. Diversity oriented microwave-assisted synthesis of (−)-staganacin aza-analogues. J. Org. Chem. 2008, 73, 7509–7516. [Google Scholar] [CrossRef]
- Hakala, U.; Wähälä, K. Expedient deuterolabeling of polyphenols in ionic liquids–DCl/D2O under microwave irradiation. J. Org. Chem. 2007, 72, 5817–5819. [Google Scholar] [CrossRef]
- Pohjoispää, M.; Hakala, U.; Silvennoinen, G.; Wähälä, K. Expedient deuterolabelling of lignans using ionic liquid–DCl/D2O under microwave heating. J. Label. Compd. Radiopharm. 2010, 53, 429–430. [Google Scholar]
- Pohjoispää, M.; Wähälä, K.; University of Helsinki, Finland. Deuterium labelling of lignans. Unpublished work. 2013. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pohjoispää, M.; Wähälä, K. Synthesis of 3,4-Dibenzyltetrahydrofuran Lignans (9,9′-Epoxylignanes). Molecules 2013, 18, 13124-13138. https://doi.org/10.3390/molecules181113124
Pohjoispää M, Wähälä K. Synthesis of 3,4-Dibenzyltetrahydrofuran Lignans (9,9′-Epoxylignanes). Molecules. 2013; 18(11):13124-13138. https://doi.org/10.3390/molecules181113124
Chicago/Turabian StylePohjoispää, Monika, and Kristiina Wähälä. 2013. "Synthesis of 3,4-Dibenzyltetrahydrofuran Lignans (9,9′-Epoxylignanes)" Molecules 18, no. 11: 13124-13138. https://doi.org/10.3390/molecules181113124



