11β-Hydroxyandrostenedione Returns to the Steroid Arena: Biosynthesis, Metabolism and Function
Abstract
:1. Introduction
2. The History of the 11OHA4 Metabolite
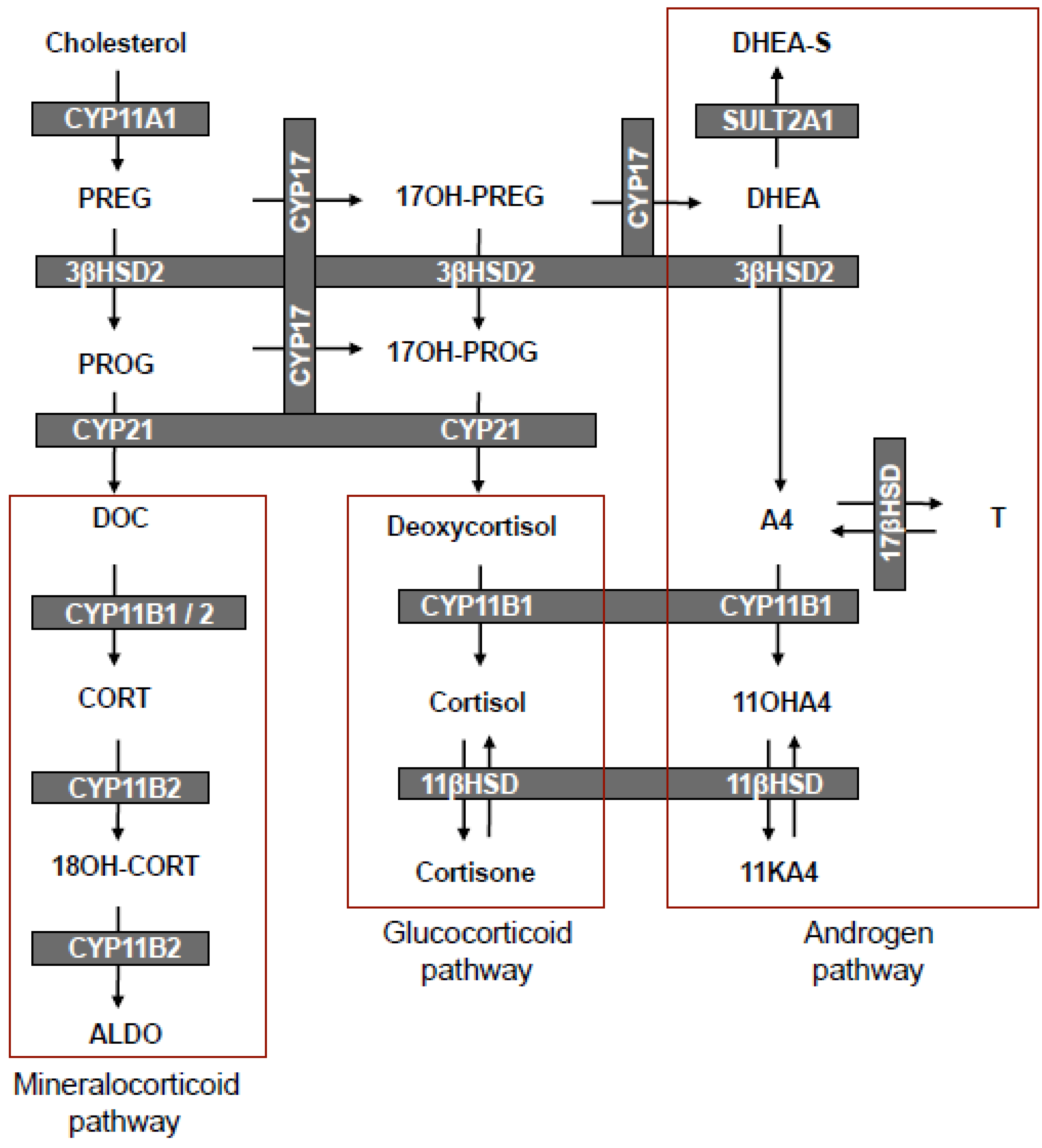
3. The Biosynthesis of 11OHA4
| Steroid metabolite | Basal | + Forskolin | |||
|---|---|---|---|---|---|
| Total ± SEM (nM) | Total ± SEM (nM) | Fold Change | |||
| PREG | 237.7 ± 12.7 | 799.5 ± 34.2 | ↑ | 3.4 | *** |
| 17OH-PREG | ND | ND | |||
| DHEA | 232.3 ± 18.2 | 464.5 ± 34.7 | ↑ | 2.0 | *** |
| DHEA-S | 3.4 ± 0.2 | 5.1 ± 0.3 | ↑ | 1.6 | *** |
| A4 | 913.2 ± 29.2 | 1338.0 ± 81.1 | ↑ | 1.4 | *** |
| 11OHA4 | 100.4 ± 5.2 | 329.9 ± 27.2 | ↑ | 3.5 | *** |
| 11KA4 | 2.8 ± 1.0 | 3.4 ± 0.8 | |||
| T | 46.0 ± 3.8 | 50.0 ± 2.4 | |||
| 11OHT | ND | ND | |||
| 11KT | ND | ND | |||
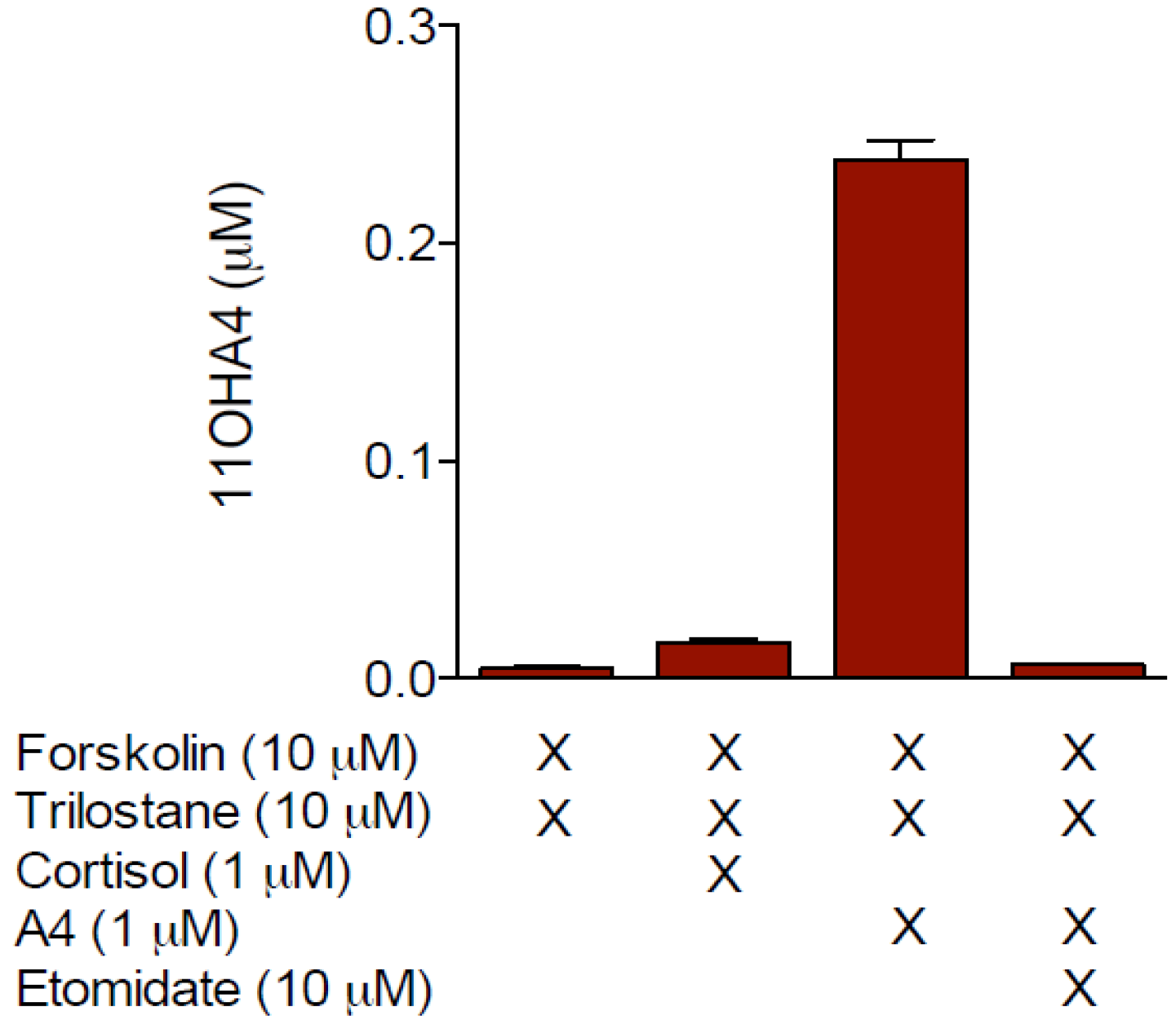
4. The Metabolism of 11OHA4
4.1. 11β-Hydroxysteroid Dehydrogenase (11βHSD)
4.2. 17β-Hydroxysteroid Dehydrogenase (17βHSD)
4.3. Steroid 5α-Reductase (SRD5A)
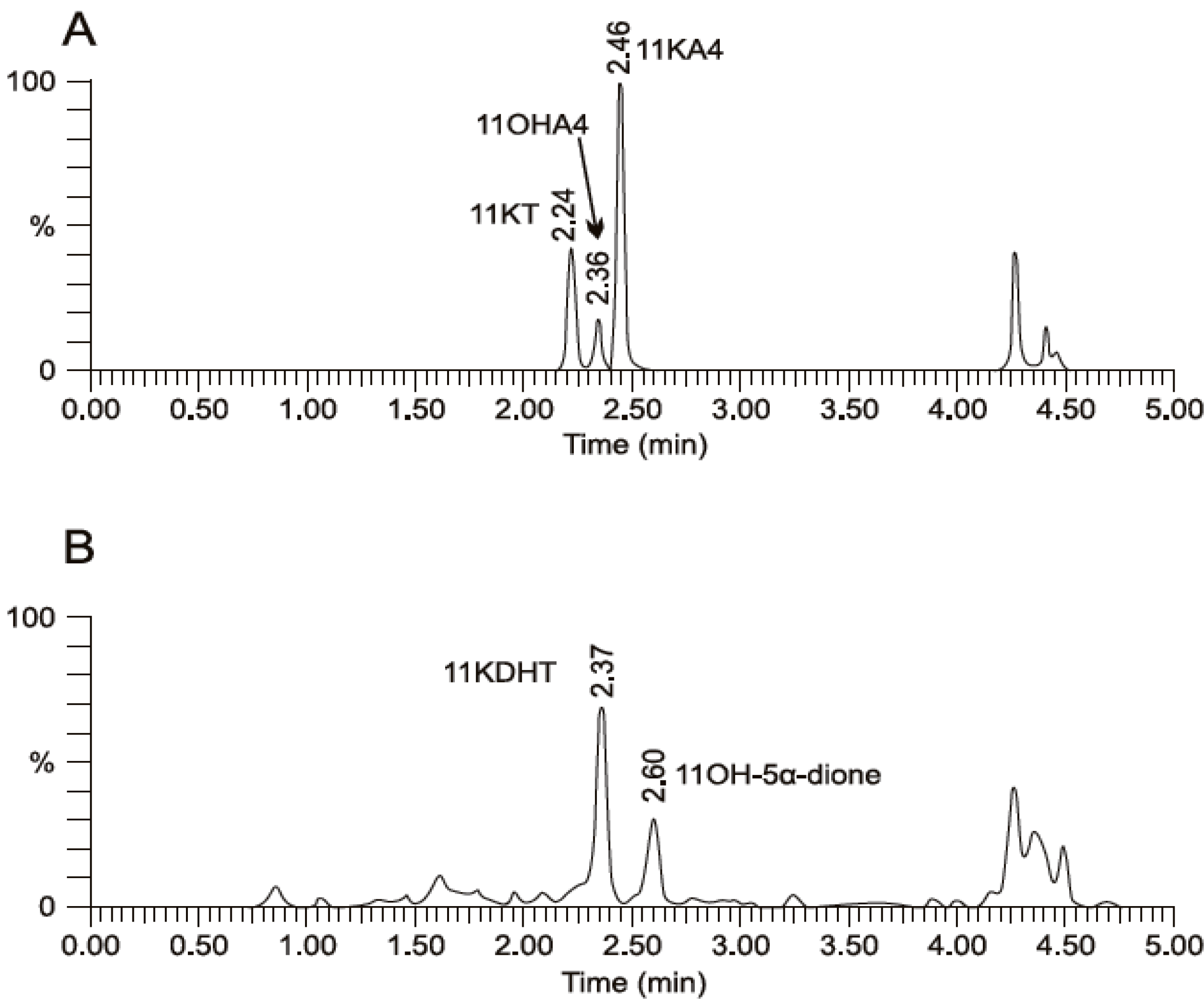
5. The Metabolism of 11OHA4 in Vivo
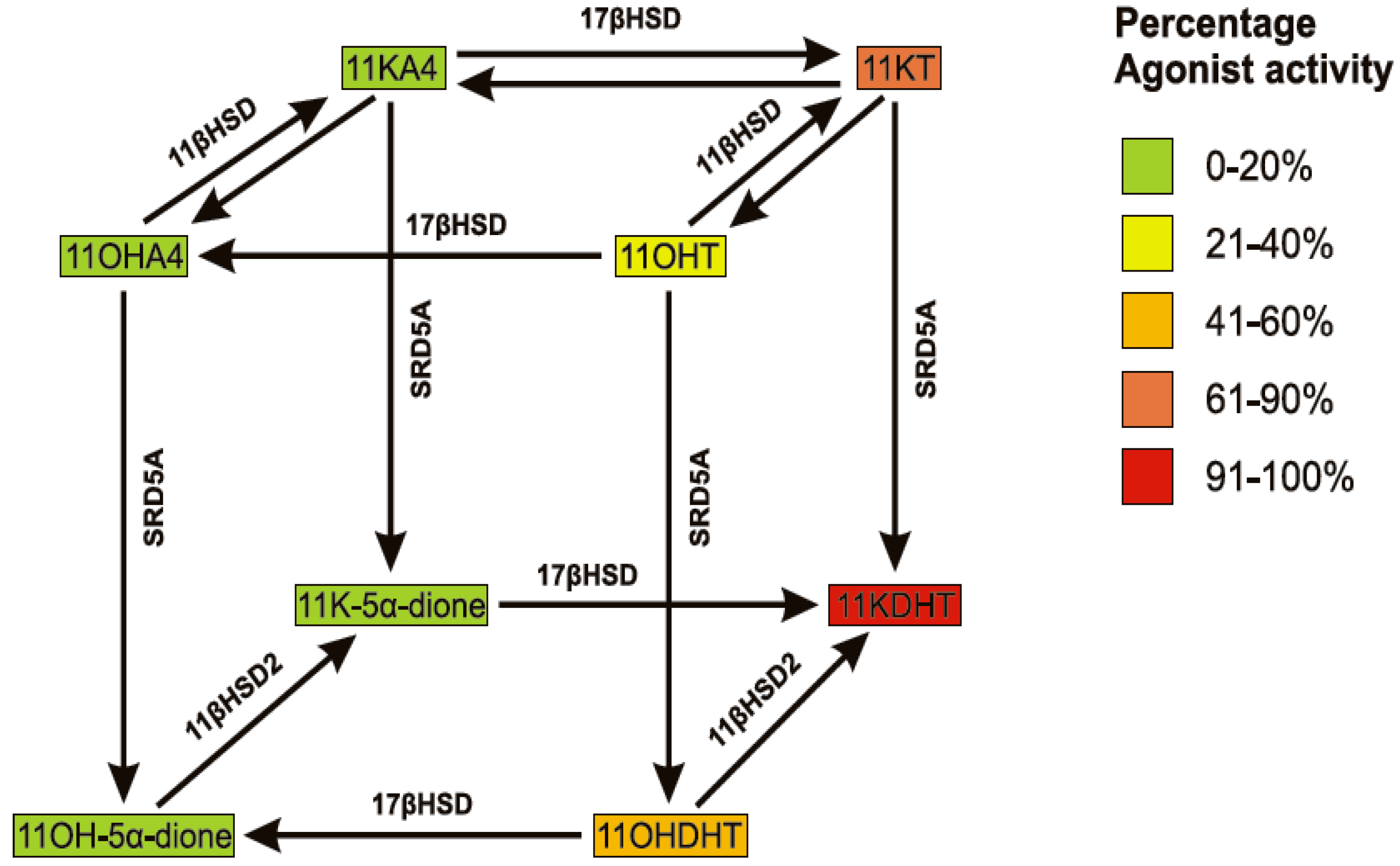
6. The Biological Significance of 11OHA4
7. Summary and Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Touchstone, H.C.; Glazer, L.; Cooper, D.Y.; Roberts, J.M. The isolation of delta 4-androstene-11 beta-ol-3,17-dione from human adrenal incubates. J. Clin. Endocrinol. Metab. 1955, 15, 382–384. [Google Scholar] [CrossRef]
- Jeanloz, R.W.; Levy, H.; Jacobsen, R.P.; Hechter, O.; Schenker, V.; Pincus, G. Chemical transformations of steroids by adrenal perfusion. III. Delta4-Androstene-3, 17-dione. J. Biol. Chem. 1953, 203, 453–461. [Google Scholar]
- Dorfman, R.I. In vivo metabolism of neutral steroid hormones. J. Clin. Endocrinol. Metab. 1954, 14, 318–325. [Google Scholar] [CrossRef]
- Masuda, M. Urinary ketosteroid excretion patterns in congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 1957, 17, 1181–1190. [Google Scholar] [CrossRef]
- Cohn, G.L.; Mulrow, P.J. Androgen release and synthesis in vitro by human adult adrenal glands. J. Clin. Invest. 1963, 42, 64–78. [Google Scholar] [CrossRef]
- Dadevoh, B.K.; Engel, L.L.; Shaw, D.; Gray, C.H. Metabolism of progesterone-4–14C by adrenal tissue from a patient with cushing’s syndrome. J. Clin. Endocrinol. Metab. 1965, 25, 784–791. [Google Scholar] [CrossRef]
- Swart, P.; Swart, A.C.; Waterman, M.R.; Estabrook, R.W.; Mason, J.I. Progesterone 16 alpha-hydroxylase activity is catalyzed by human cytochrome P450 17 alpha-hydroxylase. J. Clin. Endocrinol. Metab. 1993, 77, 98–102. [Google Scholar] [CrossRef]
- Goldzieher, J.W.; Beering, S.C. Metabolism of 11-beta-hydroxyandrostenedione, adrenosterone and hydrocortisone to urinary 11-oxy 17-ketosteroids. J. Clin. Endocrinol. Metab. 1969, 29, 171–178. [Google Scholar] [CrossRef]
- Goldzieher, J.W.; Axelrod, L.R. Precursors of urinary 11-oxy-17-ketosteroids. II. Allo-3 -tetrahydrocortisol. J. Clin. Endocrinol. Metab. 1971, 33, 176–181. [Google Scholar] [CrossRef]
- Axelrod, L.R.; Kraemer, D.C.; Burdett, J., Jr.; Goldzieher, J.W. Biosynthesis of 11-hydroxyandrostenedione by human and baboon adrenals. Acta Endocrinol. (Copenh) 1973, 72, 545–550. [Google Scholar]
- Gustafsson, J.A.; Hrycay, E.G.; Ernster, L. Sodium periodate, sodium chlorite, and organic hydroperoxides as hydroxylating agents in steroid hydroxylation reactions catalyzed by adrenocortical microsomal and mitochondrial cytochrome P450. Arch. Biochem. Biophys. 1976, 174, 440–453. [Google Scholar] [CrossRef]
- Estabrook, R.W. Steroid hydroxylations: A paradigm for cytochrome P450 catalyzed mammalian monooxygenation reactions. Biochem. Biophys. Res. Commun. 2005, 338, 290–298. [Google Scholar] [CrossRef]
- Lombardo, M.E.; Hudson, P.B. The biosynthesis of adrenocortical hormones by the human adrenal gland in vitro. Endocrinology 1959, 65, 417–425. [Google Scholar] [CrossRef]
- Hudson, R.W.; Killinger, D.W. The in vitro biosynthesis of 11 -hydroxyandrostenedione by human adrenal homogenates. J. Clin. Endocrinol. Metab. 1972, 34, 215–224. [Google Scholar] [CrossRef]
- Klein, A.; Siebenmann, R.; Curtius, H.C.; Zachmann, M. Steroid 11beta-hydroxylase activity in the microsomal fraction of human adrenals. J. Steroid Biochem. 1976, 7, 283–287. [Google Scholar] [CrossRef]
- Chang, E.; Mittelman, A.; Dao, T.L. Metabolism of 4-C-14-testosterone in normal human adrenal homogenate. J. Biol. Chem. 1963, 238, 913–917. [Google Scholar]
- Dorfman, R.I.; Rooks, W.H.; Jones, J.B.; Leman, J.D. Androgenic activity of highly purified 5alpha-androstane and 5alpha-androstan-17beta-ol. J. Med. Chem. 1966, 9, 930–931. [Google Scholar] [CrossRef]
- Rosemberg, E.; Dorfman, R.I. Biological activity of 9 alpha-fluoro-11 beta-hydroxy-delta 4-androstene-3, 17-dione. Proc. Soc. Exp. Biol. Med. 1958, 99, 336–338. [Google Scholar] [CrossRef]
- Goldzieher, J.W.; de la, P.A.; Aivaliotis, M.M. Radioimmunoassay of plasma androstenedione, testosterone and 11beta-hydroxyandrostenedione after chromatography on Lipidex-5000 (hydroxyalkoxypropyl Sephadex). J. Steroid Biochem. 1978, 9, 169–173. [Google Scholar] [CrossRef]
- Belanger, B.; Fiet, J.; Belanger, A. Effects of adrenocorticotropin on adrenal and plasma 11 beta-hydroxyandrostenedione in the guinea pig and determination of its relative androgen potency. Steroids 1993, 58, 29–34. [Google Scholar] [CrossRef]
- Labrie, C.; Belanger, A.; Labrie, F. Androgenic activity of dehydroepiandrosterone and androstenedione in the rat ventral prostate. Endocrinology 1988, 123, 1412–1417. [Google Scholar] [CrossRef]
- Miller, W.L. Molecular biology of steroid hormone synthesis. Endocr. Rev. 1988, 9, 295–318. [Google Scholar] [CrossRef]
- Sato, H.; Ashida, N.; Suhara, K.; Itagaki, E.; Takemori, S.; Katagiri, M. Properties of an adrenal cytochrome P-450 (P-45011beta) for the hydroxylations of corticosteroids. Arch. Biochem. Biophys. 1978, 190, 307–314. [Google Scholar] [CrossRef]
- Mathew, P.A.; Mason, J.I.; Trant, J.M.; Sanders, D.; Waterman, M.R. Amino acid substitutions Phe66----Leu and Ser126----Pro abolish cortisol and aldosterone synthesis by bovine cytochrome P450(11)beta. J. Biol. Chem. 1990, 265, 20228–20233. [Google Scholar]
- Curnow, K.M.; Tusie-Luna, M.T.; Pascoe, L.; Natarajan, R.; Gu, J.L.; Nadler, J.L.; White, P.C. The product of the CYP11B2 gene is required for aldosterone biosynthesis in the human adrenal cortex. Mol. Endocrinol. 1991, 5, 1513–1522. [Google Scholar] [CrossRef]
- Zhang, G.; Miller, W.L. The human genome contains only two CYP11B (P450c11) genes. J. Clin. Endocrinol. Metab. 1996, 81, 3254–3256. [Google Scholar] [CrossRef]
- Xing, Y.; Edwards, M.A.; Ahlem, C.; Kennedy, M.; Cohen, A.; Gomez-Sanchez, C.E.; Rainey, W.E. The effects of ACTH on steroid metabolomic profiles in human adrenal cells. J. Endocrinol. 2011, 209, 327–335. [Google Scholar]
- Schloms, L.; Storbeck, K.H.; Swart, P.; Gelderblom, W.C.; Swart, A.C. The influence of Aspalathus linearis (Rooibos) and dihydrochalcones on adrenal steroidogenesis: Quantification of steroid intermediates and end products in H295R cells. J. Steroid Biochem. Mol. Biol. 2012, 128, 128–138. [Google Scholar] [CrossRef]
- Denner, K.; Rainey, W.E.; Pezzi, V.; Bird, I.M.; Bernhardt, R.; Mathis, J.M. Differential regulation of 11 beta-hydroxylase and aldosterone synthase in human adrenocortical H295R cells. Mol. Cell Endocrinol. 1996, 121, 87–91. [Google Scholar] [CrossRef]
- Liakos, P.; Lenz, D.; Bernhardt, R.; Feige, J.J.; Defaye, G. Transforming growth factor beta1 inhibits aldosterone and cortisol production in the human adrenocortical cell line NCI-H295R through inhibition of CYP11B1 and CYP11B2 expression. J. Endocrinol. 2003, 176, 69–82. [Google Scholar] [CrossRef]
- Rainey, W.E.; Bird, I.M.; Mason, J.I. The NCI-H295 cell line: A pluripotent model for human adrenocortical studies. Mol. Cell Endocrinol. 1994, 100, 45–50. [Google Scholar] [CrossRef]
- Hofland, J.; de Herder, W.W.; Derks, L.; Hofland, L.J.; van Koetsveld, P.M.; de Krijger, R.R.; van Nederveen, F.H.; Horvath, A.; Stratakis, C.A.; de Jong, F.H.; et al. Regulation of steroidogenesis in a primary pigmented nodular adrenocortical disease-associated adenoma leading to virilization and subclinical Cushing’s syndrome. Eur. J. Endocrinol. 2013, 168, 67–74. [Google Scholar] [CrossRef]
- Swart, A.C.; Schloms, L.; Storbeck, K.H.; Bloem, L.M.; Toit, T.D.; Quanson, J.L.; Rainey, W.E.; Swart, P. 11beta-Hydroxyandrostenedione, the product of androstenedione metabolism in the adrenal, is metabolized in LNCaP cells by 5alpha-reductase yielding 11beta-hydroxy-5alpha-androstanedione. J. Steroid Biochem. Mol. Biol. 2013, 138C, 132–142. [Google Scholar]
- Provencher, P.; Lorrain, A.; Belanger, A.; Fiet, J. Steroid biosynthesis by zona glomerulosa-fasciculata cells in primary culture of guinea-pig adrenals. J. Steroid Biochem. 1990, 36, 589–596. [Google Scholar] [CrossRef]
- Kornel, L. Colocalization of 11 beta-hydroxysteroid dehydrogenase and mineralocorticoid receptors in cultured vascular smooth muscle cells. Am. J. Hypertens. 1994, 7, 100–103. [Google Scholar]
- Shackleton, C.H.; Neres, M.S.; Hughes, B.A.; Stewart, P.M.; Kater, C.E. 17-Hydroxylase/C17,20-lyase (CYP17) is not the enzyme responsible for side-chain cleavage of cortisol and its metabolites. Steroids 2008, 73, 652–656. [Google Scholar] [CrossRef]
- Storbeck, K.H.; Bloem, L.M.; Africander, D.; Schloms, L.; Swart, P.; Swart, A.C. 11beta-Hydroxydihydrotestosterone and 11-ketodihydrotestosterone, novel C19 steroids with androgenic activity: A putative role in castration resistant prostate cancer? Mol. Cell Endocrinol. 2013, 377, 135–146. [Google Scholar] [CrossRef]
- Mindnich, R.; Moller, G.; Adamski, J. The role of 17 beta-hydroxysteroid dehydrogenases. Mol. Cell Endocrinol. 2004, 218, 7–20. [Google Scholar] [CrossRef]
- Andersson, S.; Russell, D.W. Structural and biochemical properties of cloned and expressed human and rat steroid 5 alpha-reductases. Proc. Natl. Acad. Sci. USA 1990, 87, 3640–3644. [Google Scholar] [CrossRef]
- Bruchovsky, N.; Wilson, J.D. The conversion of testosterone to 5-alpha-androstan-17-beta-ol-3-one by rat prostate in vivo and in vitro. J. Biol. Chem. 1968, 243, 2012–2021. [Google Scholar]
- Chang, K.H.; Li, R.; Papari-Zareei, M.; Watumull, L.; Zhao, Y.D.; Auchus, R.J.; Sharifi, N. Dihydrotestosterone synthesis bypasses testosterone to drive castration-resistant prostate cancer. Proc. Natl. Acad. Sci. USA 2011, 108, 13728–13733. [Google Scholar] [CrossRef]
- Rege, J.; Nakamura, Y.; Satoh, F.; Morimoto, R.; Kennedy, M.R.; Layman, L.C.; Honma, S.; Sasano, H.; Rainey, W.E. Liquid chromatography-tandem mass spectrometry analysis of human adrenal vein 19-carbon steroids before and after ACTH stimulation. J. Clin. Endocrinol. Metab. 2013, 98, 1182–1188. [Google Scholar] [CrossRef]
- Dovio, A.; Sartori, M.L.; De, F.S.; Mussino, S.; Perotti, P.; Saba, L.; Abbadessa, G.; Racca, S.; Angeli, A. Differential expression of determinants of glucocorticoid sensitivity in androgen-dependent and androgen-independent human prostate cancer cell lines. J. Steroid Biochem. Mol. Biol. 2009, 116, 29–36. [Google Scholar] [CrossRef]
- Page, N.; Warriar, N.; Govindan, M.V. 11 beta-Hydroxysteroid dehydrogenase and tissue specificity of androgen action in human prostate cancer cell LNCaP. J. Steroid Biochem. Mol. Biol. 1994, 49, 173–181. [Google Scholar] [CrossRef]
- Nath, N.; Lakshmi, V.; Rosenthal, J.C. Presence of 11 beta-hydroxysteroid dehydrogenase enzyme in the human prostate tumor cell line LNCaP. Prostate 1993, 23, 225–233. [Google Scholar] [CrossRef]
- Aumuller, G.; Eicheler, W.; Renneberg, H.; Adermann, K.; Vilja, P.; Forssmann, W.G. Immunocytochemical evidence for differential subcellular localization of 5 alpha-reductase isoenzymes in human tissues. Acta Anat. (Basel) 1996, 156, 241–252. [Google Scholar] [CrossRef]
- Azzouni, F.; Godoy, A.; Li, Y.; Mohler, J. The 5 alpha-reductase isozyme family: A review of basic biology and their role in human diseases. Adv. Urol. 2012, 2012, 530121. [Google Scholar]
- Luu-The, V.; Belanger, A.; Labrie, F. Androgen biosynthetic pathways in the human prostate. Best Pract. Res. Clin. Endoc. Met. 2008, 22, 207–221. [Google Scholar] [CrossRef]
- Thigpen, A.E.; Cala, K.M.; Russell, D.W. Characterization of Chinese hamster ovary cell lines expressing human steroid 5 alpha-reductase isozymes. J. Biol. Chem. 1993, 268, 17404–17412. [Google Scholar]
- Yazawa, T.; Uesaka, M.; Inaoka, Y.; Mizutani, T.; Sekiguchi, T.; Kajitani, T.; Kitano, T.; Umezawa, A.; Miyamoto, K. Cyp11b1 is induced in the murine gonad by luteinizing hormone/human chorionic gonadotropin and involved in the production of 11-ketotestosterone, a major fish androgen: Conservation and evolution of the androgen metabolic pathway. Endocrinology 2008, 149, 1786–1792. [Google Scholar] [CrossRef]
- Penning, T.M. New frontiers in androgen biosynthesis and metabolism. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 233–239. [Google Scholar] [CrossRef]
- Sharifi, N.; Gulley, J.L.; Dahut, W.L. Androgen deprivation therapy for prostate cancer. JAMA-J. Am. Med. Assn. 2005, 294, 238–244. [Google Scholar] [CrossRef]
- Mostaghel, E.A.; Montgomery, B.; Nelson, P.S. Castration-resistant prostate cancer: Targeting androgen metabolic pathways in recurrent disease. Urol. Oncol. 2009, 27, 251–257. [Google Scholar] [CrossRef]
- Slominski, A.; Zbytek, B.; Nikolakis, G.; Manna, P.R.; Skobowiat, C.; Zmijewski, M.; Li, W.; Janjetovic, Z.; Postlethwaite, A.; Zouboulis, C.C.; et al. Steroidogenesis in the skin: Implications for local immune functions. J. Steroid Biochem. Mol. Biol. 2013, 137, 107–123. [Google Scholar] [CrossRef]
- Nakamura, Y.; Hornsby, P.J.; Casson, P.; Morimoto, R.; Satoh, F.; Xing, Y.; Kennedy, M.R.; Sasano, H.; Rainey, W.E. Type 5 17beta-hydroxysteroid dehydrogenase (AKR1C3) contributes to testosterone production in the adrenal reticularis. J. Clin. Endocrinol. Metab. 2009, 94, 2192–2198. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bloem, L.M.; Storbeck, K.-H.; Schloms, L.; Swart, A.C. 11β-Hydroxyandrostenedione Returns to the Steroid Arena: Biosynthesis, Metabolism and Function. Molecules 2013, 18, 13228-13244. https://doi.org/10.3390/molecules181113228
Bloem LM, Storbeck K-H, Schloms L, Swart AC. 11β-Hydroxyandrostenedione Returns to the Steroid Arena: Biosynthesis, Metabolism and Function. Molecules. 2013; 18(11):13228-13244. https://doi.org/10.3390/molecules181113228
Chicago/Turabian StyleBloem, Liezl M., Karl-Heinz Storbeck, Lindie Schloms, and Amanda C. Swart. 2013. "11β-Hydroxyandrostenedione Returns to the Steroid Arena: Biosynthesis, Metabolism and Function" Molecules 18, no. 11: 13228-13244. https://doi.org/10.3390/molecules181113228





