Stability and Antioxidant Activity of Semi-synthetic Derivatives of 4-Nerolidylcatechol
Abstract
:1. Introduction

2. Results and Discussion
2.1. Radical Scavenging and Antioxidant Activities of 4-NC and Semi-Synthetic Derivatives of 4-NC
| Compound | ABTS | DPPH• | Toxicity | ||||
|---|---|---|---|---|---|---|---|
| Brine shrimp ( Artemia franciscana) | Mouse embryonic fibroblast cells (3T3L1 line) | ||||||
| (%) * | (%) * | (LC50) | (IC50, µM) | ||||
| 4-NC | 94.82 ± 0.22 a | 96.53 ± 0.50 a | 25 M | 31.5 (25.8–38.2) | |||
| 1 | 7.97 ± 2.72 b | 4.35 ± 0.71 c | >1.0 mM † | >101 ‡ | |||
| 2 | 91.12 ± 0.68 a | 44.20 ± 0.01 b | 1.0 mM | 43.1 (37.9–49.0) | |||
| 3 | 93.31 ± 0.30 a | 48.09 ± 1.33 b | >1.2 mM † | >124 ‡ | |||
| 4 & 5 | 89.05 ± 0.44 a | 40.52 ± 0.51 b | 0.60 & 1.2 mM ** | >152 ‡ | |||
| 6 | 8.83 ± 0.23 b | 3.46 ± 0.63 c | 69 µM | >95.8 ‡ | |||
| 7 | 8.60 ± 0.10 b | 6.36 ± 1.15 c | 83 µM | ND | |||
| 8 | 0.31 ± 0.01 c | 7.39 ± 3.66 c | ND | >116 ‡ | |||
| BHT | 93.13 ± 0.18 a | 16.84 ± 0.81 c | ND | ND | |||
| BHA | 94.71 ± 0.47 a | 97.97 ± 0.10 a | ND | ND | |||
| Quercetin | 99.7 ± 0.02 a | 91.4 ± 0.12 a | ND | ND | |||
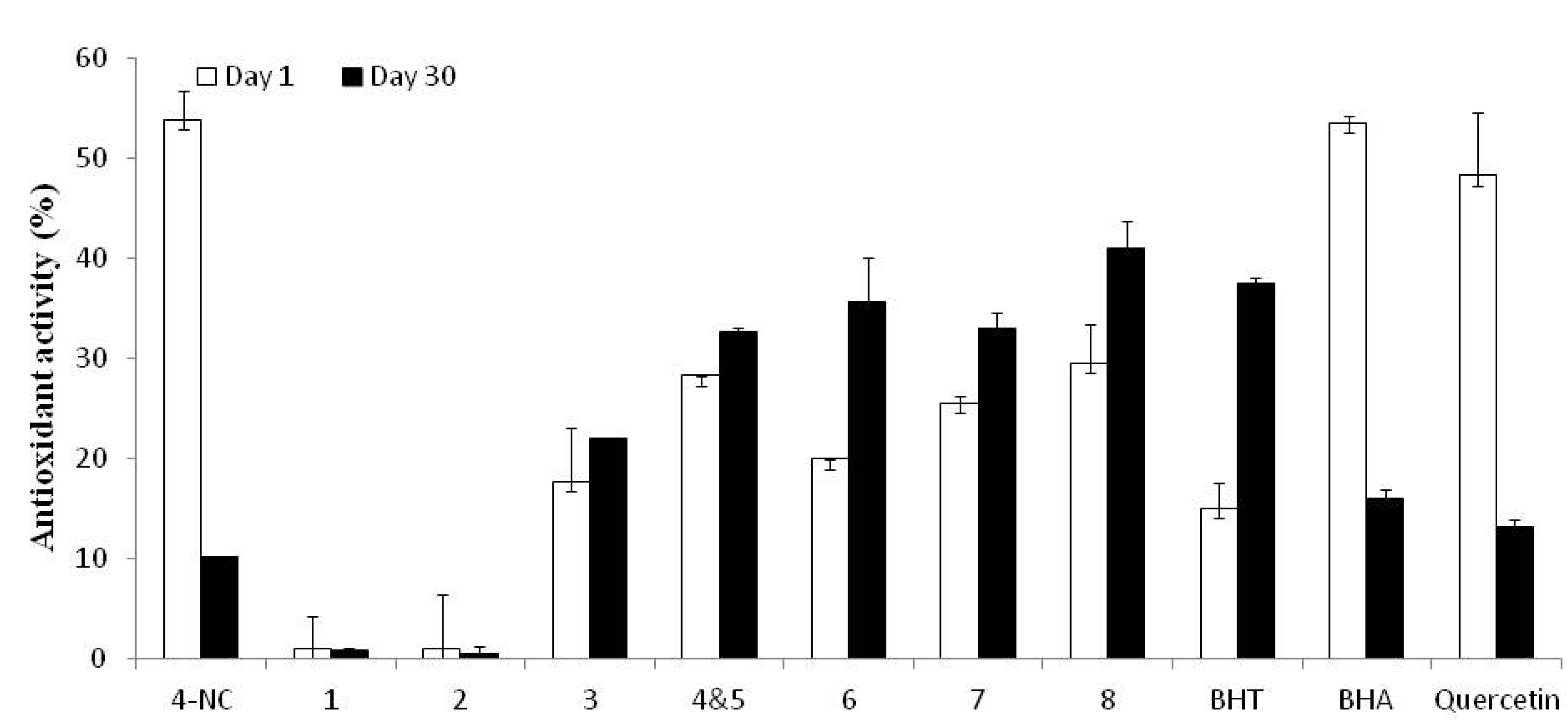
2.2. Cytotoxicity of 4-NC and Derivatives of 4-NC
2.3. Antioxidant Activity and Stability of 4-NC and Derivatives of 4-NC
2.4. Structure-Activity Relationship in 4-NC, Derivatives of 4-NC, BHT and BHA
3. Experimental
3.1. Chemicals and Reagents
3.2. Derivatives of 4-NC
3.2.1. Cultivation of Piper peltatum
3.2.2. Extraction and Isolation of 4-NC
3.2.3. Preparation of Derivatives from 4-NC and Purity of 4-NC Derivatives
3.3. Cell Culture
3.4. Antioxidant Activity Chemical Assays
3.4.1. DPPH Radical-Scavenging Activity
3.4.2. ABTS (2,2'-Azinobis-3-ethylbenzothiazoline-6-sulfonic acid) Radical-Scavenging Activity
3.5. Cell Viability Assay
3.6. Cellular Antioxidant Activity
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
- Samples Availability: The 4-NC and 4-NC derivatives 1–8 used in this scientific study are derived from an endemic plant species and so are Brazilian Genetic Heritage. Samples may be made available for further study only after authorization from the Brazilian Science, Technology and Innovation Ministry (MCTI) and/or the Brazilian Environment Ministry (MMA) is obtained.
References
- Pinto, A.C.S.; Pessoa, C.O.; Costa-Lotufo, L.V.; Moraes, M.O.; Moraes, M.E.A.; Cavalcanti, B.C.; Nunomura, S.M.; Pohlit, A.M. In vitro cytotoxicity of Pothomorphe peltata (L.) Miquel (Piperaceae), isolated 4-nerolidylcatechol and its semi-synthetic diacetyl derivative. Revista Brasileira de Plantas Medicinais 2006, 8, 205–211. [Google Scholar]
- Andrade-Neto, V.F.; Pohlit, A.M.; Pinto, A.C.S.; Silva, E.C.C.; Nogueira, K.L.; Melo, M.R.S.; Henrique, M.C.; Amorim, R.C.N.; Silva, L.F.R.; Costa, M.R.F.; et al. In vitro inhibition of Plasmodium falciparum by substances isolated from Amazonian antimalarial plants. Mem. Inst. Oswaldo Cruz 2007, 102, 359–365. [Google Scholar]
- Pinto, A.C.S.; Pena, E.A.; Pohlit, A.M.; Chaves, F.C.M. Biomass production in cultivated Pothomorphe peltata Miq. (Piperaceae) as a function of harvest time in Manaus, Amazonas State, Brazil. Rev. Bras. Plantas Med. 2006, 8, 98–101. [Google Scholar]
- Pinto, A.C.S.; Chaves, F.C.M.; Santos, P.A.; Nunes, C.V.; Tadei, W.P.; Pohlit, A.M. Piper peltatum: Biomass and 4-nerolidylcatechol production. Planta Med. 2010, 76, 1473–1476. [Google Scholar] [CrossRef]
- Desmarchelier, C.; Barros, S.; Repetto, M.; Latorre, L.R.; Kato, M.J.; Coussio, J.; Ciccia, G. 4-nerolidylcatechol from Pothomorphe spp. scavenges peroxyl radicals and inhibits Fe(II)-dependent DNA damage. Planta Med. 1997, 63, 561–563. [Google Scholar]
- Barros, S.B.M.; Teixeira, D.S.; Aznar, A.E.; Moreira, J.R.; Ishii, I.; Freitas, P.C.D. Antioxidant activity of ethanolic extracts of Pothomorphe umbellata L. Miq. (Pariparoba). Ciência e Cultura J. Braz. Assoc. Adv. Sci. 1996, 48, 114–116. [Google Scholar]
- Pasqualoto, K.F.M.; de Freitas, P.C.D.; Barros, S.B.M.; Ferreira, E.I. Estudos computacionais de mecânica quântica do 4-nerolidilcatecol e do pentadecilresorcinol. Revista Brasileira de Ciências Farmaceuticas 2001, 37, 68. [Google Scholar]
- De Freitas, P.C.D. Atividade Antioxidante de Espécies Medicinais da Família Piperaceae: Pothomorphe umbellata (L.) Miq. e Piper regnelli (Miq.) C. DC. Ph.D. Thesis, 1999. [Google Scholar]
- Valeriano, V.S.; Leal, A.F.V.B.; Rezende, K.R. Complexo de inclusão do 4-nerolidilcatecol em hidroxipropil-β-ciclodextrina. Revista Eletrônica de Farmácia 2005, 2, 224–227. [Google Scholar]
- Soares, L.A.; Leal, A.F.V.B.; Resende, K.R. Determinação da estequiometria e constante de estabilidade do complexo de inclusão do 4-nerolidilcatecol de Pothomorphe umbellata (Piperaceae) em 2-hidroxipropil-β-ciclodextrina. Revista Eletrônica de Farmácia 2007, 4, 68–71. [Google Scholar]
- Soares, L.A.; Leal, A.F.V.B; Fraceto, L.F.; Maia, E.R.; Resck, I.S.; Kato, M.K.; Gil, E.S.; Sousa, A.R.; Cunha, L.C.; Resende, K.R. Host-guest system of 4-nerolidylcatechol in 2-hydroxypropyl-β-cyclodextrin: Preparation, characterization and molecular modeling. J. Incl. Phenom. Macrocycl. Chem. 2009, 64, 23–35. [Google Scholar] [CrossRef]
- Pinto, A.C.S. Estudo Fitoquímico e Biológico de Pothomorphe peltata (L.) Miquel (Piperaceae). M.Sc. Dissertation, 2002; p. 156. [Google Scholar]
- Quignard, E.L.J.; Nunomura, S.M.; Pohlit, A.M.; Alecrim, A.M.; Pinto, A.C.S.; Portela, C.N.; Oliveira, L.C.P.; Don, L.C.; Rocha e Silva, L.F.; Henrique, M.C.; et al. Median lethal concentrations of Amazonian plant extracts in the brine shrimp assay. Pharm. Biol. 2004, 42, 253–257. [Google Scholar] [CrossRef]
- Brohem, C.A.; Sawada, T.C.H.; Massaro, R.R.; Almeida, R.L.; Rivelli, D.P.; Ropke, C.D.; Silva, V.V.; Lima, T.M.; Curi, R.; Barros, S.B.M.; et al. Apoptosis induction by 4-nerolidylcatechol in melanoma cell lines. Toxicol. In Vitro 2009, 23, 111–119. [Google Scholar] [CrossRef]
- Brohem, C.A.; Massaro, R.R.; Tiago, M.; Marinho, C.E.; Jasiulioni, M.G.; Almeida, R.L.; Rivelli, D.P.; Albuquerque, R.C.; Oliveira, T.F.; Loureiro, A.P.M.; et al. Proteasome inhibition and ROS generation by 4-nerolidylcatechol induces melanoma cell death. Pigment Cell Melanoma Res. 2012, 25, 354–369. [Google Scholar] [CrossRef]
- Mongelli, E.; Romano, A.; Desmarchelier, C.; Coussio, J.; Ciccia, G. Cytotoxic 4-nerolidylcatechol from Pothomorphe peltata inhibits topoisomerase I activity. Planta Med. 1999, 65, 376–378. [Google Scholar] [CrossRef]
- Rocha e Silva, L.F.R.; Pinto, A.C.S.; Pohlit, A.M.; Quignard, E.L.J.; Vieira, P.P.R.; Tadei, W.P.; Chaves, F.C.M.; Samonek, J.F.V.; Lima, A.J.L.; Costa, M.R.F.; et al. In vivo and in vitro antimalarial activity of 4-nerolidylcatechol. Phytother. Res. 2011, 25, 1181–1188. [Google Scholar] [CrossRef]
- Ropke, C.D.; Meirelles, R.R.; Silva, V.V.; Sawada, T.C.H.; Barros, S.B.M. Pothomorphe umbellata extract prevents α-tocopherol depletion after UV-irradiation. Photochem. Photobiol. 2003, 78, 436–439. [Google Scholar] [CrossRef]
- Ropke, C.D.; Sawada, T.C.H.; Silva, V.V.; Michalany, N.S.; Barros, S.B.M. Photoprotective effect of Pothomorphe umbellata root extract against ultraviolet radiation induced chronic skin damage in the hairless mouse. Clin. Exp. Dermatol. 2005, 30, 272–276. [Google Scholar] [CrossRef]
- Desmarchelier, C.; Slowing, K.; Ciccia, G. Anti-inflammatory activity of Pothomorphe peltata leaf methanol extract. Fitoterapia 2000, 71, 556–558. [Google Scholar] [CrossRef]
- Perazzo, F.F.; Souza, G.H.B.; Lopes, W.; Cardoso, L.G.V.; Carvalho, J.C.T.; Nanayakkara, N.P.D.; Bastos, J.K. Anti-inflammatory and analgesic properties of water-ethanolic extract from Pothomorphe umbellata (Piperaceae) aerial parts. J. Ethnopharmacol. 2005, 99, 215–220. [Google Scholar] [CrossRef]
- Pinto, A.C.S.; Rocha e Silva, L.F.; Cavalcanti, B.C.; Melo, M.R.S.; Chaves, F.C.M.; Lotufo, L.V.C.; Moraes, M.O.; Andrade-Neto, V.F.; Tadei, W.P.; Pessoa, C.O.; et al. New antimalarial and cytotoxic 4-nerolidylcatechol derivatives. Eur. J. Med. Chem. 2009, 44, 2731–2735. [Google Scholar]
- Pinto, A.C.S.; Melo, M.R.S.; Andrade Neto, V.F.; Chaves, F.C.M.; Vieira, P.P.R.; Pohlit, A.M. Derivatives of 4-nerolidylcatechol, Pharmaceutical Compositions Comprising Them and Process for Producing the Same. WO/2009/082795 9 July 2009. [Google Scholar]
- Rocha e Silva, L.F.; Pinto, A.C.S.; Pohlit, A.M. Universidade Federal do Amazonas, Manaus, Amazonas, Brazil. Unpublished work. 2012. [Google Scholar]
- Perron, N.R.; Brumaghim, J.L. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef]
- Justino, G.C.; Correia, C.F.; Mira, L.; Borges Dos Santos, R.M.; Martinho Simões, J.A.; Silva, A.M.; Santos, C.; Gigante, B. Antioxidant activity of a catechol derived from abietic acid. J. Agric. Food Chem. 2006, 54, 342–348. [Google Scholar]
- Fujisawa, S.; Kadoma, Y.; Yokoe, I. Radical-scavenging activity of butylated hydroxytoluene (BHT) and its metabolites. Chem. Phys. Lipids 2004, 130, 189–195. [Google Scholar] [CrossRef]
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS+ radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Nakayama, G.R.; Caton, M.C.; Nova, M.P.; Parandoosh, Z. Assessment of the Alamar Blue assay for cellular growth and viability in vitro. J. Immunol. Methods 1997, 204, 205–208. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lima, E.S.; Pinto, A.C.S.; Nogueira, K.L.; Silva, L.F.R.e.; Almeida, P.D.O.d.; Vasconcellos, M.C.d.; Chaves, F.C.M.; Tadei, W.P.; Pohlit, A.M. Stability and Antioxidant Activity of Semi-synthetic Derivatives of 4-Nerolidylcatechol. Molecules 2013, 18, 178-189. https://doi.org/10.3390/molecules18010178
Lima ES, Pinto ACS, Nogueira KL, Silva LFRe, Almeida PDOd, Vasconcellos MCd, Chaves FCM, Tadei WP, Pohlit AM. Stability and Antioxidant Activity of Semi-synthetic Derivatives of 4-Nerolidylcatechol. Molecules. 2013; 18(1):178-189. https://doi.org/10.3390/molecules18010178
Chicago/Turabian StyleLima, Emerson Silva, Ana Cristina Silva Pinto, Karla Lagos Nogueira, Luiz Francisco Rocha e Silva, Patricia Danielle Oliveira de Almeida, Marne Carvalho de Vasconcellos, Francisco Celio Maia Chaves, Wanderli Pedro Tadei, and Adrian Martin Pohlit. 2013. "Stability and Antioxidant Activity of Semi-synthetic Derivatives of 4-Nerolidylcatechol" Molecules 18, no. 1: 178-189. https://doi.org/10.3390/molecules18010178
APA StyleLima, E. S., Pinto, A. C. S., Nogueira, K. L., Silva, L. F. R. e., Almeida, P. D. O. d., Vasconcellos, M. C. d., Chaves, F. C. M., Tadei, W. P., & Pohlit, A. M. (2013). Stability and Antioxidant Activity of Semi-synthetic Derivatives of 4-Nerolidylcatechol. Molecules, 18(1), 178-189. https://doi.org/10.3390/molecules18010178





