Antifungal Activity of Salicylanilides and Their Esters with 4-(Trifluoromethyl)benzoic Acid
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
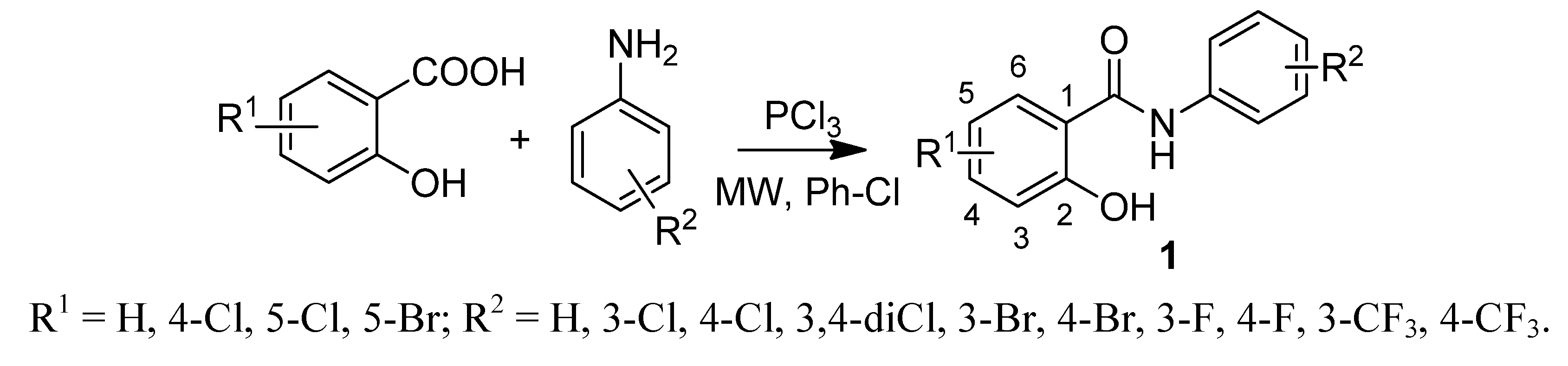
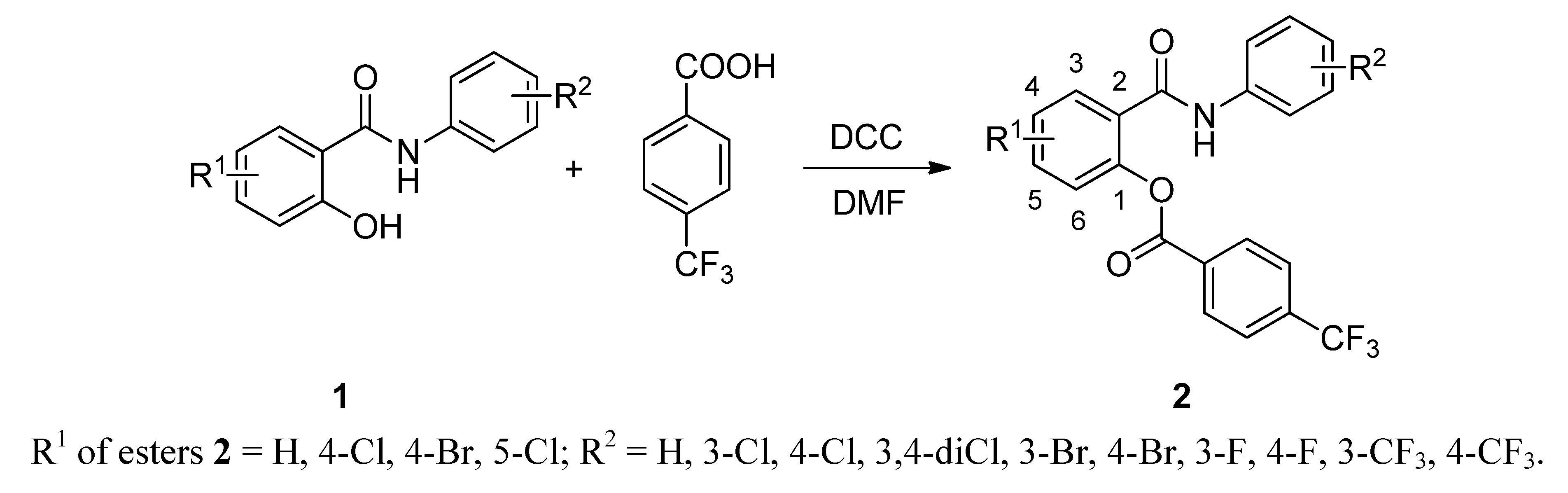
2.2. In vitro Antifungal Evaluation
| MIC/IC 80 [μmol/L] | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Candida albicans | Candida tropicalis | Candida krusei | Candida glabrata | Trichosporon asahii | |||||||||
| Code | R1 | R2 | Clog P | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h |
| 1a | 5-Cl | 3-Cl | 5.85 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 |
| 2a | 4-Cl | 3-Cl | 7.02 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 1b | 4-Cl | 3-Cl | 5.18 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 |
| 2b | 5-Cl | 3-Cl | 6.71 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 1c | 5-Cl | 4-Cl | 5.50 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 |
| 2c | 4-Cl | 4-Cl | 6.68 | 62.5 | >250 | >250 | >250 | 250 | >250 | >250 | >250 | 250 | >250 |
| 1d | 4-Cl | 4-Cl | 4.83 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 |
| 2d | 5-Cl | 4-Cl | 6.37 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 1e | 5-Cl | 3,4-diCl | 6.51 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 |
| 2e | 4-Cl | 3,4-diCl | 7.69 | >500 | >500 | 7.81 | 31.25 | 7.81 | 31.25 | >500 | >500 | >500 | >500 |
| 1f | 4-Cl | 3,4-diCl | 5.84 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | 3.9 | 15.62 |
| 2f | 5-Cl | 3,4-diCl | 7.38 | >125 | >125 | >125 | >125 | 62.5 | >125 | >125 | >125 | 31.25 | >125 |
| 1g | 5-Cl | 3-Br | 5.92 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | 15.62 | 62.5 |
| 2g | 4-Cl | 3-Br | 7.09 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 1h | 4-Cl | 3-Br | 5.25 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | 1.95 | 62.5 |
| 2h | 5-Cl | 3-Br | 6.87 | 125 | >500 | >500 | >500 | 125 | 250 | >500 | >500 | 62.5 | 250 |
| 1i | 5-Cl | 4-Br | 5.88 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | 3.9 | 3.9 |
| 2i | 4-Cl | 4-Br | 7.05 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 1j | 4-Cl | 4-Br | 5.21 | 7.81 | >125 | >125 | >125 | 7.81 | >125 | >125 | >125 | 1.95 | 7.81 |
| 2j | 5-Cl | 4-Br | 6.74 | 15.62 | 31.25 | 500 | >500 | 31.25 | 62.5 | 500 | >500 | 15.62 | 31.25 |
| 1k | 5-Cl | 3-F | 5.20 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 |
| 2k | 4-Cl | 3-F | 6.37 | 250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 |
| 1l | 4-Cl | 3-F | 4.53 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 |
| 2l | 5-Cl | 3-F | 6.06 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 1m | 5-Cl | 4-F | 5.16 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 |
| 2m | 4-Cl | 4-F | 6.33 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 1n | 4-Cl | 4-F | 4.49 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 |
| 2n | 5-Cl | 4-F | 6.02 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 1o | 5-Cl | 3-CF3 | 6.06 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 |
| 2o | 4-Cl | 3-CF3 | 7.24 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 1p | 4-Cl | 3-CF3 | 5.39 | >500 | >500 | >500 | >500 | >500 | >500 | 500 | >500 | 250 | 500 |
| 2p | 5-Cl | 3-CF3 | 6.93 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 |
| 1q | 5-Cl | 4-CF3 | 5.72 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 |
| 2q | 4-Cl | 4-CF3 | 6.90 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 |
| 1r | 4-Cl | 4-CF3 | 5.05 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | 1.95 | 15.62 |
| 2r | 5-Cl | 4-CF3 | 6.59 | 62.5 | 500 | >500 | >500 | 31.25 | 62.5 | >500 | >500 | 15.62 | 62.5 |
| 1s | 5-Br | 4-CF3 | 5.49 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | 1.95 | 7.81 |
| 2s | 4-Br | 4-CF3 | 6.50 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 1t | H | H | 3.27 | 250 | 500 | 250 | 500 | 250 | 500 | 250 | 250 | 250 | 500 |
| UND | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | 250 | >500 | |||
| FLU | 1.0 | 2.0 | 3.0 | 5.0 | >50 | >50 | 22.0 | >50 | 4.00 | 9.00 | |||
| MIC/IC80/IC50 [μmol/L] | ||||||||
|---|---|---|---|---|---|---|---|---|
| Aspergillus fumigatus | Absidia corymbifera | Trichophyton mentagrophytes | ||||||
| Code | R1 | R2 | 24 h | 48 h | 24 h | 48 h | 72 h | 150 h |
| 1a | 5-Cl | 3-Cl | >125 | >125 | 7.81 | 62.5 | 1.95 | 1.95 |
| 2a | 4-Cl | 3-Cl | ND | ND | ND | ND | ND | ND |
| 1b | 4-Cl | 3-Cl | >125 | >125 | 125 | >125 | 1.95 | 1.95 |
| 2b | 5-Cl | 3-Cl | ND | ND | ND | ND | ND | ND |
| 1c | 5-Cl | 4-Cl | >125 | >125 | 125 | >125 | 1.95 | 1.95 |
| 2c | 4-Cl | 4-Cl | >250 | >250 | 250 | 250 | 15.62 | 15.62 |
| 1d | 4-Cl | 4-Cl | >125 | >125 | 15.62 | >125 | 1.95 | 1.95 |
| 2d | 5-Cl | 4-Cl | ND | ND | ND | ND | ND | ND |
| 1e | 5-Cl | 3,4-diCl | >125 | >125 | >125 | >125 | 31.25 | 31.25 |
| 2e | 4-Cl | 3,4-diCl | >500 | >500 | >500 | >500 | 15.62 | 125 |
| 1f | 4-Cl | 3,4-diCl | >125 | >125 | 3.9 | 7.81 | 3.9 | 3.9 |
| 2f | 5-Cl | 3,4-diCl | >125 | >125 | >125 | >125 | >125 | >125 |
| 1g | 5-Cl | 3-Br | >125 | >125 | 15.62 | 31.25 | 1.95 | 1.95 |
| 2g | 4-Cl | 3-Br | ND | ND | ND | ND | ND | ND |
| 1h | 4-Cl | 3-Br | >125 | >125 | >125 | >125 | 1.95 | 1.95 |
| 2h | 5-Cl | 3-Br | >500 | >500 | 250 | >500 | 0.98 | 1.95 |
| 1i | 5-Cl | 4-Br | >500 | >500 | 15.62 | 31.25 | 0.98 | 0.98 |
| 2i | 4-Cl | 4-Br | ND | ND | ND | ND | ND | ND |
| 1j | 4-Cl | 4-Br | >125 | >125 | 7.81 | 7.81 | 1.95 | 1.95 |
| 2j | 5-Cl | 4-Br | 15.62 | 31.25 | 7.81 | 15.62 | 0.49 | 0.98 |
| 1k | 5-Cl | 3-F | >125 | >125 | >125 | >125 | 3.9 | 3.9 |
| 2k | 4-Cl | 3-F | >250 | >250 | 250 | >250 | 62.5 | 62.5 |
| 1l | 4-Cl | 3-F | >125 | >125 | >125 | >125 | 3.9 | 3.9 |
| 2l | 5-Cl | 3-F | ND | ND | ND | ND | ND | ND |
| 1m | 5-Cl | 4-F | >125 | >125 | >125 | >125 | 7.81 | 7.81 |
| 2m | 4-Cl | 4-F | ND | ND | ND | ND | ND | ND |
| 1n | 4-Cl | 4-F | >125 | >125 | >125 | >125 | 62.5 | 62.5 |
| 2n | 5-Cl | 4-F | ND | ND | ND | ND | ND | ND |
| 1o | 5-Cl | 3-CF3 | >125 | >125 | 3.9 | 3.9 | 3.9 | 3.9 |
| 2o | 4-Cl | 3-CF3 | ND | ND | ND | ND | ND | ND |
| 1p | 4-Cl | 3-CF3 | >500 | >500 | >500 | >500 | >500 | >500 |
| 2p | 5-Cl | 3-CF3 | >500 | >500 | >500 | >500 | 15.62 | 31.25 |
| 1q | 5-Cl | 4-CF3 | >125 | >125 | >125 | >125 | 3.9 | 7.81 |
| 2q | 4-Cl | 4-CF3 | >125 | >125 | >125 | >125 | 3.9 | 15.62 |
| 1r | 4-Cl | 4-CF3 | >125 | >125 | 3.9 | 3.9 | 3.9 | 3.9 |
| 2r | 5-Cl | 4-CF3 | 15.62 | 15.62 | 15.62 | 15.62 | 0.49 | 1.95 |
| 1s | 5-Br | 4-CF3 | >125 | >125 | 3.9 | 7.81 | 7.81 | 7.81 |
| 2s | 4-Br | 4-CF3 | ND | ND | ND | ND | ND | ND |
| 1t | H | H | 500 | 500 | 250 | 500 | 31.25 | 125 |
| UND | >500 | >500 | >500 | >500 | 250 | 250 | ||
| FLU | >50 | >50 | >50 | >50 | 17.0 | 26.0 | ||
3. Experimental
3.1. General Methods
3.2. Synthesis of Salicylanilides
3.3. Synthesis of Salicylanilide 4-(Trifluoromethyl)benzoates
3.4. Antifungal Activity Determination
4. Conclusions
Conflict of Interest
Acknowledgements
References
- Lopez-Martinez, R. Candidosis, a new challenge. Clin. Dermatol. 2010, 6, 178–184. [Google Scholar] [CrossRef]
- Silva, S.; Negri, M.; Henriques, M.; Oliveira, R.; Williams, D.W.; Azeredo, J. Candida glabrata, Candida parapsilosis and Candida tropicalis: Biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol. Rev. 2012, 36, 288–305. [Google Scholar] [CrossRef]
- Morschhäuser, J. Regulation of multidrug resistance in pathogenic fungi. Fungal Genet. Biol. 2010, 47, 94–106. [Google Scholar] [CrossRef]
- Chandrasekar, P. Management of invasive fungal infections: A role for polyenes. J. Antimicrob. Chemother. 2011, 66, 457–465. [Google Scholar] [CrossRef]
- Krátký, M.; Vinšová, J.; Buchta, V.; Horvati, K.; Bösze, S.; Stolaříková, J. New amino acid esters of salicylanilides active against MDR-TB and other microbes. Eur. J. Med. Chem. 2010, 45, 6106–6113. [Google Scholar]
- Krátký, M.; Vinšová, J. Antiviral activity of substituted salicylanilides-a review. Mini-Rev. Med. Chem. 2011, 11, 956–967. [Google Scholar]
- Krátký, M.; Vinšová, J. Salicylanilide ester prodrugs as potential antimicrobial agents-a review. Curr. Pharm. Des. 2011, 17, 3494–3505. [Google Scholar] [CrossRef]
- Waisser, K.; Pešina, M.; Holý, P.; Pour, M.; Bureš, O.; Kuneš, J.; Klimešová, V.; Buchta, V.; Kubanová, P.; Kaustová, J. Antimycobacterial and Antifungal Isosters of Salicylamides. Arch. Pharm. Pharm. Med. Chem. 2003, 336, 322–335. [Google Scholar] [CrossRef]
- Otevrel, J.; Mandelova, Z.; Pesko, M.; Guo, J.; Kralova, K.; Sersen, F.; Vejsova, M.; Kalinowski, D.S.; Kovacevic, Z.; Coffey, A.; et al. Investigating the Spectrum of Biological Activity of Ring-Substituted Salicylanilides and Carbamoylphenylcarbamates. Molecules 2010, 15, 8122–8142. [Google Scholar]
- Ienacu, I.M.C.; Lupea, A.X.; Hadaruga, D.; Hadaruga, N.; Popescu, I.M. The Antimicrobial Activity and Quantitative Structure-Biological Activity Relationships Evaluation of Some Novel 2-Hydroxybenzamide Derivatives. Rev. Chim. 2008, 59, 247–250. [Google Scholar]
- Pastor, L.; García-Domenech, R.; Gálvez, J.; Wolski, S.; García, M.D. New antifungals selected by molecular topology. Bioorg. Med. Chem. Lett. 1998, 8, 2577–2582. [Google Scholar] [CrossRef]
- Daidone, G.; Maggio, B.; Schillaci, D. Salicylanilide and its heterocyclic analogues. A comparative study of their antimicrobial activity. Pharmazie 1990, 45, 441–442. [Google Scholar]
- Kumar, A.; Narasimhan, B.; Kumar, D. Synthesis, antimicrobial, and QSAR studies of substituted benzamides. Bioorg. Med. Chem. 2007, 15, 4113–4124. [Google Scholar] [CrossRef]
- Skála, P.; Macháček, M.; Vejsová, M.; Kubicová, L.; Kuneš, J.; Waisser, K. Synthesis and antifungal evaluation of Hydroxy-3-phenyl-2H-1,3-benzoxazine-2,4(3H)-diones and their thioanalogs. J. Heterocycl. Chem. 2009, 46, 873–880. [Google Scholar] [CrossRef]
- Sivakumar, P.M.; Seenivasan, S.P.; Kumar, V.; Doble, M. Novel 1,3,5-triphenyl-2-pyrazolines as anti-infective agents. Bioorg. Med. Chem. Lett. 2010, 20, 3169–3172. [Google Scholar]
- Vinsova, J.; Imramovsky, A.; Buchta, V.; Ceckova, M.; Dolezal, M.; Staud, F.; Jampilek, J.; Kaustova, J. Salicylanilide acetates: Synthesis and antibacterial evaluation. Molecules 2007, 12, 1–12. [Google Scholar] [CrossRef]
- Krátký, M.; Vinšová, J.; Buchta, V. In vitro antibacterial and antifungal activity of salicylanilide benzoates. Sci. World J. 2012, 12. [Google Scholar] [CrossRef]
- Krátký, M.; Vinšová, J.; Buchta, V. In vitro antibacterial and antifungal activity of salicylanilide pyrazine-2-carboxylates. Med. Chem. 2012, 8, 732–741. [Google Scholar] [CrossRef]
- De Vita, D.; Scipione, L.; Tortorella, S.; Mellini, P.; Di Rienzo, B.; Simonetti, G.; D’Auria, F.D.; Panella, S.; Cirilli, R.; Di Santo, R.; et al. Synthesis and antifungal activity of a new series of 2-(1H-imidazol-1-yl)-1-phenylethanol derivatives. Eur. J. Med. Chem. 2012, 49, 334–342. [Google Scholar] [CrossRef]
- Imramovský, A.; Férriz, J.M.; Pauk, K.; Krátký, M.; Vinšová, J. Synthetic route for the preparation of 2-hydroxy-N-[1-(2-hydroxyphenylamino)-1-oxoalkan-2-yl]benzamides. J. Comb. Chem. 2010, 12, 414–416. [Google Scholar] [CrossRef]
- Crawford, F.; Hollis, S. Topical treatments for fungal infections of the skin and nails of the foot. Cochrane Db. Syst. Rev. 2007. [Google Scholar] [CrossRef]
- Norrington, F.E.; Hyde, R.M.; Williams, S.G.; Wotton, R. Physicochemical-Activity relations inpractice. 1. Rational and self-consistent data bank. J. Med. Chem. 1975, 18, 604–607. [Google Scholar]
- Imramovsky, A.; Vinsova, J.; Ferriz, J.M.; Buchta, V.; Jampilek, J. Salicylanilide esters of N-protected amino acids as novel antimicrobial agents. Bioorg. Med. Chem. Lett. 2009, 19, 348–351. [Google Scholar] [CrossRef]
- Waisser, K.; Bureš, O.; Holý, P.; Kuneš, J.; Oswald, R.; Jirásková, J.; Pour, M.; Klimešová, V.; Kubicová, L.; Kaustová, J. Relationship between the structure and antimycobacterial activity of substituted salicylanilides. Arch. Pharm. Pharm. Med. Chem. 2003, 336, 53–71. [Google Scholar] [CrossRef]
- Wood, R.D.; Welsh, W.J.; Ekins, S.; Ai, N. Glutamate receptor modulators and therapeutic agents. U.S. Patent 2009/0239919, 24 September 2009. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI), Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. Approved Standard, Third Edition. CSLI document M27–A3, 3rd ed; CLSI: Wayne, PA, USA, 2008; Volume 28.
- Clinical and Laboratory Standards Institute (CLSI), Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi. Approved Standard, Second Edition. CSLI document M38–A2, 2nd ed; CLSI: Wayne, PA, USA, 2008; Volume 28.
- Sample Availability: Samples of the compounds 1a-t and 2a-s are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Krátký, M.; Vinšová, J. Antifungal Activity of Salicylanilides and Their Esters with 4-(Trifluoromethyl)benzoic Acid. Molecules 2012, 17, 9426-9442. https://doi.org/10.3390/molecules17089426
Krátký M, Vinšová J. Antifungal Activity of Salicylanilides and Their Esters with 4-(Trifluoromethyl)benzoic Acid. Molecules. 2012; 17(8):9426-9442. https://doi.org/10.3390/molecules17089426
Chicago/Turabian StyleKrátký, Martin, and Jarmila Vinšová. 2012. "Antifungal Activity of Salicylanilides and Their Esters with 4-(Trifluoromethyl)benzoic Acid" Molecules 17, no. 8: 9426-9442. https://doi.org/10.3390/molecules17089426
APA StyleKrátký, M., & Vinšová, J. (2012). Antifungal Activity of Salicylanilides and Their Esters with 4-(Trifluoromethyl)benzoic Acid. Molecules, 17(8), 9426-9442. https://doi.org/10.3390/molecules17089426





