Thermal Hazard Evaluation of Lauroyl Peroxide Mixed with Nitric Acid
Abstract
:Abbreviations:
| Ea | Activation energy (kJ/mol) |
| R | Gas constant (J/(mol k)) |
| Q | Heat power (W/g) |
| T0 | Exothermic onset temperature (°C) |
| T | Absolute temperature (K) |
| ∆Hd | Heat of decomposition (J/g) |
| TMRiso | Isothermal time to maximum rate (min, h, or day) |
| N | Thermal power or heat production rate (W = J/s) |
| ∆Hiso | Heat of decomposition under isothermal condition (J/g) |
1. Introduction
2. Results and Discussion
2.1. Thermal Analysis by DSC
| Sample | Mass (mg) | β (°C/min) | Tmax (°C) | T0 (°C) | ΔHd (J/g) | n | Ea (kJ/mol) | ΔHtotal (mJ/g) |
|---|---|---|---|---|---|---|---|---|
| LPO 95 mass% + HNO3 (0.1 N, 3.8 mg) | 18.3 | 4 | 108 | 68 | 202 | 1.17 | 113 | 3,682 |
| LPO 95 mass% + HNO3 (1 N, 1.6 mg) | 10.9 | 4 | 108 | 68 | 279 | 1.23 | 115 | 4,504 |
| LPO 95 mass% + HNO3 (2 N, 1.2 mg) | 7.51 | 4 | 109 | 65 | 600 | 1.25 | 129 | 4,504 |
| LPO 95 mass% + HNO3 (6 N, 0.5 mg) | 5.37 | 4 | 110 | 60 | 851 | 4.32 | 260 | 5,024 |
| LPO 95 mass% + HNO3 (12 N, 0.8 mg) | 6.07 | 4 | 160 | 58 | 8162 | 2.36 | 147 | 49,550 |
2.1.1. Mechanism A




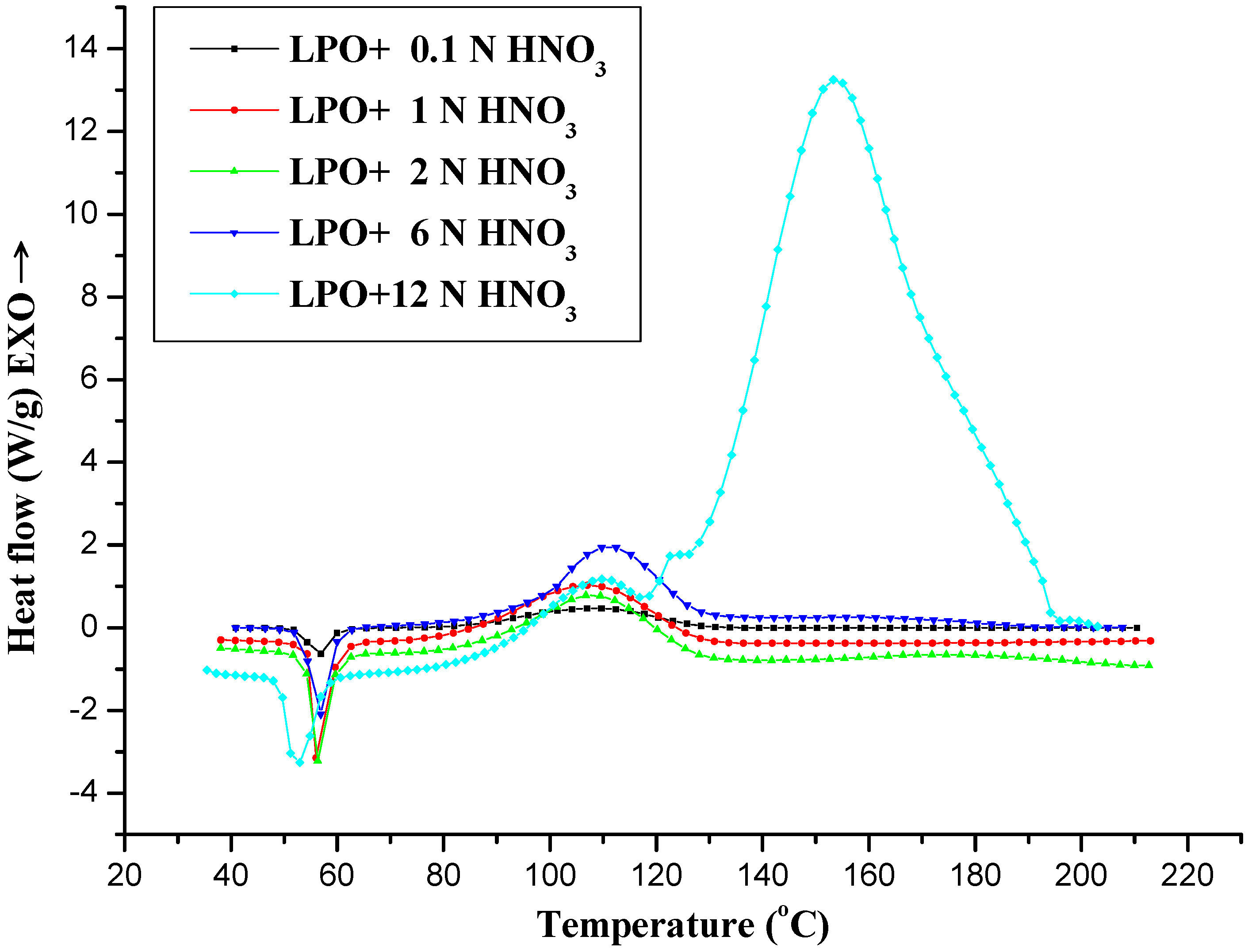
2.1.2. Mechanism B



2.1.3. Mechanism C



2.2. Thermal Decomposition Analysis of LPO Mixed with Inorganic Acids by TAM III
| Sample | Mass/(LPO/contaminant) (mg) | Cell | TMRiso (min) | Heat power ∆Hiso (W/g) (J/g) |
|---|---|---|---|---|
| LPO + 1 N HNO3 | 52.8/15.2 | Glass | 696 | 0.0027 793.07 |
| LPO + 6 N HNO3 | 52.7/24.6 | Glass | 570.6 | 0.0022 1,013.04 |
| LPO +12 N HNO3 | 52.3/15.2 | Glass | 240.6 | 0.0048 2,059.98 |
| Sample | Mass/(LPO/contaminant) (mg) | Cell | TMRiso (min) | Heat power ∆Hiso (W/g) (J/g) |
|---|---|---|---|---|
| LPO + 1 N HNO3 | 59.8/16.0 | Glass | 94.70 | 0.0103 894.12 |
| LPO + 6 N HNO3 | 57.4/13.3 | Glass | 62.04 | 0.012 976.21 |
| LPO +12 N HNO3 | 52.3/15.2 | Glass | 38.35 | 0.023 1,203.64 |
| Sample | Mass/(LPO/contaminant) (mg) | Cell | TMRiso (min) | Heat power ∆Hiso (W/g) (J/g) |
|---|---|---|---|---|
| LPO + 1 N HNO3 | 57.4/14.2 | Glass | 12.09 | 0.046 872.0 |
| LPO + 6 N HNO3 | 56.4/10.5 | Glass | 17.14 | 0.043 952.8 |
| LPO +12 N HNO3 | 57.1/13.7 | Glass | 12.8 | 0.063 1,072.05 |
| Sample | Mass/(LPO/contaminant) (mg) | Cell | TMRiso (min) | Heat power ∆Hiso (W/g) (J/g) |
|---|---|---|---|---|
| LPO + 1 N HNO3 | 60.1/11.9 | Glass | 8.58 | 0.13 573.06 |
| LPO + 6 N HNO3 | 60.6/10.06 | Glass | 10.62 | 0.12 734.08 |
| LPO + 12 N HNO3 | 60.1/8.5 | Glass | 8.07 | 0.15 655.04 |
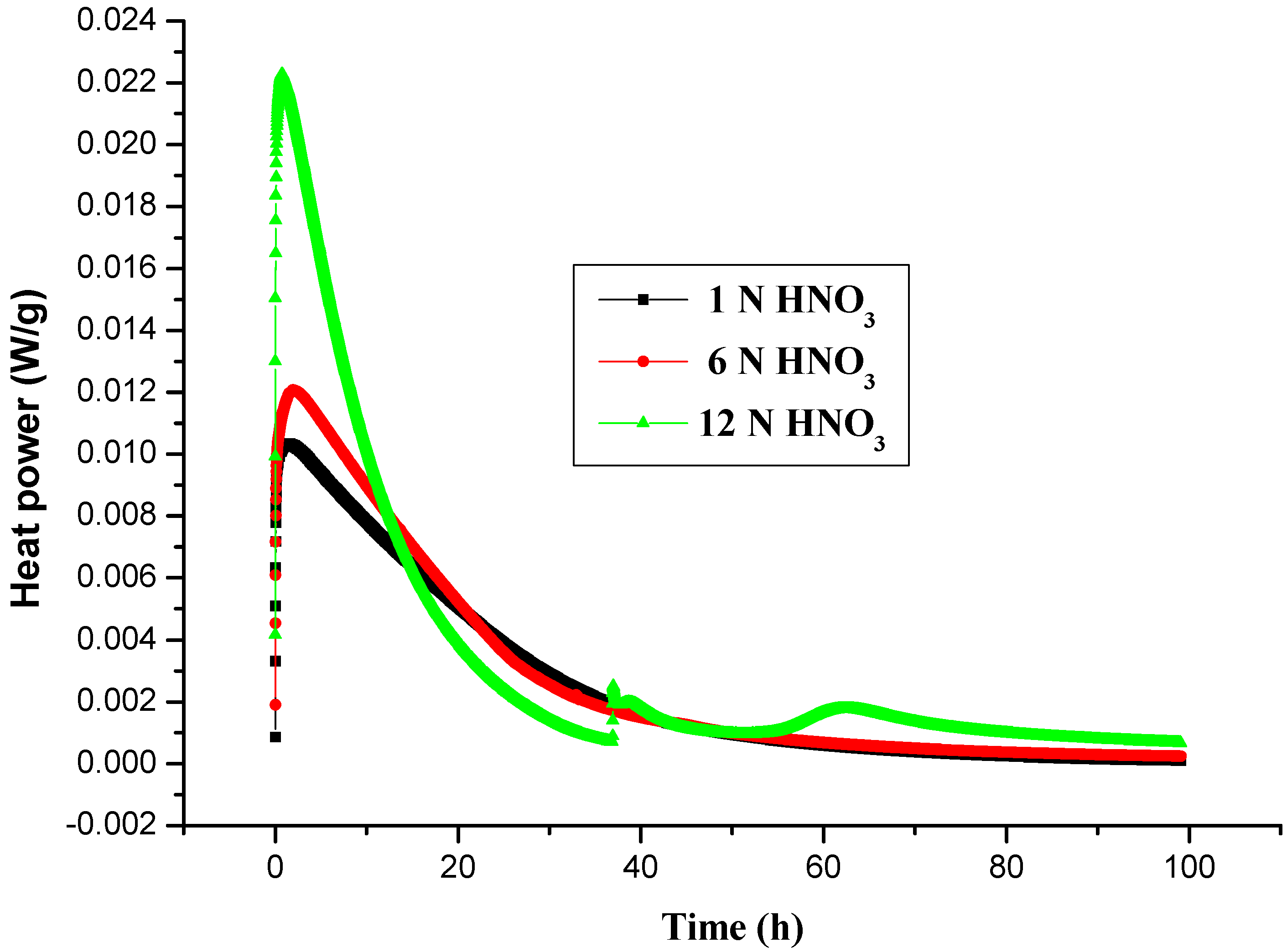
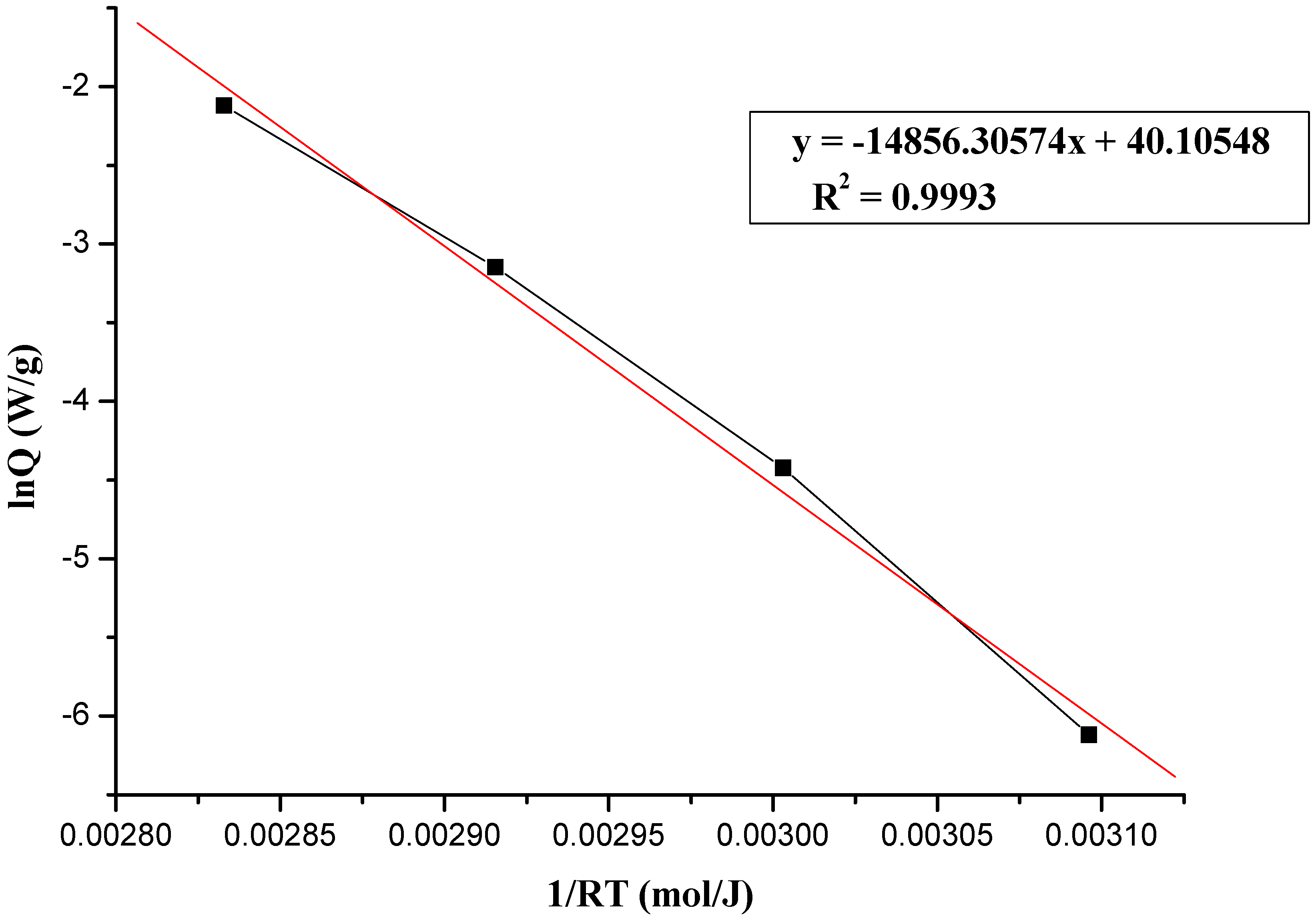
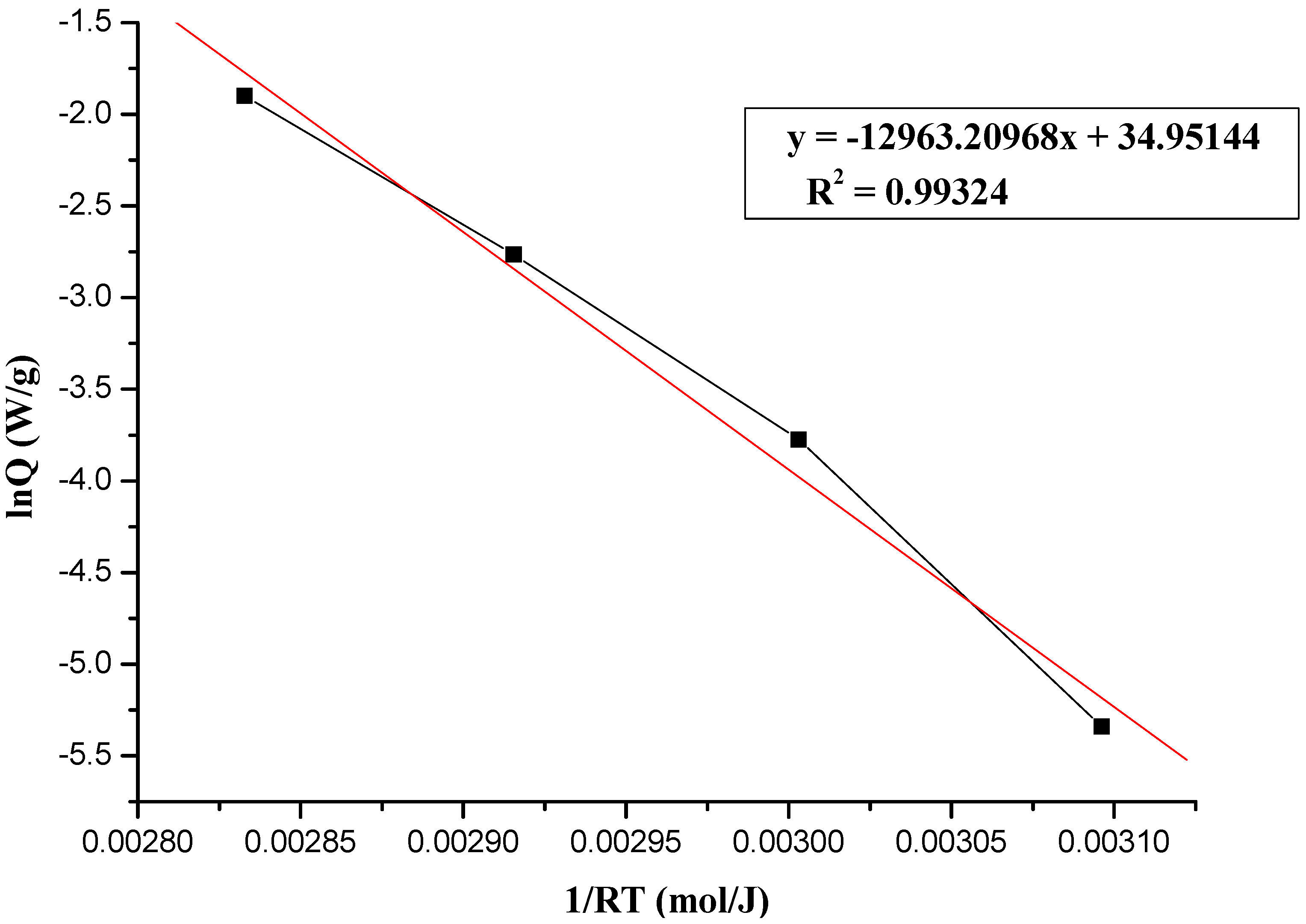
2.2.1. The Calculation of Thermokinetic Parameters

3. Experimental
3.1. Sample Preparations

3.2. Differential Scanning Calorimetry (DSC)
3.3. Gas Chromatography/Mass Spectrometer (GC/MS)
3.4. Thermal Activity Monitor III (TAM III)
4. Conclusions
Acknowledgments
References
- Gagosz, F.; Mourtrille, C.; Zard, Z.Z. A new tin-free source of amidyl radicals. Org. Lett. 2002, 4, 2707–2709. [Google Scholar] [CrossRef]
- Bevington, J.C.; Hunt, B.J. The use of stabilized radicals with monomers and lauroyl peroxide. Eur. Polym. J. 2004, 40, 103–108. [Google Scholar] [CrossRef]
- Tsubota, T.K.; Ida, S.T.; Hirabayashi, O.; Nagaoka, S.; Nagata, M.N.; Matsumoto, Y.M. Chemical modification of diamond surface using a diacyl peroxide as radical initiator and CN group-containing compounds for the introduction of the CN group. Phys. Chem. Chem. Phys. 2002, 4, 3881–3886. [Google Scholar]
- You, M.L.; Liu, M.Y.; Wu, S.H.; Chi, J.H.; Shu, C.M. Thermal explosion and runaway reaction simulation of lauroyl peroxide by DSC tests. J. Therm. Anal. Calorim. 2009, 96, 777–782. [Google Scholar] [CrossRef]
- Tseng, J.M.; Shu, C.M.; Gupta, J.P.; Lin, Y.F. Evaluation and modeling runaway reaction of methyl ethyl ketone peroxide mixed with nitric acid. Ind. Eng. Chem. Res. 2007, 46, 8738–8745. [Google Scholar] [CrossRef]
- You, M.L.; Tseng, J.M.; Liu, M.Y.; Shu, C.M. Runaway reaction of lauroyl peroxide with nitric acid by DSC. J. Therm. Anal. Calorim. 2010, 102, 535–539. [Google Scholar] [CrossRef]
- Wei, J.M.; You, M.L.; Chu, Y.C.; Shu, C.M. Evaluation of thermal hazard for lauroyl peroxide by VSP2 and TAM III. J. Therm. Anal. Calorim. 2012. [Google Scholar] [CrossRef]
- Hou, H.Y.; Tsai, T.L.; Shu, C.M. Reaction of cumene hydroperoxide mixed with sodium hydroxide. J. Hazard. Mater. 2008, 152, 1214–1219. [Google Scholar] [CrossRef]
- Yuan, M.H.; Shu, C.M.; Kossoy, A.A. Kinetics and hazards of thermal decomposition of methyl ethyl ketone peroxide by DSC. Thermochim. Acta 2005, 430, 67–71. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Tseng, J.M.; Lin, S.Y.; Gupta, J.P.; Shu, C.M. Runaway reaction on tert-butyl peroxybenzoate. J. Therm. Anal. Calorim. 2008, 93, 121–126. [Google Scholar] [CrossRef]
- Russel, M.; Yao, J.; Chen, H.; Wang, F.; Zhou, Y.; Choi, M.M.F.; Zaray, G.; Trebse, P. Different technique of microcalorimetry and their applications to environmental sciences: A review. J. Am. Sci. 2009, 5, 194–208. [Google Scholar]
- Akinade, K.A.; Campbell, R.M.; Compton, A.C. The use of a simultaneous TGA/DSC/FT-IR system as a problem-solving tool. J. Mater. Sci. 1994, 29, 3802–3812. [Google Scholar] [CrossRef]
- Dong, H.B.; Hunt, J.D. A numerical model for a heat flux DSC: Determining heat transfer coefficients within a DSC. Mater. Sci. Eng. 2005, 413–414, 470–473. [Google Scholar]
- Liu, J.; Tang, X.; Zhang, Y.; Zhao, W. Determination of the volatile composition in brown millet, milled millet and millet bran by gas chromatography/mass spectrometry. Molecules 2012, 17, 2271–2282. [Google Scholar]
- Karasek, F.W.; Clement, R.E. Basic Gas Chromatography-Mass Spectrometry: Principles and Techniques; Elsevier: New York, NY, USA, 1988. [Google Scholar]
- National Institute of Advanced Industrial Science and Technology (AIST). Spectral Database for Organic Compounds SDBS. Available online: http://riodb01.ibase.aist.go.jp/sdbs/cgi-bin/direct_frame_top.cgi (accessed on 14 June 2012).
- Muller, M.D.; Simon, W. The identification of anthocyanins by pyrolysis mass spectrometry and pyrolysis-GC/MS. Mikrochimica Acta 1979, 2, 389–396. [Google Scholar] [CrossRef]
- Pavlova, A.; Ivanova, R. GC methods for quantitative determination of benzene in gasoline. Acta Chromatogr. 2003, 13, 215–225. [Google Scholar]
- Hosaka, A.H.; Watanabe, C.C.; Tsuge, S. Rapid determination of decabromodiphenyl ether in polystyrene by thermal desorption-GC/MS. Anal. Sci. 2005, 21, 1145–1147. [Google Scholar] [CrossRef]
- Guillet, J.E.; Gilmer, J.C. Decomposition of lauroyl, decanoyl, and octanoyl peroxide in solution. Can. J. Chem. 1969, 47, 4405–4411. [Google Scholar] [CrossRef]
- Tanimoto, Y.F.; Nishino, M.T.; Itoh, M.Y. Magnetic-field effect on the thermal decompositon of dilauroyl peroxide. Bull. Chem. Soc. Jpn. 1985, 58, 3365–3366. [Google Scholar]
- Cooper, R.A.; Lawler, R.G.; Ward, H.R. Radical pair substitution in benzoyl peroxide thermolyses observed by chemically induced dynamic nuclear polarization. J. Am. Ceram. Soc. 1971, 94, 2, 552–558. [Google Scholar]
- Ray, R.; Thorpe, R.B. A comparison of gasification with pyrolysis for the recycling of plastic containing wastes. Int. J. Chem. Reactor Eng. 2007, 5, A85. [Google Scholar]
- Advancing the Chemical Sciences (RSC). ChemSpider. Available online: http://www.chemspider.com/InChIKey=MQEMKUTWMALMCC-UHFFFAOYAN (accessed on 14 June 2012).
- Tseng, J.M.; Liu, M.Y.; Chen, S.L.; Horng, J.J.; Hwang, W.T.; Gupta, J.P.; Shu, C.M. Runaway effects of nitric acid on methyl ethyl ketone peroxide by TAM III tests. J. Therm. Anal. Calorim. 2009, 96, 789–793. [Google Scholar] [CrossRef]
- Townsend, D.I.; Tou, J.C. Thermal hazard evaluation by an accelerating rate calorimeter. Thermochim. Acta 1980, 37, 1–30. [Google Scholar] [CrossRef]
- Fu, Z.M.; Li, X.R.; Koseki, H.; Mok, Y.S. Evaluation on thermal hazard of methyl ethylketone peroxide by using adiabatic method. J. Loss. Prev. Process Ind. 2003, 16, 389–393. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tsai, L.-C.; You, M.-L.; Ding, M.-F.; Shu, C.-M. Thermal Hazard Evaluation of Lauroyl Peroxide Mixed with Nitric Acid. Molecules 2012, 17, 8056-8067. https://doi.org/10.3390/molecules17078056
Tsai L-C, You M-L, Ding M-F, Shu C-M. Thermal Hazard Evaluation of Lauroyl Peroxide Mixed with Nitric Acid. Molecules. 2012; 17(7):8056-8067. https://doi.org/10.3390/molecules17078056
Chicago/Turabian StyleTsai, Lung-Chang, Mei-Li You, Mei-Fang Ding, and Chi-Min Shu. 2012. "Thermal Hazard Evaluation of Lauroyl Peroxide Mixed with Nitric Acid" Molecules 17, no. 7: 8056-8067. https://doi.org/10.3390/molecules17078056






