Self Assembled Films of Porphyrins with Amine Groups at Different Positions: Influence of Their Orientation on the Corrosion Inhibition and the Electrocatalytic Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure of the Porphyrins

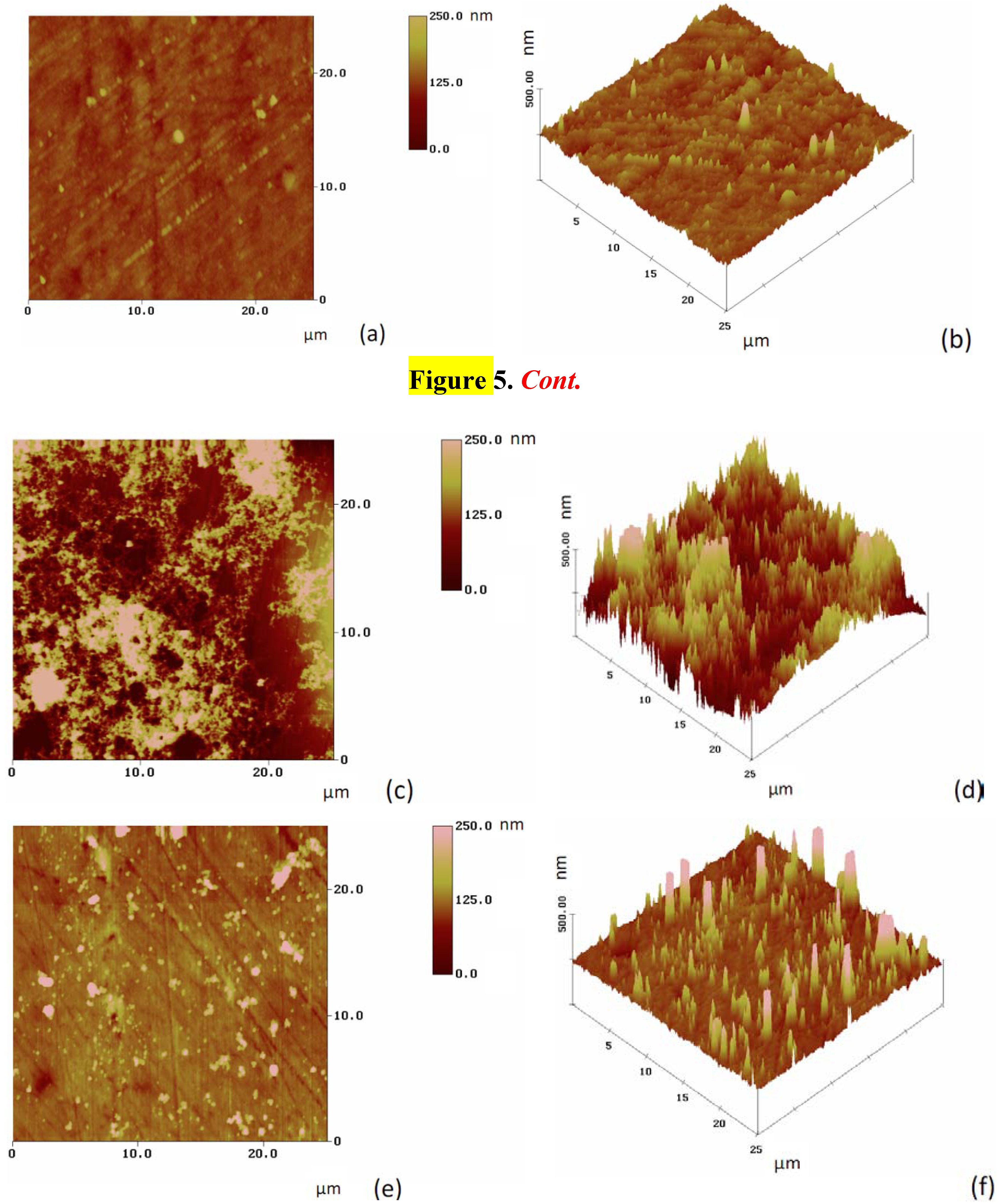
2.2. Characterization of the Modified Gold Surfaces
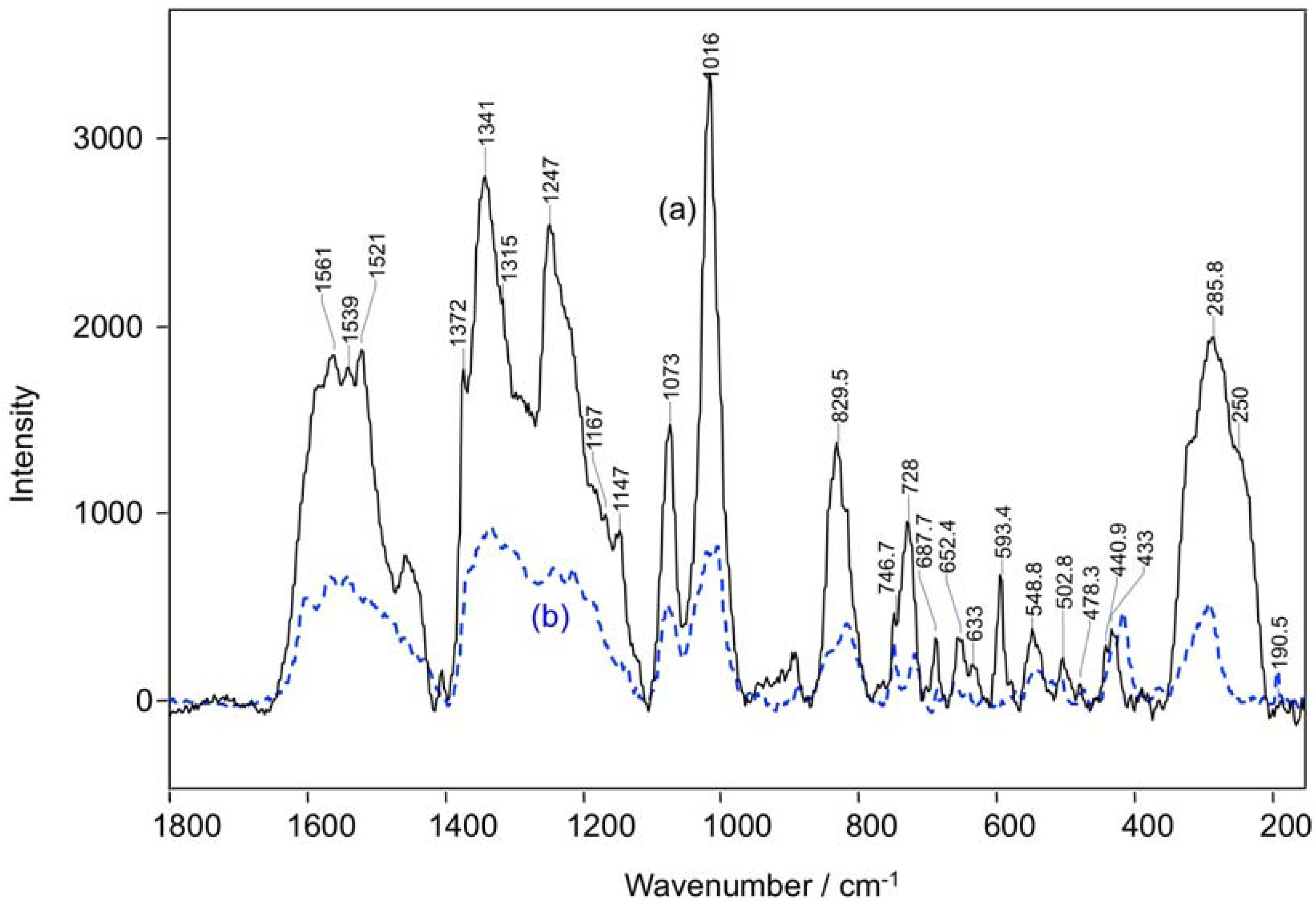
| Raman shift, cm−1 | Assignments [ν, Stretching; δ, Bending] |
|---|---|
| 1561 | ν (CβCβ) |
| 1540 | ν (CαCm)sym |
| 1372 | ν (CαCβ), ν (CβCs), ν (pyr half-ring)sym |
| 1341 | ν (pyr half-ring)sym |
| 1247 | δ (CmH) |
| 1073 | ν (CβCβ)asym |
| 1016 | ν (CβCβ)asym |
| 830 | ν (pyr breathing) |
| 747 | ν (pyr breathing) |
| 728 | δ (pyr def)sym |
| 688 | δ (pyr def)sym |
| 593 | δ (pyr def)asym |
| 549 | δ (pyr rot) |
| 250 | δ (CβCβ)sym |



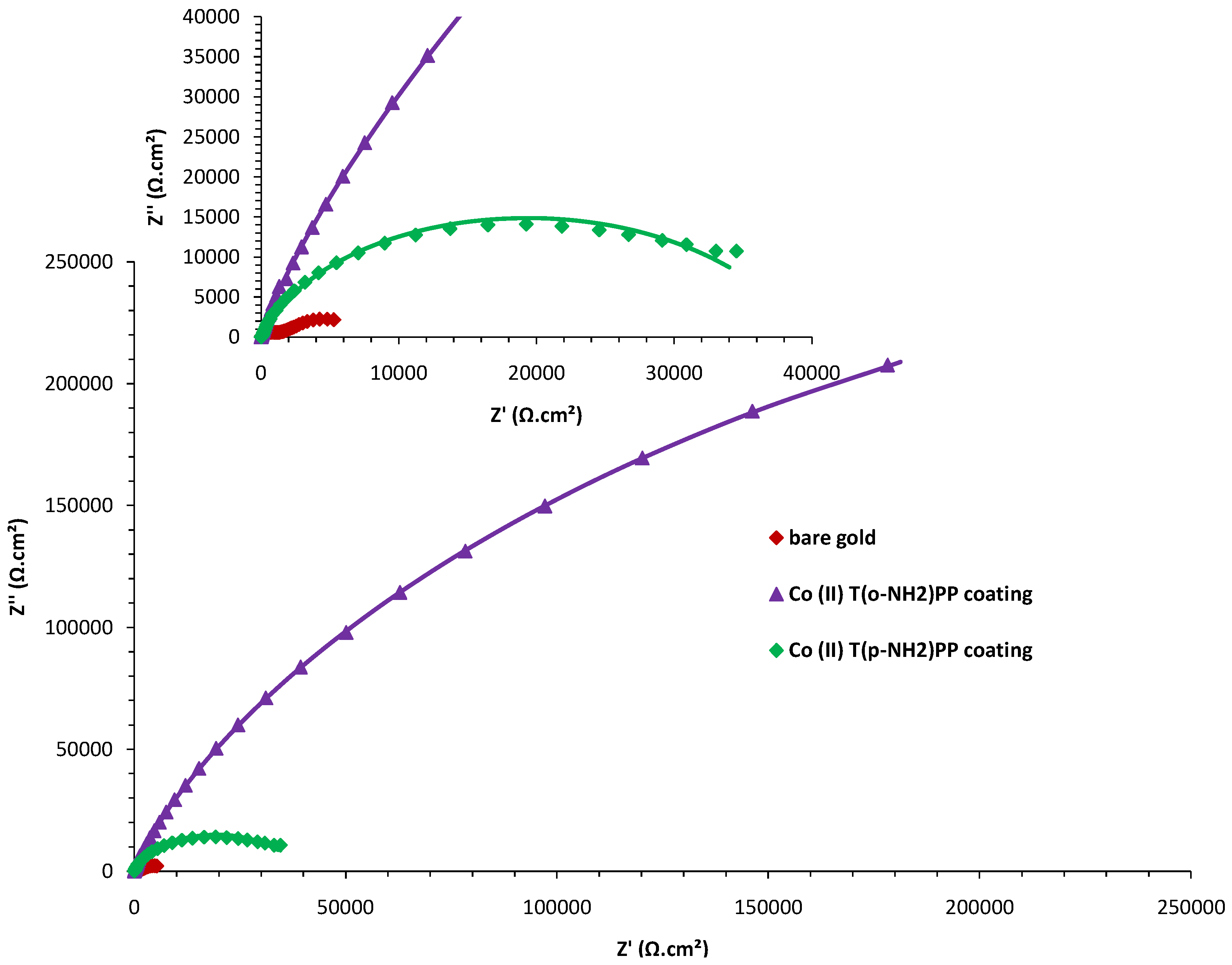
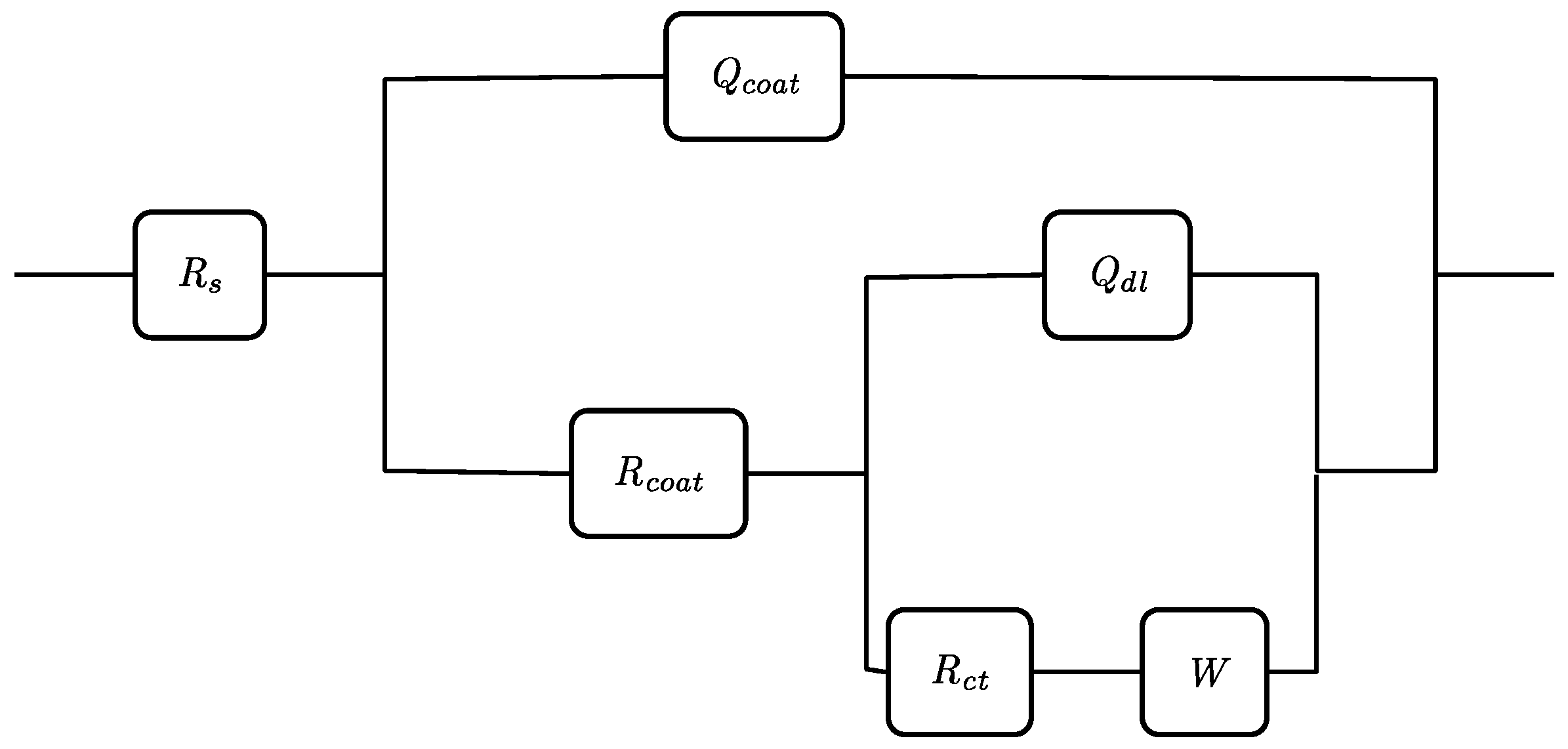
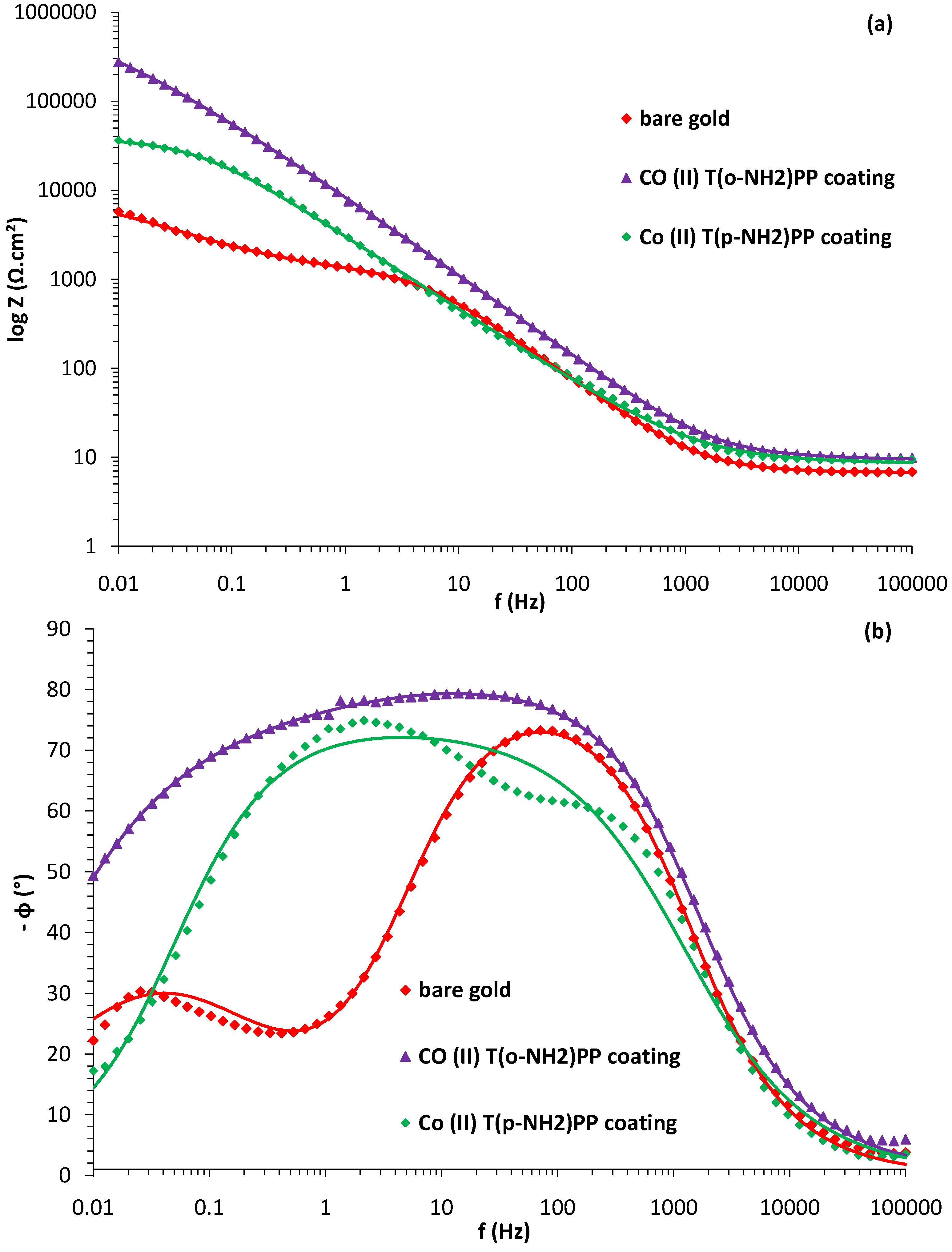
| Rs (Ωcm2) | Ccoat (µFcm−2) | Rcoat (Ωcm−2) | Cdl (µFcm−2) | Rct (.103 Ωcm2) | W (.10−3 Ω−1cm−2 s−1/2) | |
|---|---|---|---|---|---|---|
| bare gold | 6.65 | 38.11 | 1262.5 | 925.37 | 7.32 | 10.69 |
| Co(II) (T(p-NH2)PP) | 6.61 | 28.75 | 513.4 | 24.53 | 844.20 | 7.96 |
| Co(II) (T(o-NH2)PP) | 6.70 | 12.58 | 295.5 | 15.82 | 3002.94 | 4.36 |


2.4. Explanation of the Porphyrin Film Formation
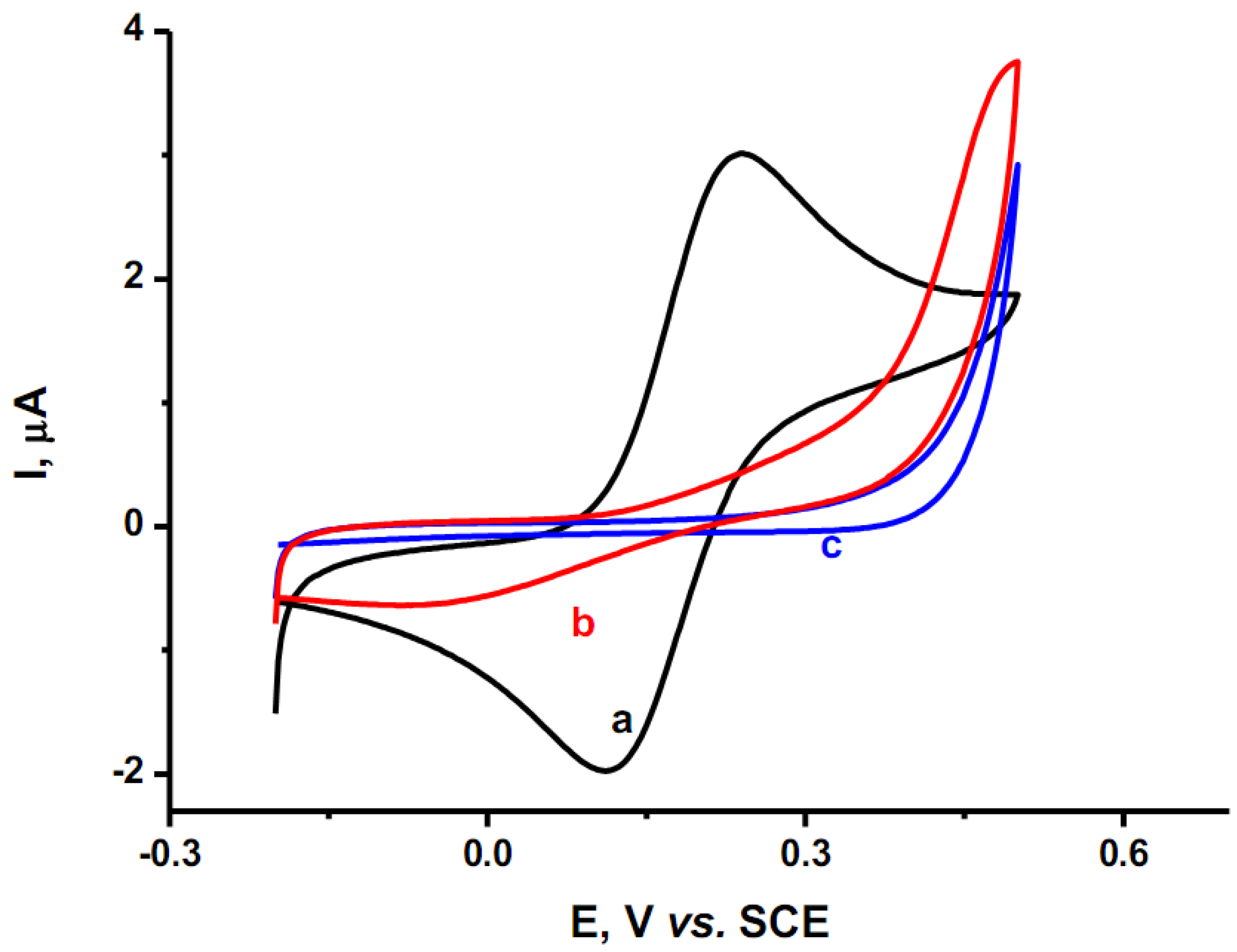
2.5. Electrocatalytic Activity

3. Experimental
3.1. Materials
3.2. Formation of a Self Assembled Film of Porphyrin on Gold
3.3. Instrumentation and Experimental Details
4. Conclusions
Acknowledgements
References
- Biesaga, M.; Pyrzyńska, K.; Trojanowicz, M. Porphyrins in analytical chemistry. A review. Talanta 2000, 51, 209–224. [Google Scholar] [CrossRef]
- Xiao, J.; Meyerhoff, M.E. Retention behavior of amino acids and peptides on protoporphyrin-silica stationary phases with varying metal ion centers. Anal. Chem. 1996, 68, 2818–2825. [Google Scholar] [CrossRef]
- Shi, Z.; Fu, C. Porphyrins as ligands for trace metal analysis by high-performance liquid chromatography. Talanta 1997, 44, 593–604. [Google Scholar] [CrossRef]
- Atunasov, P.; Gamburzev, S.; Wiikins, E. Needle-type glucose biosensors based on a pyrolyzed cobalt-tetramethoxy-phenylporphyrin catalytic electrode. Electroanalysis 1996, 8, 158–164. [Google Scholar] [CrossRef]
- Barondeau, D.P.; Kassmann, C.J.; Tainer, J.A.; Getzoff, E.D. Structural chemistry of a green fluorescent protein Zn biosensor. J. Am. Chem. Soc. 2002, 124, 3522–3524. [Google Scholar]
- Zhang, Y.; Yang, R.H.; Liu, F.; Li, K.A. Fluorescent sensor for imidazole derivatives based on monomer-dimer equilibrium of a zinc porphyrin complex in a polymeric film. Anal. Chem. 2004, 76, 7336–7345. [Google Scholar] [CrossRef]
- Lokesh, K.S.; de Wael, K.; Adriaens, A. Self-Assembled supramolecular array of polymeric phthalocyanine on gold for the determination of hydrogen peroxide. Langmuir 2010, 26, 17665–17673. [Google Scholar] [CrossRef]
- Song, E.; Shi, C.; Anson, F.C. Comparison of the behavior of several cobalt porphyrins as electrocatalysts for the reduction of O2 at graphite electrodes. Langmuir 1998, 14, 4315–4321. [Google Scholar] [CrossRef]
- Imahori, H.; Norieda, H.; Ozawa, S.; Ushida, K.; Yamada, H.; Azuma, T.; Tamaki, K.; Sakata, Y. Chain length effect on photocurrent from polymethylene-linked porphyrins in self-assembled monolayers. Langmuir 1998, 14, 5335–5338. [Google Scholar] [CrossRef]
- Imahori, H.; Hasobe, T.; Yamada, H.; Nishimura, Y.; Yamazaki, I.; Fukuzumi, S. Concentration effects of porphyrin monolayers on the structure and photoelectrochemical properties of mixed self-assembled monolayers of porphyrin and alkanethiol on gold electrodes. Langmuir 2001, 17, 4925–4931. [Google Scholar]
- Offord, D.A.; Sachs, S.B.; Ennis, M.S.; Emberspacher, T.A.; Griffin, J.H.; Chidsey, C.E.; Collman, J.P. Synthesis and properties of metalloporphyrin monolayers and stacked multilayers bound to an electrode via site specific axial ligation to a self-assembled monolayer. J. Am. Chem. Soc. 1998, 120, 4478–4487. [Google Scholar]
- Lyons, M.E.G. Transport and kinetics in electroactive polymers. Adv. Chem. Phys. Polym. Sys. 1996, 94, 297–624. [Google Scholar]
- Gorton, L. Chemically modified electrodes for the electrocatalytic oxidation of nicotinamide coenzymes. J. Chem. Soc. Farad. Trans. 1 1986, 82, 1245–1258. [Google Scholar] [CrossRef]
- Gorton, L.; Torstensson, A.; Jaegfeldt, H.; Johansson, G. Electrocatalytic oxidation of reduced nicotinamide coenzymes by graphite electrodes modified with an adsorbed phenoxazinium salt, meldola blue. J. Electroanal. Chem. Interf. Electrochem. 1984, 161, 103–120. [Google Scholar] [CrossRef]
- Lyons, M.E.G.; Fitzgerald, C.A.; Smyth, M.R. Glucose oxidation at ruthenium dioxide based electrodes. Analyst 1994, 119, 855–861. [Google Scholar] [CrossRef]
- Lyons, M.E.G.; Lyons, C.H.; Michas, A.; Bartlett, P.N. Heterogeneous redox catalysis at hydrated oxide layers. J. Electroanal. Chem. 1993, 351, 245–258. [Google Scholar] [CrossRef]
- Ulman, A. Formation and structure of self-assembled monolayers. Chem. Rev. 1996, 96, 1533–1554. [Google Scholar] [CrossRef]
- Cook, M.J. Thin film formulations of substituted phthalocyanines. J. Mater. Chem. 1996, 6, 677–689. [Google Scholar] [CrossRef]
- Bourgoin, J.P.; Doublet, F.; Palacin, S.; Vandevyver, M. High in-plane anisotropy in phthalocyanine LB films. Langmuir 1996, 12, 6473–6479. [Google Scholar] [CrossRef]
- Sauer, T.; Arndt, T.; Batchelder, D.N.; Kalachev, A.A.; Wegner, G. The structure of langmuir-blodgett films from substituted phthalocyaninato-polysiloxanes. Thin Solid Films 1990, 187, 357–374. [Google Scholar] [CrossRef]
- Cook, M.J.; Mayes, D.A.; Poynter, R.H. Spectroscopic monitoring of thermally induced molecular reorganisations within spin-coated and Langmuir-Blodgett films of mesogenic phthalocyanines. J. Mater. Chem. 1995, 5, 2233–2238. [Google Scholar] [CrossRef]
- Fujiki, M.; Tabei, H.; Kurihara, T. In-plane dichroisms of phthalocyanine Langmuir-Blodgett films. Langmuir 1988, 4, 1123–1128. [Google Scholar] [CrossRef]
- Vukusic, P.S.; Sambles, J.R. Cobalt phthalocyanine as a basis for the optical sensing of nitrogen dioxide using surface plasmon resonance. Thin Solid Films 1992, 221, 311–317. [Google Scholar] [CrossRef]
- Cheng, Z.H.; Gao, L.; Deng, Z.T.; Liu, Q.; Jiang, N.; Lin, X.; He, B.; Du, S.X.; Gao, H.J. Epitaxial growth of iron phthalocyanine at the initial stage on Au(111) surface. J. Phys. Chem. C 2007, 111, 2656–2660. [Google Scholar]
- Cheng, Z.H.; Gao, L.; Deng, Z.T.; Jiang, N.; Liu, Q.; Shi, D.X.; Du, S.X.; Guo, H.M.; Gao, H.J. Adsorption behavior of iron phthalocyanine on Au(111) surface at submonolayer coverage. J. Phys. Chem. C 2007, 111, 9240–9244. [Google Scholar]
- Borras, A.; Aguirre, M.; Groening, O.; Lopez-Cartes, C.; Groening, P. Synthesis of supported single-crystalline organic nanowires by physical vapor deposition. Chem. Mater. 2008, 20, 7371–7373. [Google Scholar] [CrossRef]
- Scudiero, L.; Barlow, D.E.; Hipps, K.W. Physical properties and metal ion specific scanning tunneling microscopy images of metal(ii) tetraphenylporphyrins deposited from vapor onto gold (111). J. Phys. Chem. B. 2000, 104, 11899–11905. [Google Scholar] [CrossRef]
- Huc, V.; Armand, F.; Bourgoin, J.P.; Palacin, S. Covalent anchoring of phthalocyanines on silicon dioxide surfaces:Building up mono- and multilayers. Langmuir 2001, 17, 1928–1935. [Google Scholar] [CrossRef]
- Hutchison, J.E.; Postlethwaite, T.A.; Murray, R.W. Molecular films of thiol-derivatized tetraphenylporphyrins on gold: Film formation and electrocatalytic dioxygen reduction. Langmuir 1993, 9, 3277–3283. [Google Scholar] [CrossRef]
- Malem, F.; Mandler, D. Self-assembled monolayers in electroanalytical chemistry: Application of .omega.-mercapto carboxylic acid monolayers for the electrochemical detection of dopamine in the presence of a high concentration of ascorbic acid. Anal. Chem. 1993, 65, 37–41. [Google Scholar]
- Xiao, Y.; Ju, H.X.; Chen, H.Y. A reagentless hydrogen peroxide sensor based on incorporation of horseradish peroxidase in poly(thionine) film on a monolayer modified electrode. Anal. Chim. Acta 1999, 391, 299–306. [Google Scholar] [CrossRef]
- Zak, J.; Yuan, H.; Ho, M.; Woo, L.K.; Porter, M.D. Thiol-derivatized metalloporphyrins: Monomolecular films for the electrocatalytic reduction of dioxygen at gold electrodes. Langmuir 1993, 9, 2772–2774. [Google Scholar] [CrossRef]
- Oh, S.Y.; Yun, Y.J.; Kim, D.Y.; Han, S.H. Formation of a self-assembled monolayer of diaminododecane and a heteropolyacid monolayer on the ITO surface. Langmuir 1999, 15, 4690–4692. [Google Scholar] [CrossRef]
- Gallardo, I.; Pinson, J.; Vila, N.J. Spontaneous attachment of amines to carbon and metallic surfaces. J. Phys. Chem. B 2006, 110, 19521–19529. [Google Scholar]
- Somashekarappa, M.P.; Sampath, S. Orientation dependent electrocatalysis using self assembled molecular fims. Chem. Commun. 2002, 1262–1263. [Google Scholar] [CrossRef]
- Sivanesan, A.; Abraham John, S. Amino group position dependent orientation of self-assembled monomolecular films of tetraaminophthalocyanatocobalt(II) on Au surfaces. Langmuir 2008, 24, 2186–2190. [Google Scholar] [CrossRef]
- Arima, V.; Blyth, R.I.R.; Della Sala, F.; del Sole, R.; Matino, F.; Mele, G.; Vasapollo, G.; Cingolani, R.; Rinaldi, R. Long-range order induced by cobalt porphyrin adsorption on aminothiophenol-functionalized Au(111): The influence of the induced dipole. Mat. Sci. Eng. C 2004, 24, 569–573. [Google Scholar] [CrossRef]
- Collman, J.P.; Denisevich, P.; Konai, Y.; Marrocco, M.; Koval, C.; Anson, F.C. Electrode catalysis of the four-electron reduction of oxygen to water by dicobalt face-to-face porphyrins. J. Am. Chem. Soc. 1980, 102, 6027–6036. [Google Scholar]
- Durand, R.R.; Bencosme, C.S.; Collman, J.P.; Anson, F.C. Mechanistic aspects of the catalytic reduction of dioxygen by cofacial metalloporphyrins. J. Am. Chem. Soc. 1983, 105, 2710–2718. [Google Scholar] [CrossRef]
- Lukasczyk, T.; Flechtner, K.; Merte, L.R.; Jux, N.; Maier, F.; Gottfried, J.M.; Steinrück, H.M. Interaction of cobalt(ii) tetraarylporphyrins with a Ag(111) surface studied with photoelectron spectroscopy. J. Phys. Chem. C 2007, 111, 3090–3098. [Google Scholar]
- Buchner, F.; Flechtner, F.; Bai, Y.; Zillner, E.; Keller, I.; Steinrück, H.P.; Marbach, H.; Gottfried, J.M. Coordination of iron atoms by tetraphenylporphyrin monolayers and multilayers on Ag(111) and formation of iron-tetraphenylporphyrin. J. Phys. Chem. C 2008, 112, 15458–15465. [Google Scholar]
- Wang, C.; Liu, C.; Yan, X.; He, J.; Zhang, M.; Shen, T. Investigation on the behavior of porphyrins at the surface of the colloidal silver particles. J. Photochem. Photobiol. A 1997, 104, 159–163. [Google Scholar] [CrossRef]
- Czernuszewicz, R.S.; Maes, E.M.; Rankin, J.G. Resonance Raman Spectroscopyof Petroporphyrins. In The Porphyrin Handbook, Theoretical and Physical Characterization; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; Academic Press: San Diego, CA, USA, 2003; Volume 7, pp. 293–338. [Google Scholar]
- Creighton, J.A.; Blatchford, C.G.; Albrecht, M.G. Plasma resonance enhancement of Raman scattering by pyridine adsorbed on silver or gold sol particles of size comparable to the excitation wavelength. J. Chem. Soc. Farad. Trans. 2 1979, 75, 790–798. [Google Scholar] [CrossRef]
- Creighton, J. The Selection Rules for Surface-Enhanced Raman Spectroscopy. In Spectroscopy of Surfaces: Advances in Spectroscopy; Clarke, R.J.H., Hester, R.E., Eds.; Wiley and Sons: New York, NY, USA, 1988; Volume 16. [Google Scholar]
- Collyer, S.D.; Davis, F.; Lucke, A.; Stirling, C.J.M.; Higson, S.P.J. The electrochemistry of the ferri/ferrocyanide couple at a calix[4]resorcinarenetetrathiol-modified gold electrode as a study of novel electrode modifying coatings for use within electro-analytical sensors. J. Electroanal. Chem. 2003, 549, 119–127. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Zhang, L.; Li, M.; Wang, X.; Zhang, Y.; Liu, X.; Zuo, G. Electrochemical characterization of self-assembled thiol-porphyrin monolayers on gold electrodes by SECM. ChemPhysChem 2006, 7, 854–862. [Google Scholar] [CrossRef]
- Amirudin, A.; Thierry, D. Application of electrochemical impedance spectroscopy to study the degradation of polymer-coated metals. Prog. Org. Coat. 1995, 26, 1–28. [Google Scholar] [CrossRef]
- Hettiarachichi, S.; Chan, Y.W.; Wilson, R.B.Jr.; Agarwala, V.S. Macrocyclic corrosion inhibitors for steel in acid chloride environments. Corrosion 1989, 45, 30–34. [Google Scholar] [CrossRef]
- Maranhao, S.L.A.; Guedes, I.C.; Anaissi, F.J.; Toma, H.E.; Aoki, I.V. Electrochemical and corrosion studies of poly(nickel-tetraaminophthalocyanine) on carbon steel. Electrochim. Acta 2006, 52, 519–526. [Google Scholar] [CrossRef]
- Khaled, K.; Amin, M.A. Electrochemical and molecular dynamics simulation studies on the corrosion inhibition of aluminum in molar hydrochloric acid using some imidazole derivatives. J. Appl. Electrochem. 2009, 39, 2553–2568. [Google Scholar] [CrossRef]
- Loveday, D.; Peterson, P.; Rodgers, B. Evaluation of organic coatings with electrochemical impedance spectroscopy—Part 2: Application of EIS to coatings. J. Coating Technol. 2004, 1, 88–93. [Google Scholar]
- Grandle, J.; Taylor, S. Electrochemical impedance spectroscopy of coated aluminum beverage containers: Part 1—Determination of an optimal parameter for large sample evaluation. Corrosion 1994, 50, 792–803. [Google Scholar] [CrossRef]
- Frignani, A.; Grassi, V.; Zucchi, F.; Zanotto, F. Mono-carboxylate conversion coatings for AZ31 Mg alloy protection. Mater. Corros. 2010, 61, 1–8. [Google Scholar]
- Buchner, F.; Warnick, K.G.; Wölfe, T.; Görling, A.; Heinrück, H.P.; Heiringer, W.; Marbach, H. Chemical fingerprints of large organic molecules in scanning tunneling microscopy: Imaging adsorbate-substrate coupling of metalloporphyrin. J. Phys. Chem. C 2009, 113, 16540–16457. [Google Scholar]
- Lokesh, K.S.; de Keersmaecker, M.; Elia, A.; Depla, D.; Dubruel, P.; Vandenabeele, P.; van Vlierberghe, S.; Adriaens, A. Adsorption of cobalt (II) 5,10,15,20-tetrakis(2-aminophenyl)-porphyrin onto copper substrates: Characterization and impedance studies for corrosion inhibition. Corros. Sci. 2012, 62, 73–82. [Google Scholar] [CrossRef]
- Buchner, F.; Schwald, V.; Comanici, K.; Steinrück, H.P.; Marbach, H. Microscopic evidence of the metalation of a free-base porphyrin monolayer with iron. Chem. Phys. Chem. 2007, 8, 241–243. [Google Scholar] [CrossRef]
- Buchner, F.; Keller, I.; Heiringer, W.; Görling, A.; Steinrück, H.P.; Marbach, H. Ordering aspects and intramolecular conformation of tetraphenylporphyrins on Ag(111). Phys. Chem. Chem. Phys. 2010, 12, 13082–13090. [Google Scholar]
- Lu, X.; Lv, B.; Xue, Z.; Li, M.; Zhang, L.; Kang, J. Self-assembled monolayers of a thiol-derivatized porphyrin on gold electrode: Film formation and electrocatalytic dioxygen reaction. Thin Solid Films 2005, 488, 230–235. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lokesh, K.S.; De Keersmaecker, M.; Adriaens, A. Self Assembled Films of Porphyrins with Amine Groups at Different Positions: Influence of Their Orientation on the Corrosion Inhibition and the Electrocatalytic Activity. Molecules 2012, 17, 7824-7842. https://doi.org/10.3390/molecules17077824
Lokesh KS, De Keersmaecker M, Adriaens A. Self Assembled Films of Porphyrins with Amine Groups at Different Positions: Influence of Their Orientation on the Corrosion Inhibition and the Electrocatalytic Activity. Molecules. 2012; 17(7):7824-7842. https://doi.org/10.3390/molecules17077824
Chicago/Turabian StyleLokesh, Koodlur Sannegowda, Michel De Keersmaecker, and Annemie Adriaens. 2012. "Self Assembled Films of Porphyrins with Amine Groups at Different Positions: Influence of Their Orientation on the Corrosion Inhibition and the Electrocatalytic Activity" Molecules 17, no. 7: 7824-7842. https://doi.org/10.3390/molecules17077824





