3. Experimental
3.1. General
Melting points were recorded on a Griffin melting point apparatus and are reported uncorrected. IR spectra were recorded using KBr disks using a Perkin-Elmer System 2000 FT-IR spectrophotometer.
1H-NMR (400 MHz) or (600 MHz) and
13C-NMR (100 MHz) or (150 MHz) spectra were recorded at 25 °C or as reported in CDCl
3 or DMSO-
d6 as solvent with TMS as internal standard on a Bruker DPX 400 or 600 super-conducting NMR spectrometer. Chemical shifts are reported in ppm. Mass spectra were measured using a high resolution GC-MS (DFS) Thermo spectrometer with EI (70 EV). Microanalyses were performed on a LECO CHNS-932 Elemental Analyzer. The crystal structures were determined by a Rigaku R-AXIS RAPID diffractometer and Bruker X8 Prospector at Kuwait University. The compounds 5-amino-
N-(2-chloro-5-nitrophenyl)-1
H-pyrazole-4-carboxamide and 5-amino-
N-(4,6-dimethylpyrimidin-2-yl)-1
H-pyrazole-4-carboxamide were prepared according to the literature procedure [
38].
3.2. General Procedure for the Preparation of 2-Chloro-N-(heteroaryl)acetamides 2a–d
A solution of the appropriate amines 1a–d (10 mmol) and chloroacetyl chloride (1.12 g, 10 mmol) in chloroform (50 mL) was refluxed in the presence of K2CO3 (15 mmol) for about 10 h. Then the solvent was removed in vacuo and the residue was stirred with water (100 mL) and filtered. The solid product is then washed with 5% NaHCO3 solution and subsequently with water. The crude product is dried and crystallized from appropriate solvent to furnish pure solid product.
2-Chloro-N-(2-chloro-5-nitrophenyl)acetamide (2a). Recrystallized from isopropyl alcohol as pale yellow crystals, yield: 93%, m.p. 120–121 °C; IR (KBr): 𝑣/cm−1 3354 (NH), 1686 (CO); 1H-NMR (DMSO-d6): δ = 4.47 (s, 2H, CH2), 7.84 (d, J = 8.0 Hz, 1H, Ar-H), 8.06 (d, J = 8.0 Hz, 1H, Ar-H), 8.73 (s, 1H, Ar-H), and 10.23 ppm (s, 1H, NH); 13C-NMR (DMSO-d6): δ = 43.03 (CH2), 115.96, 119.91, 129.44, 129.77, 134.58, 147.17 and 164.18 ppm (Ar-C and CO) MS (EI): m/z (%) 248 (M+, 11.55), 249 (M++1, 3.75). Anal. calcd. for C8H6Cl2N2O3 (249.05): C, 38.58; H, 2.43; N, 11.25. Found: C, 38.66; H, 2.50; N, 11.18.
N-(4-Acetylphenyl)-2-chloroacetamide (2b). Recrystallized from EtOH as yellow crystals, yield: 89%, m.p. 145–146 °C; IR (KBr): 𝑣/cm−1 3286 (NH), 1707, 1658 (2CO); 1H-NMR (DMSO-d6): δ = 2.53 (s, 3H, CH3), 4.31 (s, 2H, CH2), 7.73 (d, J = 8.4 Hz, 2H, Ar-H), 7.94 (d, J = 8.4 Hz, 2H, Ar-H) and 10.63 ppm (s, 1H, NH); m/z (%) 211 (M+, 39.20), 212 (M++1, 7.65). Anal. calcd. for C10H10ClNO2 (211.65): C, 56.75; H, 4.76; N, 6.62. Found: C, 56.84; H, 4.72; N, 6.58.
N-Benzothiazol-2-yl-2-chloroacetamide (
2c) [
39]. Recrystallized from EtOH as white crystals, yield: 96%, m.p. 145–146 °C; IR (KBr): 𝑣/cm
−1 3348 (NH), 1665 (CO);
1H-NMR (DMSO-
d6): δ = 4.47 (s, 2H, CH
2), 7.32 (t,
J = 7.6 Hz, 1H, Ar-H), 7.45 (t,
J = 7.6 Hz, 1H, Ar-H), 7.77 (d,
J = 7.6 Hz, 1H, Ar-H), 7.99 (d,
J = 7.6 Hz, 1H, Ar-H) and 12.74 ppm (s, 1H, NH);
13C-NMR (DMSO-
d6): δ = 43.02 (CH
2), 121.16, 122.26, 124.26, 126.70, 131.94, 148.87, 158.06 and 166.43 ppm (Ar-C and CO); MS (EI):
m/
z (%) 226 (M
+, 26.4), 227 (M
++1, 5.6). Anal. calcd. for C
9H
7ClN
2OS (226.69): C, 47.69; H, 3.11; N, 12.36; S, 14.14. Found: C, 47.77; H, 3.09; N, 12.44; S, 14.23.
Ethyl-4-(2-chloroacetamido)benzoate (
2d). Recrystallized from EtOH as white crystals, yield: 91%, m.p. 106–107 °C; IR (KBr): 𝑣/cm
−1 3275 (NH), 1722, 1678 (2CO);
1H-NMR (CDCl
3): δ = 1.36 (t,
J = 7.2 Hz, 3H, CH
3), 4.17 (s, 2H, CH
2), 4.34 (q,
J = 7.2 Hz, 2H, CH
2), 7.63 (d,
J = 8.0 Hz, 2H, Ar-H), 8.01 (d,
J = 8.0 Hz, 2H, Ar-H) and 8.56 ppm (s, 1H, NH);
13C-NMR (CDCl
3): δ = 14.22 (CH
3), 42.84 (CH
2), 60.93 (CH
2), 119.12, 126.74, 130.68, 140.75, 164.15 and 165.92 ppm (Ar-C and CO); MS (EI):
m/
z (%) 241 (M
+, 68.84), 242 (M
++1, 21.72). Anal. calcd. for C
11H
12ClNO
3 (241.68): C, 54.67; H, 5.00; N, 5.80. Found: 54.75; H, 4.93; N, 5.71. Crystallographic Analysis for
2d: The crystals were mounted on a glass fiber. All measurements were performed on Bruker X8 Prospector. The data were collected at a temperature of 20 ± 1 °C to a maximum θ value of 66.61° using the ω scanning technique. The structure was solved by direct method using SHELXS-97 (Sheldrick, 2008) and refined by Full-matrix least-squares on F
2. The non-hydrogen atoms were refined anisotropically. Data were corrected for absorption effects using the multi-scan method (SADABS). Crystal Data: C
11H
12ClNO
3, M = 241.68, monoclinic, a = 4.7349(4) Å, b = 28.541(2) Å, c = 9.1098(6) Å, V = 1203.41(16) Å
3, α = γ = 90.00°, β = 102.173(4)°, space group: P 1 21/n 1, Z = 4, D
calc = 1.334 Mg cm
−3, No. of reflections measured 4589, θ
max = 66.34°, R1 = 0.06.
Figure 1 illustrates the structure as determined. Full data can be obtained on request from the CCDC [
28].
3.3. General Procedure for the Synthesis of 2-(Arylimino)thiazolidin-4-ones 3a–d
A solution of 2-chloro-N-(heteroaryl or aryl)acetamides 2a–d (10 mmol) and ammonium thiocyanate (15 mmol) in absolute ethanol (30 mL) was refluxed for 4 h and allowed to stand overnight. The formed precipitate was filtered off, washed with water and then recrystallised from the appropriate solvent.
(Z)-2-(2-Chloro-5-nitrophenylimino)thiazolidin-4-one (
3a). Recrystallized from EtOH as yellow crystals, yield: 76%, m.p. 198–199 °C; IR (KBr): 𝑣/cm
−1 3136 (NH), 1738 (CO);
1H-NMR (DMSO-
d6): δ = 4.09 (s, 2H, CH
2), 7.81 (d,
J = 8.4 Hz, 1H, Ar-H), 7.89 (s, 1H, Ar-H), 7.98 (d,
J = 8.4 Hz, 1H, Ar-H) and 12.23 ppm (s, 1H, NH);
13C-NMR (DMSO-
d6): δ = 34.57 (CH
2), 116.96, 119.99, 131.12, 133.19, 146.50, 146.78, 160.11 and 173.73 (Ar-C and CO); MS (EI):
m/
z (%) 271 (M
+, 100), 272 (M
++1, 32.25). Anal. calcd. for C
9H
6ClN
3O
3S (271.68): C, 39.79; H, 2.23; N, 15.47; S, 11.80; Found: C, 39.86; H, 2.14; N, 15.36; S, 11.88. Crystallographic Analysis for
3a: The crystals were mounted on a glass fiber. All measurements were performed on a Rigaku R-AXIS RAPID diffractometer using filtered Mo-Kα radiation. The data were collected at a temperature of 20 ± 1 °C to a maximum 2θ value of 55.0° using the ω scanning technique. The structure was solved by charge flipping method and expanded using Fourier techniques. The non-hydrogen atoms were refined anisotropically. Hydrogen atoms were refined using the riding model. Crystal Data: C
9H
6ClN
3O
3S, M = 271.68, orthorhombic, a = 20.1723(7) Å, b = 11.3976(5) Å, c = 20.145(2) Å, V = 4631.6(5) Å
3, α = β = γ = 90.00°, space group: Pbca (#61), Z = 16, D
calc = 1.558 g cm
−3, No. of reflections measured 5236, 2θ
max = 54.9°, R1 = 0.0683.
Figure 2 shows the structure as determined. Full data can be obtained on request from the CCDC [
29].
(Z)-2-(4-Acetylphenylimino)thiazolidin-4-one (3b). Recrystallized from EtOH/dioxane (1:1) as buff crystals, yield: 81%, m.p. 260–261 °C; IR (KBr): 𝑣/cm−1 3271 (NH), 1717, 1666 (2CO); 1H-NMR (DMSO-d6): δ = 2.55 (s, 3H, CH3), 4.03 (s, 2H, CH2), 7.06 (d, J = 8.0 Hz, 1H, Ar-H), 7.86 (d, J = 8.0 Hz, 1H, Ar-H), 7.96 (d, J = 8.0 Hz, 2H, Ar-H) and 11.92 ppm (s, 1H, NH); 13C-NMR (DMSO-d6): δ = 26.49 (CH3), 37.39 (CH2), 118.56, 129.57, 132.26, 142.57, 164.90, 174.01 and 196.49 (Ar-C and CO); MS (EI): m/z (%) 234 (M+, 78.0), 235 (M++1, 15.80). Anal. calcd. for C11H10N2O2S (234.28): C, 56.40; H, 4.30; N, 11.96; S,13.69. Found: C, 56.33; H, 4.26; N, 11.85; S,13.55.
(Z)-2-(Benzothiazol-2-ylimino)thiazolidin-4-one (
3c) [
36]. Recrystallized from an EtOH/dioxane (1:1) mixture pale yellow crystals, yield: 73%, m.p. 201–202 °C; IR (KBr): 𝑣/cm
−1 3145 (NH), 1734 (CO);
1H-NMR (DMSO-
d6): δ = 4.12 (s, 2H, CH
2), 7.39 (t,
J = 7.6 Hz, 1H, Ar-H), 7.51 (t,
J = 7.6 Hz, 1H, Ar-H), 7.85 (d,
J = 7.6 Hz, 1H, Ar-H), 8.01 (d,
J = 7.6 Hz, 1H, Ar-H) and 12.35 ppm (s, 1H, NH);
13C-NMR (DMSO-
d6): δ = 35.68 (CH
2), 121.82, 122.40, 124.65, 126.82, 133.49, 151.30, 166.56, 169.29 and 174.78 (Ar-C and CO); MS (EI):
m/
z (%) 249 (M
+, 100), 250 (M
++1, 14.6). Anal. calcd. for C
10H
7N
3OS
2 (249.31): C, 48.18; H, 2.83; N, 16.85; S, 25.72. Found: C, 48.09; H, 2.94; N, 16.94; S, 25.65. Crystallographic Analysis for
3c: The crystals were mounted on a glass fiber. All measurements were performed on a Rigaku R-AXIS RAPID diffractometer using filtered Mo-Kα radiation. The data were collected at a temperature of 20 ± 1 °C to a maximum 2θ value of 55.0° using the ω scanning technique. The structure was solved by charge flipping method and expanded using Fourier techniques. The non-hydrogen atoms were refined anisotropically. Hydrogen atoms were refined using the riding model. Crystal Data: C
10H
7N
3OS
2, M = 249.31, monoclinic, a = 18.289(2) Å, b = 5.0485(1) Å, c = 11.5483(4) Å, V = 1066.30(9) Å
3, α = γ = 90.00°, β = 90.077(6)°, space group: P2
1/c, Z = 4, D
calc = 1.553 g cm
−3. No. of reflections measured 2444, 2θ
max = 54.9°, R1 = 0.0305.
Figure 3 illustrates the structure as determined. Full data can be obtained on request from the CCDC [
30].
(Z)-Ethyl-4-(4-oxothiazolidin-2-ylideneamino)benzoate (
3d). Recrystallized from EtOH as pale yellow crystals, yield: 80%, m.p. 186–187 °C; IR (KBr): 𝑣/cm
−1 3200 (NH), 1719, 1672 (2CO);
1H-NMR (DMSO-
d6): δ = 1.31 (t,
J = 7.2 Hz, 3H, CH
3), 4.03 (s, 2H, CH
2), 4.29 (q,
J = 7.2 Hz, 2H, CH
2), 7.06 (d,
J = 7.8 Hz, 1H, Ar-H), 7.85 (d,
J = 7.8 Hz, 1H, Ar-H), 7.96 (d,
J = 7.8 Hz, 2H, Ar-H) and 11.54 ppm (br, 1H, NH);
13C-NMR (DMSO-
d6): δ = 14.20 (CH
3), 34.42 (CH
2), 60.58 (CH
2), 119.71, 121.31, 125.51, 130.48, 142.80, 157.92, 165.72 and 174.33 (Ar-C and CO); MS (EI):
m/
z (%) 264 (M
+, 100), 265 (M
++1, 17.80). Anal. calcd. For C
12H
12N
2O
3S (264.31): C, 54.53; H, 4.58; N, 10.60; S, 12.13; Found: 54.64; H, 4.49; N, 10.67; S, 12.06. Crystallographic Analysis for
3d. The crystals were mounted on a glass fiber. All measurements were performed on Bruker X8 Prospector. The data were collected at a temperature of 20 ± 1 °C to a maximum θ value of 66.61° using the ω scanning technique. The structure was solved by direct method using SHELXS-97 (Sheldrick, 2008) and refined by Full-matrix least-squares on F
2. The non-hydrogen atoms were refined anisotropically. Data were corrected for absorption effects using the multi-scan method (SADABS). Crystal Data. C
12H
12N
2O
3S, M = 264.31, triclinic, a = 4.0829(3) Å, b = 5.5436(4) Å, c = 26.974(2) Å, V = 605.71(8) Å
3, α = 84.625(6)°, β = 89.051(6)°, γ = 85.220(6)°, space group: P-1, Z = 2, D
calc = 1.449 Mg cm
−3, No. of reflections measured 5850, θ
max = 66.0°, R1 = 0.0623.
Figure 4 shows the structure as determined. Full data can be obtained on request from the CCDC [
31].
3.4. General Procedure for the Synthesis of 2-Arylimino-5-arylidene-4-thiazolidinones 7a–p
Method A: Independent mixtures of 2-(heteroaryl or arylimino)thiazolidin-4-ones 3a–d (5 mmol) and the appropriate arylidene malononitrile 5 (0.77 g, 5 mmol) in ethanol (30 mL) containing few drops of piperidine (5 drops) were stirred at reflux for 3 h. Then, the reaction mixtures were cooled to room temperature. The solid which formed was collected by filtration, washed with hot ethanol, and recrystallized from the appropriate solvent to afford 7a–p respectively, as pure substances.
Method B: A mixture of 2-(heteroaryl or arylimino)thiazolidin-4-ones 3a–d (5 mmol) and the appropriate arylaldehyde (5 mmol) in acetic acid (25 mL) containing sodium acetate (10 mmol) was refluxed for 5 h. The reaction mixture was then cooled to room temperature and poured into ice-cold water. The precipitate was filtered off and washed with water and the resulting crude product was purified by recrystallization from the appropriate solvent.
(2Z,5Z)-5-Benzylidene-2-(2-chloro-5-nitrophenylimino)thiazolidin-4-one (7a). Recrystallized from dioxane/DMF (1:2) mixture as creamy crystals; yield: (A: 84%, B: 71%), m.p. 240–241 °C; IR (KBr): 𝑣/cm−1 3121 (NH), 1729 (CO); 1H-NMR (DMSO-d6): δ = 7.42–7.49 (m, 5H, Ar-H), 7.71 (s, 1H, olefinic CH), 7.82 (d, J = 7.6 Hz, 1H, Ar-H), 8.02–8.05 (m, 2H, Ar-H) and 12.86 ppm (s, 1H, NH); 13C-NMR (DMSO-d6): δ = 117.28, 120.51, 122.10, 129.25, 129.80, 130.15, 130.62, 131.18, 133.01, 146.07, 146.93, 154.28, 160.98 and 167.22 ppm (Ar-C and CO); MS (EI): m/z (%) 359 (M+, 82.3), 360 (M++1, 20.22). Anal. calcd. for C16H10ClN3O3S (359.79): C, 53.41; H, 2.80; N, 11.68; S, 8.91. Found: C, 53.34; H, 2.68; N, 11.75; S, 8.84.
(2Z,5Z)-2-(2-Chloro-5-nitrophenylimino)-5-(4-methylbenzylidene)thiazolidin-4-one (7b). Obtained from dioxane/DMF (1:1) mixture as yellowish white crystals; yield: (A: 83%, B: 68%), m.p. 250–251 °C; IR (KBr): 𝑣/cm−1 3132 (NH), 1731 (CO); 1H-NMR (DMSO-d6): δ = 3.32 (s, 3H, CH3), 7.27 (d, J = 8.0 Hz, 2H, Ar-H), 7.39 (d, J = 8.0 Hz, 2H, Ar-H), 7.68 (s, 1H, olefinic CH), 7.84 (d, J = 7.6 Hz, 1H, Ar-H), 8.02–8.04 (m, 2H, Ar-H) and 12.82 ppm (s, 1H, NH); 13C-NMR (DMSO-d6): δ = 21.03(CH3), 117.25, 120.43, 120.81, 129.84, 130.20, 130.69, 131.15, 133.01,140.36, 146.10, 146.89, 154.28, 160.84 and 167.25 ppm (Ar-C and CO); MS (EI): m/z (%) 373 (M+, 71.75), 374 (M++1, 18.60). Anal. calcd. for C17H12ClN3O3S (373.82): C, 54.62; H, 3.24; N, 11.24; S, 8.58. Found: C, 54.70; H, 3.17; N, 11.31; S, 8.66.
(2Z,5Z)-2-(2-Chloro-5-nitrophenylimino)-5-(4-methoxybenzylidene)thiazolidin-4-one (
7c). Obtained from DMSO as yellow crystals; yield: (A: 77%, B: 65%), m.p. 247–248 °C; IR (KBr): 𝑣/cm
−1 3136 (NH), 1726 (CO);
1H-NMR (DMSO-
d6): δ = 3.78 (s, 3H, OCH
3), 7.03 (d,
J = 8.4 Hz, 2H, Ar-H), 7.49 (d,
J = 8.4 Hz, 2H, Ar-H), 7.69 (s, 1H, olefinic CH), 7.87 (d,
J = 8.0 Hz, 1H, Ar-H), 8.04–8.10 (m, 2H, Ar-H) and 12.77 ppm (s, 1H, NH);
13C-NMR (DMSO-
d6): δ = 55.39 (CH
3), 114.83, 117.32, 118.88, 120.42, 125.47, 130.65, 131.18, 131.84, 133.06, 146.24, 146.93, 154.46, 160.75 and 167.38 ppm (Ar-C and CO); MS (EI):
m/
z (%) 389 (M
+, 66.25), 390 (M
++1, 13.94). Anal. calcd. for C
17H
12ClN
3O
4S (389.82): C, 52.38; H, 3.10; N, 10.78; S, 8.23. Found: C, 52.43; H, 3.03; N, 10.84; S, 8.17. Crystallographic Analysis for
7c: The crystals were mounted on a glass fiber. All measurements were performed on a Rigaku R-AXIS RAPID diffractometer using filtered Mo-Kα radiation. The data were collected at a temperature of 20 ± 1 °C to a maximum 2θ value of 55.0° using the ω scanning technique. The structure was solved by charge flipping method and expanded using Fourier techniques. The non-hydrogen atoms were refined anisotropically. Hydrogen atoms were refined using the riding model. Crystal Data: C
17H
12ClN
3O
4S, M = 389.82, monoclinic, a = 21.540(3) Å, b = 8.1718(9) Å, c = 25.489(3) Å, V = 4170.4(8) Å
3, α = γ = 90.00°, β = 111.639(8)°, space group: P2
1/c, Z = 8, D
calc = 1.490 g cm
−3, No. of reflections measured 6925, 2θ
max = 50.0°, R1 = 0.0470.
Figure 5 illustrates the structure as determined. Full data can be obtained on request from the CCDC [
34].
(2Z,5Z)-2-(2-Chloro-5-nitrophenylimino)-5-(4-nitrobenzylidene)thiazolidin-4-one (7d). Recrystallized from dioxane/DMF (1:2) mixture as yellow crystals; yield: (A: 73%, B: 59%), m.p. 263–264 °C; IR (KBr): 𝑣/cm−1 3292 (NH), 1733 (CO); 1H-NMR (DMSO-d6): δ = 7.78 (d, J = 8.4 Hz, 2H, Ar-H), 7.83 (s, 1H, olefinic CH), 7.88 (d, J = 8.0 Hz, 1H, Ar-H), 8.06–8.08 (m, 2H, Ar-H), 8.27 (d, J = 8.4 Hz, 2H, Ar-H) and 13.07 ppm (s, 1H, NH); 13C-NMR (DMSO-d6): δ = 117.27, 120.69, 124.22, 126.62, 128.02, 130.76, 131.26, 132.99, 139.32; 145.88, 146.93, 147.24, 153.67 and 166.87 ppm (Ar-C and CO); MS (EI): m/z (%) 404 (M+, 55.30), 405 (M++1, 14.62). Anal. calcd. For C16H9ClN4O5S (404.79): C, 47.48; H, 2.24; N, 13.84; S, 7.92. Found: C, 47.56; H, 2.27; N, 13.91; S, 7.86.
(2Z,5Z)-5-(4-Chlorobenzylidene)-2-(2-chloro-5-nitrophenylimino)thiazolidin-4-one (7e). Recrystallized from DMF as yellowish white crystals; yield: (A: 75%, B: 66%), m.p. 253–254 °C; IR (KBr): 𝑣/cm−1 3128 (NH), 1724 (CO); 1H-NMR (DMSO-d6): δ = 7.51–7.52 (m, 4H, Ar-H), 7.71 (s, 1H, olefinic CH), 7.84 (d, J = 8.0 Hz, 1H, Ar-H), 8.03–8.05 (m, 2H, Ar-H) and 12.89 ppm (s, 1H, NH); MS (EI): m/z (%) 393 (M+, 45.30), 394 (M++1, 12.95). Anal. calcd. for C16H9Cl2N3O3S (394.24): C, 48.75; H, 2.30; N, 10.66; S, 8.13. Found: C, 48.82; H, 2.23; N, 10.58; S, 8.19.
(2Z,5Z)-2-(2-Chloro-5-nitrophenylimino)-5-(thiophen-2-ylmethylidene)thiazolidin-4-one (7f). Obtained from dioxane as pale brown crystals; yield: (A: 81%, B: 70%), m.p. 266–267 °C; IR (KBr): 𝑣/cm−1 3112 (NH), 1727 (CO); 1H-NMR (DMSO-d6): δ = 7.24 (t, J = 4.8 Hz, 1H, Ar-H), 7.62 (d, J = 4.8 Hz, 1H, Ar-H), 7.86 (s, 1H, olefinic CH), 7.90 (d, J = 4.8 Hz, 1H, Ar-H), 8.00 (s, 1H, Ar-H), 8.05–8.07 (m, 2H, Ar-H) and 12.83 ppm (s, 1H, NH); 13C-NMR (DMSO-d6): δ = 117.26, 119.64, 120.51, 123.98, 128.85, 131.18, 132.45, 133.10, 133.94, 137.16, 146.10, 146.88, 153.71 and 166.94 ppm (Ar-C and CO); MS (EI): m/z (%) 365 (M+, 86.77), 366 (M++1, 23.48). Anal. calcd. For C14H8ClN3O3S2 (365.82): C, 45.97; H, 2.20; N, 11.49; S, 17.53. Found: C, 46.05; H, 2.26; N, 11.43; S, 17.44.
(2Z,5Z)-Ethyl-4-(5-benzylidene-4-oxothiazolidin-2-ylideneamino)benzoate (7g). Recrystallized from DMF as yellow crystals; yield: (A: 74%, B: 69%), m.p. 291–292 °C; IR (KBr): 𝑣/cm−1 3199 (NH), 1709, 1678 (2CO); 1H-NMR (DMSO-d6): δ = 1.33 (t, J = 6.8 Hz, 3H, CH3), 4.32 (q, J = 6.8 Hz, 2H, CH2), 7.15 (d, J = 7.2 Hz, 2H, Ar-H), 7.47–8.00 (m, 8H, Ar-H and olefinic CH) and 12.34 ppm (br, 1H, NH); MS (EI): m/z (%) 352 (M+, 19.50), 353 (M++1, 5.20). Anal. calcd. for C19H16N2O3S (352.41): C, 64.76; H, 4.58; N, 7.95; S, 9.10; Found: C, 64.84; H, 4.65; N, 8.01; S, 9.18.
(2Z,5Z)-2-(4-Acetylphenylimino)-5-benzylidenethiazolidin-4-one (7h). Recrystallized from DMF as pale brown crystals; yield: (A: 90%, B: 79%), m.p. above 300 °C; IR (KBr): 𝑣/cm−1 3199 (NH), 1711, 1676 (CO); 1H-NMR (DMSO-d6 at 110 °C): δ = 2.58 (s, 3H, CH3), 7.41–7.43 (m, 3H, Ar-H), 7.47–7.56 (m, 4H, Ar-H), 7.69 (s, 1H, olefinic CH), 8.00 (d, J = 8.0 Hz, 2H, Ar-H) and 12.34 ppm (s, 1H, NH); MS (EI): m/z (%) 322 (M+, 15.45), 323 (M++1, 4.15). Anal. calcd. for C18H14N2O2S (322.39): C, 67.06; H, 4.38; N, 8.69; S, 9.95. Found: C, 66.98; H, 4.51; N, 8.63; S, 10.03.
(2Z,5Z)-2-(4-Acetylphenylimino)-5-(4-methylbenzylidene)thiazolidin-4-one (7i). Recrystallized from DMF as pale brown crystals; yield: (A: 93%, B: 81%), m.p. above 300 °C; IR (KBr): 𝑣/cm−1 3196 (NH), 1710, 1674 (2CO); 1H-NMR (DMSO-d6 at 110 °C): δ = 2.35 (s, 3H, CH3), 2.56 (s, 3H, CH3), 7.30 (d, J = 7.6 Hz, 2H, Ar-H), 7.43–7.45 (m, 4H, Ar-H), 7.65 (s, 1H, olefinic CH), 7.98 (d, J = 8.0 Hz, 2H, Ar-H) and 11.84 ppm (s, 1H, NH); MS (EI): m/z (%) 336 (M+, 55.0), 337 (M++1, 13.50). Anal. calcd. for C19H16N2O2S (336.42): C, 67.84; H, 4.79; N, 8.33; S, 9.53. Found: C, 67.91; H, 4.75; N, 8.39; S, 9.45.
(2Z,5Z)-2-(4-Acetylphenylimino)-5-(4-methoxybenzylidene)thiazolidin-4-one (7j). Recrystallized from DMF as yellow crystals; yield: (A: 89%, B: 75%), m.p. above 300 °C; IR (KBr): 𝑣/cm−1 3192 (NH), 1713, 1672 (2CO); 1H-NMR (DMSO-d6 at 110 °C): δ = 2.56 (s, 3H, CH3), 3.82 (s, 3H, OCH3), 7.04 (d, J = 8.4 Hz, 2H, Ar-H), 7.39–7.49 (m, 4H, Ar-H), 7.64 (s, 1H, olefinic CH), 7.98 (d, J = 8.0 Hz, 2H, Ar-H) and 11.81 ppm (s, 1H, NH); 13C-NMR (DMSO-d6 at 110 °C): δ = 26.68 (CH3), 55.96 (CH3), 115.09, 115.43, 1121.44, 126.75, 130.03, 130.26, 130.76, 131.91, 134.11, 143.95, 161.31, 166.74 and 196.84 ppm (Ar-C and CO); MS (EI): m/z (%) 352 (M+, 46.20), 353 (M++1, 12.15). Anal. calcd. for C19H16N2O3S (352.41): C, 64.76; H, 4.58; N, 7.95; S, 9.10. Found: C, 64.79; H, 4.65; N, 7.86; S, 8.99.
(2Z,5Z)-2-(4-Acetylphenylimino)-5-(4-nitrobenzylidene)thiazolidin-4-one (7k). Recrystallized from DMF as yellow crystals; yield: (A: 92%, B: 84%), m.p. above 300 °C; IR (KBr): 𝑣/cm−1 3198 (NH), 1718, 1674 (2CO); 1H-NMR (DMSO-d6 at 110 °C): δ = 2.57 (s, 3H, CH3), 7.48–7.78 (m, 4H, Ar-H), 7.98 (s, 1H, olefinic CH), 8.10–8.27 (m, 4H, Ar-H) and 12.09 ppm (s, 1H, NH); MS (EI): m/z (%) 367 (M+, 100), 368 (M++1, 23.80). Anal. calcd. for C18H13N3O4S (367.39): C, 58.85; H, 3.57; N, 11.44; S, 8.73. Found: C, 58.77; H, 3.66; N, 11.48; S, 8.79.
(2Z,5Z)-2-(4-Acetylphenylimino)-5-(4-chlorobenzylidene)thiazolidin-4-one (7l). Recrystallized from DMF as pale brown crystals; yield: Yield: (A: 95%, B: 86%), m.p. above 300 °C; IR (KBr): 𝑣/cm−1 3196 (NH), 1715, 1678 (2CO); 1H-NMR (DMSO-d6 at 110 °C): δ = 2.56 (s, 3H, CH3), 7.40–7.54 (m, 6H, Ar-H), 7.67 (s, 1H, olefinic CH), 7.98 (d, J = 8.4 Hz, 2H, Ar-H) and 11.94 ppm (s, 1H, NH); MS (EI): m/z (%) 356 (M+, 78.50), 357 (M++1, 19.95). Anal. calcd. for C18H13ClN2O2S (356.83): C, 60.59; H, 3.67; N, 7.85; S, 8.99. Found: C, 60.67; H, 3.64; N, 7.88; S, 8.93.
(2Z,5Z)-2-(4-Acetylphenylimino)-5-(thiophen-2-ylmethylidene)thiazolidin-4-one (7m). Recrystallized from DMF as yellow crystals; yield: (A: 87%, B: 78%), m.p. above 300 °C; IR (KBr): 𝑣/cm−1 3195 (NH), 1716, 1672 (2CO); 1H-NMR (DMSO-d6 at 110 °C): δ = 2.58 (s, 3H, CH3), 7.20–8.00 (m, 8H, Ar-H and olefinic CH) and 11.95 ppm (s, 1H, NH); MS (EI): m/z (%) 328 (M+, 49.55), 329 (M++1, 10.25). Anal. calcd. for C16H12N2O2S2 (328.41): C, 58.52; H, 3.68; N, 8.53; S, 19.53. Found: C, 58.59; H, 3.76; N, 8.56; S, 19.44.
(2Z,5Z)-2-(Benzothiazol-2-ylimino)-5-(4-methylbenzylidene)thiazolidin-4-one (7n). Recrystallized from dioxane as yellow crystals; yield: (A: 86%, B: 73%), m.p. 247–248 °C; IR (KBr): 𝑣/cm−1 3125 (NH), 1696 (CO); 1H-NMR (DMSO-d6): δ = 2.37 (s, 3H, CH3), 7.35–7.39 (m, 3H, Ar-H), 7.50 (t, J = 7.6 Hz, 1H, Ar-H), 7.58 (d, J = 8.0 Hz, 2H, Ar-H), 7.74 (s, 1H, olefinic CH), 7.93 (d, J = 7.6 Hz, 1H, Ar-H), 7.99 (d, J = 7.6 Hz, 1H, Ar-H) and 12.88 ppm (s, 1H, NH); MS (EI): m/z (%) 351 (M+, 52.6), 352 (M++1,12.2). Anal. calcd. for C18H13N3OS2 (351.45): C, 61.52; H, 3.73; N, 11.96; S, 18.25 Found: C, 61.45; H, 3.81; N, 12.02; S, 18.32.
(2Z,5Z)-2-(Benzothiazol-2-ylimino)-5-(thiophen-2-ylmethylidene)thiazolidin-4-one (7o). Obtained from dioxane as yellow crystals; yield: (A: 86%, B: 79%), m.p. 276–277 °C; IR (KBr): 𝑣/cm−1 3136 (NH), 1692 (CO); 1H-NMR (DMSO-d6): δ = 7.33 (t, J = 5.4 Hz, 1H, Ar-H), 7.39 (t, J = 7.6 Hz, 1H, Ar-H), 7.52 (t, J = 7.6 Hz, 1H, Ar-H), 7.73 (s, 1H, olefinic CH), 7.91 (d, J = 7.6 Hz, 1H, Ar-H), 8.01 (d, J = 7.6 Hz, 1H, Ar-H), 8.06–8.10 (m, 2H, Ar-H) and 12.89 ppm (s, 1H, NH); 13C-NMR (DMSO-d6): δ = 121.67, 121.94, 122.12, 124.48, 125.81, 126.56, 129.00, 133.16, 133.43, 134.92, 137.38, 150.75, 158.06, 166.83 and 168.22, ppm (Ar-C and CO); MS (EI): m/z (%) 343 (M+, 80.5), 344 (M++1, 15.7). Anal. calcd. for C15H9N3OS3 (343.45): C, 52.46; H, 2.64; N, 12.23; S, 28.01. Found: C, 52.38; H, 2.73; N, 12.31; S, 27.94.
(2Z,5Z)-2-(Benzothiazol-2-ylimino)-5-[4-(dimethylamino)benzylidene]thiazolidin-4-one (7p). Obtained from dioxane as orange crystals; yield: (A: 90%, B: 77%), m.p. 299–300 °C; IR (KBr): 𝑣/cm−1 3129 (NH), 1711 (CO); 1H-NMR (DMSO-d6): δ = 3.04 (s, 6H, 2CH3), 6.87 (d, J = 8.4 Hz, 2H, Ar-H), 7.36 (t, J = 8.0 Hz, 1H, Ar-H), 7.50 (t, J = 8.0 Hz, 1H, Ar-H), 7.55 (d, J = 8.4 Hz, 2H, Ar-H), 7.66 (s, 1H, olefinic CH), 7.93 (d, J = 8.0 Hz, 1H, Ar-H), 8.00 (d, J = 8.0 Hz, 1H, Ar-H) and 12.68 ppm (s, 1H, NH); MS (EI): m/z (%) 380 (M+, 26.75), 381 (M++1, 7.65). Anal. calcd. for C19H16N4OS2 (380.49): C, 59.98; H, 4.24; N, 14.72; S, 16.85. Found: C,60.07; H, 4.16; N, 14.65; S, 16.89.
3.5. General Procedure for the Synthesis of the Enamines 8
Mixture of 3a or 3c (5 mmol), N,N-dimethylformamide dimethylacetal (DMF-DMA) (0.6 g, 5 mmol) in dioxane (20 mL) were stirred at reflux for 12 h. The separated solid product obtained on standing at room temperature was collected by filtration, washed by EtOH and recrystallized from dioxane to afford the corresponding enamines 8 as orange crystal.
(2Z,5Z)-2-(2-Chloro-5-nitrophenylimino)-5-[(dimethylamino)methylidene]thiazolidin-4-one (8a). Yield: 75%, m.p. 260–261 °C; IR (KBr): 𝑣/cm−1 2383 (NH), 1708 (CO); 1H-NMR (DMSO-d6): δ = 3.01 (s, 6H, 2CH3), 7.49 (s, 1H, olefinic CH), 7.79 (d, J = 8.4 Hz, 1H, Ar-H), 7.89–795 (m, 2H, Ar-H), and 11.77 ppm (s, 1H, NH); 13C-NMR (DMSO-d6): δ = 42.22 (2CH3), 84.91, 117.90, 119.92, 131.56, 133.97, 144.76, 147.41, 147.81, 156.48 and 168.40 ppm (Ar-C and CO); MS (EI): m/z (%) 326 (M+, 57.25), 327 (M++1, 11.50). Anal. calcd. for C12H11ClN4O3S (326.76): C, 44.11; H, 3.39; N, 17.15; S, 9.81. Found: C, 44.18; H, 3.32; N, 17.24; S, 9.90.
(2Z,5Z)-2-(Benzothiazol-2-ylimino)-5-[(dimethylamino)methylidene]thiazolidin-4-one (
8b). Yield: 79%, m.p. 205–206 °C; IR (KBr): 𝑣/cm
−1 3133 (NH), 1670 (CO);
1H-NMR (DMSO-
d6): δ = 3.18 (s, 6H, 2CH
3), 7.25 (t,
J = 7.6 Hz, 1H, Ar-H), 7.39 (t,
J = 7.6 Hz, 1H, Ar-H), 7.63 (s, 1H, olefinic CH), 7.75 (d,
J = 7.6 Hz, 1H, Ar-H), 7.88 (d,
J = 7.6 Hz, 1H, Ar-H) and 12.07 ppm (s, 1H, NH);
13C-NMR (DMSO-
d6): δ = 41.71 (2CH
3), 87.52, 120.76, 121.69, 123.48, 126.03, 132.53, 146.55, 151.19, 160.35, 167.19 and 168.97 ppm (Ar-C and CO); MS (EI):
m/
z (%) 304 (M
+, 76.40), 305 (M
++1, 15.30). Anal. calcd. for C
13H
12N
4OS
2 (304.39): C, 51.30; H, 3.97; N, 18.41; S, 21.07. Found: C, 51.38; H, 4.05; N, 18.48; S, 21.13. Crystallographic Analysis for
8b: The crystals were mounted on a glass fiber. All measurements were performed on Bruker X8 Prospector. The data were collected at a temperature of 20 ± 1 °C to a maximum θ value of 66.61° using the ω scanning technique. The structure was solved by direct method using SHELXS-97 (Sheldrick, 2008) and refined by Full-matrix least-squares on F
2. The non-hydrogen atoms were refined anisotropically. Data were corrected for absorption effects using the multi-scan method (SADABS). Crystal Data: C
13H
12N
4OS
2, M = 304.39, monoclinic, a = 7.9497(5) Å, b = 7.2661(5) Å, c = 24.1772(17) Å, V = 1384.30(16) Å
3, α = γ = 90.00°, β = 97.597(3)°, space group: P 1 21/c 1, Z = 4, D
calc = 1.461 Mg cm
−3, No. of reflection measured 7648, θ
max = 66.69°, R1 = 0.0317.
Figure 6 illustrates the structure as determined. Full data can be obtained on request from the CCDC [
35].
3.6. General Procedure for the Synthesis of the Enamines 9
Mixtureof 3a or 3c (5 mmol), N,N-dimethylformamide dimethylacetal (DMF-DMA) (1.2 g, 10 mmol) in toluene (30 mL) were stirred at reflux for 6 h. The separated solid product obtained on standing at room temperature was collected by filtration, washed by EtOH and recrystallized from EtOH/dioxane (1:2) mixture to afford the corresponding enamines 9 as orange crystal.
(2Z,5Z)-2-(2-Chloro-5-nitrophenylimino)-5-[(dimethylamino)methylidene]-3-methylthiazolidin-4-one (9a). Yield: 72%, m.p. 199–200 °C; IR (KBr): 𝑣/cm−1 1703 (CO); 1H-NMR (DMSO-d6): δ = 3.04 (s, 6H, 2CH3), 3.25 (s, 3H, CH3), 7.63 (s, 1H, olefinic CH), 7.81 (d, J = 8.4 Hz, 1H, Ar-H), 7.90 (s, 1H, Ar-H) and 7.95 ppm (d, J = 8.4 Hz, 1H, Ar-H); 13C-NMR (DMSO-d6): δ = 28.85 (CH3), 41.93 (2CH3), 82.92, 117.29, 119.58, 131.16, 133.46, 144.92, 146.94, 147.09, 155.87 and 166.40 ppm (Ar-C and CO); MS (EI): m/z (%) 340 (M+, 41.55), 341 (M++1, 9.70). Anal. calcd. for C13H13ClN4O3S (340.79): C, 45.82; H, 3.85; N, 16.44; S, 9.41. Found: C, 45.91; H, 3.79; N, 16.37; S, 9.36.
(2Z,5Z)-2-(Benzothiazol-2-ylimino)-5-[(dimethylamino)methylidene]-3-methylthiazolidin-4-one (9b). Yield: 79%, m.p. 211–212 °C; IR (KBr): 𝑣/cm−1 1684 (CO); 1H-NMR (DMSO-d6): δ = 3.20 (s, 6H, 2CH3), 3.23 (s, 3H, CH3), 7.27 (t, J = 7.6 Hz, 1H, Ar-H), 7.40 (t, J = 7.6 Hz, 1H, Ar-H), 7.64 (s, 1H, olefinic CH), 7.76 (d, J = 7.6 Hz, 1H, Ar-H) and 7.90 ppm (d, J = 7.6 Hz, 1H, Ar-H); 13C-NMR (DMSO-d6): δ = 29.44 (CH3), 42.61 (2CH3), 85.93, 120.96, 121.72, 123.51, 126.06, 132.76, 146.58, 151.13, 159.38, 165.92 and 168.71 ppm (Ar-C and CO); MS (EI): m/z (%) 318 (M+, 33.9), 319 (M++1, 7.45). Anal. calcd. for C14H14N4OS2 (318.42): C, 52.81; H, 4.43; N, 17.60; S, 20.14. Found: C, 52.74; H, 4.49; N, 17.52; S, 20.25.
3.7. (2Z,5Z)-2-(4-Acetylphenylimino)-5-[(dimethylamino)methylidene]thiazolidin-4-one (10)
A mixture of 3b (10 mmol), N,N-dimethylformamide dimethylacetal (DMF-DMA) (1.2 g, 10 mmol) in toluene (25 mL) was stirred at reflux for 5 h. The separated solid product obtained on standing at room temperature was collected by filtration, washed by EtOH and recrystallized from dioxane/DMF (1:1) mixture as reddish brown crystals. Yield: 86%, m.p. 299–300 °C; IR (KBr): 𝑣/cm−1 3194, 1678, 1657 (2CO); 1H-NMR (DMSO-d6): δ = 2.55 (s, 3H, COCH3), 3.07 (s, 6H, 2CH3), 7.23 (d, J = 8.4 Hz, 2H, Ar-H), 7.57 (s, 1H, olefinic CH), 7.95 (d, J = 8.4 Hz, 2H, Ar-H) and 11.13 ppm (s, 1H, NH); MS (EI): m/z (%) 289 (M+, 55.50), 290 (M++1, 10.80). Anal. calcd. for C14H15N3O2S (289.36): C, 58.11; H, 5.23; N, 14.52; S, 11.08. Found: C, 58.24; H, 5.17; N, 14.43; S, 10.95.
3.8. (2Z,5Z)-5-[(Dimethylamino)methylidene]-3-methyl-2-{4-[(E)-4-methlypent-2-enoyl]phenylimino} thiazolidin-4-one (11)
A mixture of
3b (10 mmol),
N,N-dimethylformamide dimethylacetal (DMF-DMA) (3.6 g, 30 mmol) in toluene (75 mL) was stirred at reflux for 72 h. The separated solid product obtained on standing at room temperature was collected by filtration, washed with EtOH and recrystallized from EtOH/dioxane (2:1) mixture to afford the corresponding enaminone
11 as deep orange crystals. Yield: 81%, m.p. 236–238 °C; IR (KBr): 𝑣/cm
−1 1686, 1677 (2CO);
1H-NMR (DMSO-
d6): δ = 2.91 (s, 3H, CH
3), 3.02 (s, 6H, 2CH
3), 3.14 (s, 3H, CH
3), 3.21 (s, 3H, CH
3), 5.85 (d,
J = 12 Hz, 1H, olefinic
CH=CH), 7.00 (d,
J = 8.4 Hz, 2H, Ar-H), 7.55 (s, 1H, olefinic CH), 7.71 (d,
J = 12 Hz, 1H, olefinic CH=
CH), 7.90 (d,
J = 8.4 Hz, 2H, Ar-H);
13C-NMR (DMSO-
d6): δ = 28.87 (CH
3), 37.14 (CH
3), 41.80 (2CH
3), 24.53 (CH
3) 83.57, 90.71, 121.03, 128.72, 135.72, 144.14, 151.63, 152.80, 153.93, 166.65 and 184.84 ppm (Ar-C and CO); MS (EI):
m/
z (%) 358 (M
+, 100), 359 (M
++1, 22.55). Anal. calcd. for C
18H
22N
4O
2S (358.47): C, 60.31; H, 6.19; N, 15.63; S, 8.94. Found: C, 60.25; H, 6.23; N, 15.57; S, 8.96. Crystallographic Analysis for
11: The crystals were mounted on a glass fiber. All measurements were performed on a Rigaku R-AXIS RAPID diffractometer using filtered Mo-Kα radiation. The data were collected at a temperature of 20 ± 1 °C to a maximum 2θ value of 55.0° using the ω scanning technique. The structure was solved by charge flipping method and expanded using Fourier techniques. The non-hydrogen atoms were refined anisotropically. Hydrogen atoms were refined using the riding model. Crystal Data: C
18H
22N
4O
2S, M = 358.47, triclinic, a = 7.275(2) Å, b = 10.923(2) Å, c = 24.1772(17) Å, V = 887.0(3) Å
3, α = 105.825(8)°, β = 99.679(7)°, γ = 95.624(7)°, space group: P-1 (#2), Z = 2, D
calc = 1.342 g cm
−3, No. of reflections measured 3236, θ
max = 50.7°, R1 = 0.0558.
Figure 7 illustrates the structure as determined. Full data can be obtained on request from the CCDC [
36].
3.9. (2Z)-2-(Benzothiazol-2-ylimino)-5-(phenylhydrazono)thiazolidin-4-one (12)
A cold solution of benzenediazonium chloride (10 mmol) was prepared by adding a solution of sodium nitrite (1.4 g dissolved in 10 mL water) to cold solution of aniline hydrochloride (0.93 g of aniline in 10 mL, 6M HCl) with stirring. The resulting solution of benzenediazonium chloride was then added to a cold solution of 4-thiazolidinone 3c (2.49 g, 10 mmol) in ethanol (100 mL) in the presence of sodium hydroxide (6.0 g, 15 mmol). The reaction mixture was stirred at room temperature for 1h and poured into ice cold water, the formed solid product was collected by filtration and washed with water then recrystallized from an EtOH to afford 12 as pale brown crystals, yield: 68%, m.p. 154–155 °C; IR (KBr): 𝑣/cm−1 3250, 3125 (2NH), 1723 (CO); 1H-NMR (DMSO-d6): δ = 6.98 (t, J = 8.0 Hz, 1H, Ar-H), 7.33–7.41 (m, 4H, Ar-H), 7.46–7.53 (m, 2H, Ar-H), 7.98 (d, J = 8.0 Hz, 1H, Ar-H), 8.01 (d, J = 8.0 Hz, 1H, Ar-H), 11.01 (s, 1H, NH) and 12.67 ppm (s, 1H, NH); MS (EI): m/z (%) 353 (M+, 39.70), 354 (M++1, 7.33). Anal. calcd. for C16H11N5OS2 (353.43): C, 54.38; H, 3.14; N, 19.82; S, 18.14. Found: C, 54.50; H, 3.25; N, 19.75; S, 18.20.
3.10. General Procedure for the Synthesis of Azolopyrimidines 13–18
Independent mixtures of 11 (1.075 g, 3 mmol) and the appropriate heteroaromatic amine (3 mmol) in pyridine (20 mL) were stirred at reflux for 24 h. The reaction mixtures were cooled to room temperature and poured into ice cold water then acidified with hydrochloric acid (2 N), forming solids that were collected by filtration and washed with water then MeOH and recrystallized from the indicated solvent.
(2Z,5Z)-5-[1-(Dimethylamino)methylidene]-3-methyl-2-[4-(1,2,4-triazolo[1,5-a]pyrimidin-7-yl)phenyl-imino]thiazolidin-4-one (13). Recrystallized from EtOH as yellow crystals, yield: 95%, m.p. 212–213 °C; IR (KBr): 𝑣/cm−1 1701 (CO); 1H-NMR (DMSO-d6): δ = 3.05 (s, 6H, 2CH3), 3.24 (s, 3H, CH3), 7.22 (d, J = 8.4 Hz, 2H, Ar-H), 7.58 (s, 1H, olefinic CH), 7.66 (d, J = 4.8 Hz, 1H, pyrimidine H), 8.30 (d, J = 8.4 Hz, 2H, Ar-H), 8.75 (s, 1H, triazole H) and 8.92 ppm (d, J = 4.8 Hz, 1H, pyrimidine H); 13C-NMR (DMSO-d6): δ = 28.87 (CH3), 41.65 (2CH3), 83.26, 108.91, 121.66, 124.54, 131.03, 144.34, 146.92, 152.37, 153.27, 154.83, 155.42, 155.86 and 166.50 (Ar-C and CO); MS (EI): m/z (%) 379 (M+, 100), 380 (M++1, 23.50). Anal. calcd. for C18H17N7OS (379.45): C, 56.98; H, 4.52; N, 25.84; S, 8.45. Found: C, 56.92; H, 4.44; N, 25.73; S, 8.54.
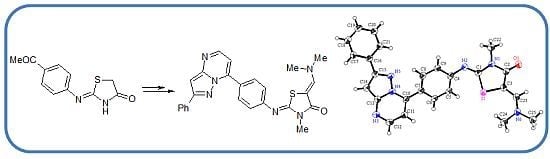
(2Z,5Z)-5-[1-(Dimethylamino)methylidene]-3-methyl-2-[4-(2-phenylpyrazolo[1,5-a]pyrimidin-7-yl)- phenylimino]thiazolidin-4-one (
14). Recrystallized from dioxane as yellow crystals, yield: 92%, m.p. 251–252 °C; IR (KBr): 𝑣/cm
−1 1690 (CO);
1H-NMR (DMSO-
d6): δ = 3.06 (s, 6H, 2CH
3), 3.25 (s, 3H, CH
3), 7.23 (d,
J = 8.4 Hz, 2H, Ar-H), 7.30–7.33 (m, 2H, pyrimidine
H and pyrazole H), 7.45 (t,
J = 7.6 Hz, 1H, Ar-H), 7.51 (t,
J = 7.6 Hz, 2H, Ar-H), 7.59 (s, 1H, olefinic CH), 8.07 (d,
J = 7.6 Hz, 2H, Ar-H), 8.34 (d,
J = 8.4 Hz, 2H, Ar-H) and 8.58 ppm (d,
J = 4.8 Hz, 1H, pyrimidine
H);
13C-NMR (DMSO-
d6 at 110 °C): δ = 29.23 (CH
3), 42.28 (2CH
3), 84.92, 93.65, 107.45, 121.93, 126.24, 126.85, 129.17, 129.29, 131.11, 133.34, 144.57, 145.56, 149.82, 151.57, 152.16, 153.31, 155.35 and 167.13 (Ar-C and CO); MS (EI):
m/
z (%) 454 (M
+, 100), 455 (M
++1, 21.95). Anal. calcd. for C
25H
22N
6OS (454.56): C, 66.06; H, 4.88; N, 18.49; S, 7.05. Found: C, 66.11; H, 4.91; N, 18.55; S, 7.12. Crystallographic Analysis for
14: The crystals were mounted on a glass fiber. All measurements were performed on a Rigaku R-AXIS RAPID diffractometer using filtered Mo-Kα radiation. The data were collected at a temperature of 20 ± 1 °C to a maximum 2θ value of 55.0° using the ω scanning technique. The structure was solved by charge flipping method and expanded using Fourier techniques. The non-hydrogen atoms were refined anisotropically. Hydrogen atoms were refined using the riding model. Crystal Data: C
25H
22N
6OS, M = 454.56, monoclinic, a = 7.6658(8) Å, b = 10.612(2) Å, c =29.279(4) Å, V = 2378.2(5) Å
3, α = γ = 90.00°, β = 93.200(7)°, space group: P2
1/c (#14), Z = 4, D
calc = 1.392 g cm
−3, No. of reflection measured 4333, θ
max = 50.6°, R1 = 0.066.
Figure 8 illustrates the structure as determined. Full data can be obtained on request from the CCDC [
37].
7-{4-[(Z)5-(1-Dimethylaminomethylidene)-3-methyl-4-oxo-thiazolidin-(2Z)-ylideneamino]phenyl}-N-(2-chloro-5-nitrophenyl)pyrazolo[1,5-a]pyrimidine-3-carboxamide (15). Recrystallized from dioxane/ DMF (2:1) mixture as orange crystals, yield: 87%, m.p. 294–295 °C; IR (KBr): 𝑣/cm−1 3246 (NH), 1697, 1680 (2CO); 1H-NMR (DMSO-d6 at 110 °C): δ = 3.07 (s, 6H, 2CH3), 3.27 (s, 3H, CH3), 7.24 (d, J = 8.4 Hz, 2H, Ar-H), 7.55 (s, 1H, olefinic CH), 7.59 (d, J = 4.8 Hz, 1H, pyrimidine H), 7.86 (d, J = 7.8 Hz, 1H, Ar-H), 7.97 (d, J = 7.8 Hz, 1H, Ar-H), 8.29 (d, J = 8.4 Hz, 2H, Ar-H), 8.81 (s, 1H, pyrazole H), 8.94 (d, J = 4.8 Hz, 1H, pyrimidine H), 9.48 (s, 1H, Ar-H), and 10.86 ppm (s, 1H, NH); MS (EI): m/z (%) 576 (M+, 100), 577 (M++1, 44.85). Anal. calcd. for C26H21ClN8O4S (577.03): C, 54.12; H, 3.67; N, 19.42; S, 5.56. Found: C, 54.19; H, 3.65; N, 19.38; S, 5.64.
7-{4-[(Z)5-(1-Dimethylaminomethylidene)-3-methyl-4-oxo-thiazolidin-(2Z)-ylideneamino]phenyl}-N-(4,6-dimethylpyrimidin-2-yl)pyrazolo[1,5-a]pyrimidine-3-carboxamide (16). Recrystallized from EtOH/ dioxane (1:1) mixture as orange crystals, yield: 93%, m.p. above 300 °C; IR (KBr): 𝑣/cm−1 3277 (NH), 1688, 1676 (2CO); 1H-NMR (DMSO-d6): δ = 2.42 (s, 6H, 2CH3), 3.06 (s, 6H, 2CH3), 3.24 (s, 3H, CH3), 7.01 (s, 1H, 4,6-dimethylpyrimidin H), 7.23 (d, J = 8.4 Hz, 2H, Ar-H), 7.60 (s, 1H, olefinic CH), 7.62 (d, J = 4.8 Hz, 1H, pyrimidine H), 8.26 (d, J = 8.4 Hz, 2H, Ar-H), 8.79 (s, 1H, pyrazole H), 8.98 (d, J = 4.8 Hz, 1H, pyrimidine H) and 10.60 ppm (s, 1H, NH); 13C-NMR (DMSO-d6): δ = 23.49 (2CH3), 28.91 (CH3), 42.18 (2CH3), 83.31, 104.76, 109.01, 115.59, 121.59, 124.62, 131.40, 144.46, 146.17, 146.72, 147.36, 152.36, 152.43, 153.25, 157.07, 157.97, 166.53 and 167.84 (Ar-C and CO); MS (EI): m/z (%) 527 (M+, 94.80), 528 (M++1, 30.15). Anal. calcd. for C26H25N9O2S (527.61): C, 59.19; H, 4.78; N, 23.89; S, 6.08. Found: C, 59.25; H, 4.71; N, 23.98; S, 6.15.
(2Z,5Z)-2-[4-(Benzimidazo[1,2-a]pyrimidine-4-yl)phenylimino]-5-[1-(dimethylamino)methylidene]-3-methylthiazolidin-4-one (17). Recrystallized from EtOH/ dioxane (1:1) mixture as yellow crystals, yield: 96%, m.p. 281–282 °C; IR (KBr): 𝑣/cm−1 1692 (CO); 1H-NMR (DMSO-d6): δ = 3.09 (s, 6H, 2CH3), 3.27 (s, 3H, CH3), 6.69 (d, J = 8.0 Hz, 1H, Ar-H), 7.06–7.10 (m, 2H, 1Ar-H and pyrimidine H), 7.27 (d, J = 8.4 Hz, 2H, Ar-H), 7.48 (t, J = 8.0 Hz, 1H, Ar-H), 7.61 (s, 1H, olefinic CH), 7.71 (d, J = 8.4 Hz, 2H, Ar-H), 7.88 (d, J = 8.0 Hz, 1H, Ar-H) and 8.86 ppm (d, J = 4.8 Hz, 1H, pyrimidine H); 13C-NMR (DMSO-d6): δ = 28.93 (CH3), 42.25 (2CH3), 83.47, 108.24, 114.75, 119.58, 120.76, 122.31, 125.77, 127.06, 127.20, 129.75, 144.25, 144.35, 149.54, 151.36, 151.64, 153.51, 155.86 and 166.65 (Ar-C and CO); MS (EI): m/z (%) 428 (M+, 100), 429 (M++1, 24.10). Anal. calcd. for C23H20N6OS (428.52): C, 64.47; H, 4.70; N, 19.61; S, 7.48. Found: C, 64.54; H, 4.69; N, 19.64; S, 7.52.
2((E)-3-{4-[(Z)5-(1-Dimethylaminomethylidene)-3-methyl-4-oxo-thiazolidin-(2Z)-ylideneamino]phen- yl}-3-oxoprop-1-enylamino)-4-phenylthiophene-3-carbonitrile (18). Recrystallized from dioxane as orange crystals, yield: 81%, m.p. 270–271 °C; IR (KBr): 𝑣/cm−1 3116 (NH), 2206 (CN), 1699, 1654 (2CO); 1H-NMR (DMSO-d6): δ = 3.04 (s, 6H, 2CH3), 3.22 (s, 3H, CH3), 6.43 (d, J = 8.8 Hz, 1H, olefinic CH=CH), 7.10 (t, J = 7.6 Hz, 2H, Ar-H), 7.31 (s, 1H, thiophene H), 7.43–7.53 (m, 3H, Ar-H), 7.58 (s, 1H, olefinic CH), 7.60–7.65 (m, 2H, Ar-H), 7.83 (d, J = 8.8 Hz, 1H, olefinic CH=CH), 7.90 (d, J = 7.6 Hz, 1H, Ar-H), 8.04 (d, J = 7.6 Hz, 1H, Ar-H) and 12.97 ppm (brd, 1H, NH); MS (EI): m/z (%) 513 (M+, 100), 514 (M++1, 29.80). Anal. calcd. for C27H23N5O2S2 (513.64): C, 63.14; H, 4.51; N, 13.63; S, 12.48. Found: C, 63.21; H, 4.44; N, 13.57; S, 12.39.
3.11. (2Z,5Z)-2-(Benzothiazol-2-ylimino)-5-[(1H-1,2,4-triazol-3-ylamino)methylidene]thiazolidin-4-one (19)
A mixture of the enamine 8b (1.25 g, 5 mmol) and 3-amino-1,2,4-triazol (0.42 g, 5 mmol) in acetic acid (20 mL) containing anhydrous sodium acetate (10 mmol) was refluxed for 24 h. The reaction mixture was then cooled to room temperature and then poured into ice-cold water. The precipitate was filtered off and washed with water and the resulting crude product was purified by recrystallization from dioxane as brown crystals, yield: 74%, m.p. above 300 °C; IR (KBr): 𝑣/cm−1 3195, 3142, 3115 (3NH), 1683 (CO); 1H-NMR (DMSO-d6): δ = 7.32 (t, J = 7.6 Hz, 1H, Ar-H), 7.46 (t, J = 7.6 Hz, 1H, Ar-H), 7.75 (d, J = 7.6 Hz, 1H, Ar-H), 7.95 (d, J = 7.6 Hz, 1H, Ar-H), 7.63 (d, J = 10.8 Hz, 1H, CHNH), 8.44 (br d, 1H, triazole H), 11.03 (s, 1H, NH), 12.24 (s, 1H, NH) and 13.76 ppm (br, 1H, NH); 13C-NMR (DMSO-d6): δ = 96.37, 120.91, 121.98, 123.94, 126.38, 132.88, 135.05, 143.78, 151.19, 158.97, 160.10, 167.66 and 168.94 ppm (Ar-C and CO); MS (EI): m/z (%) 343 (M+, 7.90), 344 (M++1, 2.08). Anal. calcd. for C13H9N7OS2 (343.39): C, 45.47; H, 2.64; N, 28.55; S, 18.67. Found: C, 45.53; H, 2.73; N, 28.62; S, 18.54.