3.2. General Procedure for the Synthesis of Compounds 7a,c–g
A mixture of 1a–d (0.01 mmol), and active methylenenitrile derivatives 5a–l (0.01 mmol) in the presence of piperidine (5 drops) and ethanol (10 mL) as a solvent was refluxed for 1–2 h. The reaction mixture was evaporated. The solid product, so formed, was crystallized from a suitable solvent.
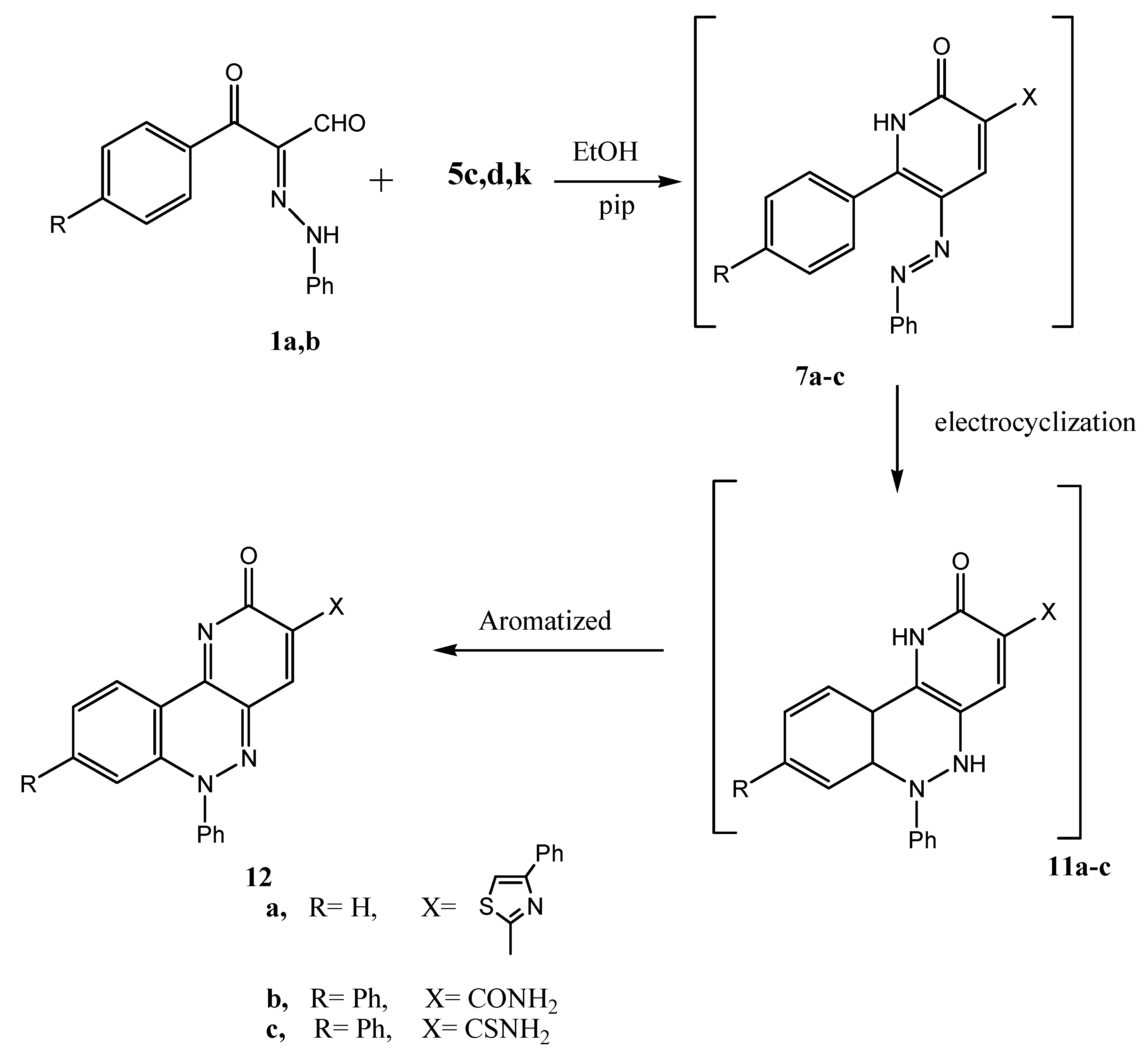
3-(4-Oxo-4,5-dihydrothiazol-2-yl)-6-phenyl-5-phenylazo-1H-pyridin-2-one (7a). Red crystals from ethanol, yield 95%; m.p. up 300 °С; Anal. Calcd. for C20H14N4O2S (374) calcd: C, 64.16; H, 3.77; N, 14.96. Found: C, 64.00; H, 3.54; N, 14.83; IR (KBr) υmax: 1,629 (CN), 1,670 (CO); 1H-NMR (DMSO-d6): δ = 1.3 (s, 2H, CH2); 7.0–8.1 (m, 10H, Ph-H); 10.0 (br, 1H, NH, D2O exchangeable); 13C-NMR (DMSO-d6): δ = 163.7, 162.9, 143.0, 137.0, 134.9, 129.0, 128.4, 127.7, 126.2, 124, 100.0, 39.0; MS: m/z (%) 373 (M+, 10), 299 (85), 224 (5), 140 (20).
2-Oxo-6-phenyl-5-phenylazo-1,2-dihydropyridine-3-carbonitrile (7b). Dark yellow crystals from ethanol, yield 95%; m.p. 153 °С; Anal. Calcd. for C18H12N4O (300): C, 71.99; H, 4.03; N, 18.66. Found: C, 71.80; H, 3.99; N, 18.53; IR (KBr): υmax: 3,264 (NH), 1,660 (CO); 13C-NMR (DMSO-d6): δ = 162.9, 156.9, 137, 134.9, 129.0, 128.4, 127.7, 126.2, 117.2, 106.7, 100.0; MS: m/z (%) 301 (M+, 50), 275 (20), 194 (15).
2-Oxo-6-phenyl-5-phenylazo-1,2-dihydropyridine-3-carboxylic acid amide (7d). Orange crystals from ethanol, yield 98%, m.p. 190 °С; Anal. Calcd. for C18H14N4OS (334): C, 64.65; H, 4.22; N, 16.75. Found: C, 64.59; H, 4.21; N, 16.62; IR (KBr): υmax: 3,399–3,266 (NH2), 1,614(CN), 1,680 (CO); 1H-NMR (DMSO-d6): δ = 7.4–7.9 (m, 10H, Ph-H); 10.6 (br, 2H, NH2, D2O exchangeable); 13C-NMR (DMSO-d6): δ = 164.7, 135.7, 133.0, 130.3, 128.1, 126.4; MS: m/z (%) 334 (M+, 100), 105 (30), 77 (25).
6-Biphenyl-4-yl-2-oxo-5-phenylazo-1,2-dihydropyridine-3-carbonitrile (7e). Green crystals from AcOH, yield 95%; m.p. 145 °С; Anal. Calcd. for C24H16N4O (376.13): C, 76.58; H, 4.28; N, 14.88. Found: C, 76.57; H, 4.21; N, 14.62; IR (KBr) υmax: 3,343 (NH), 2,202 (CN), 1,655 (CO); 13C-NMR (DMSO-d6): δ = 162.0, 156.9, 137.0, 136.6, 135.8, 132.8, 129.0, 127.4, 126.7, 117.2, 106.7, 100.0; MS: m/z (%) 377 (M+, 90), 244 (20), 152 (50), 77 (30).
6-Biphenyl-4-yl-2-oxo-5-phenylazo-1,2-dihydropyridine-3-carboxylic acid hydrazide (7f). Buff crystals from ethanol, yield 95%; m.p. 237 °С; Anal. Calcd. For C24H19N5O2 (409): C, 70.40; H, 4.68; N, 17.10. found: C, 70.39; H, 4.61; N, 17.02; IR (KBr): υmax: 3,412–3,331 (NH2), 1,660 (CO); 13C-NMR (DMSO-d6): δ = 165.9, 162.9, 148.5, 137.0, 136.6, 135.8, 133.8, 131.3, 129.0, 127.4, 126.7, 100.0; MS: m/z (%) 409 (M+, 50) 324 (80), 181 (75), 77 (70).
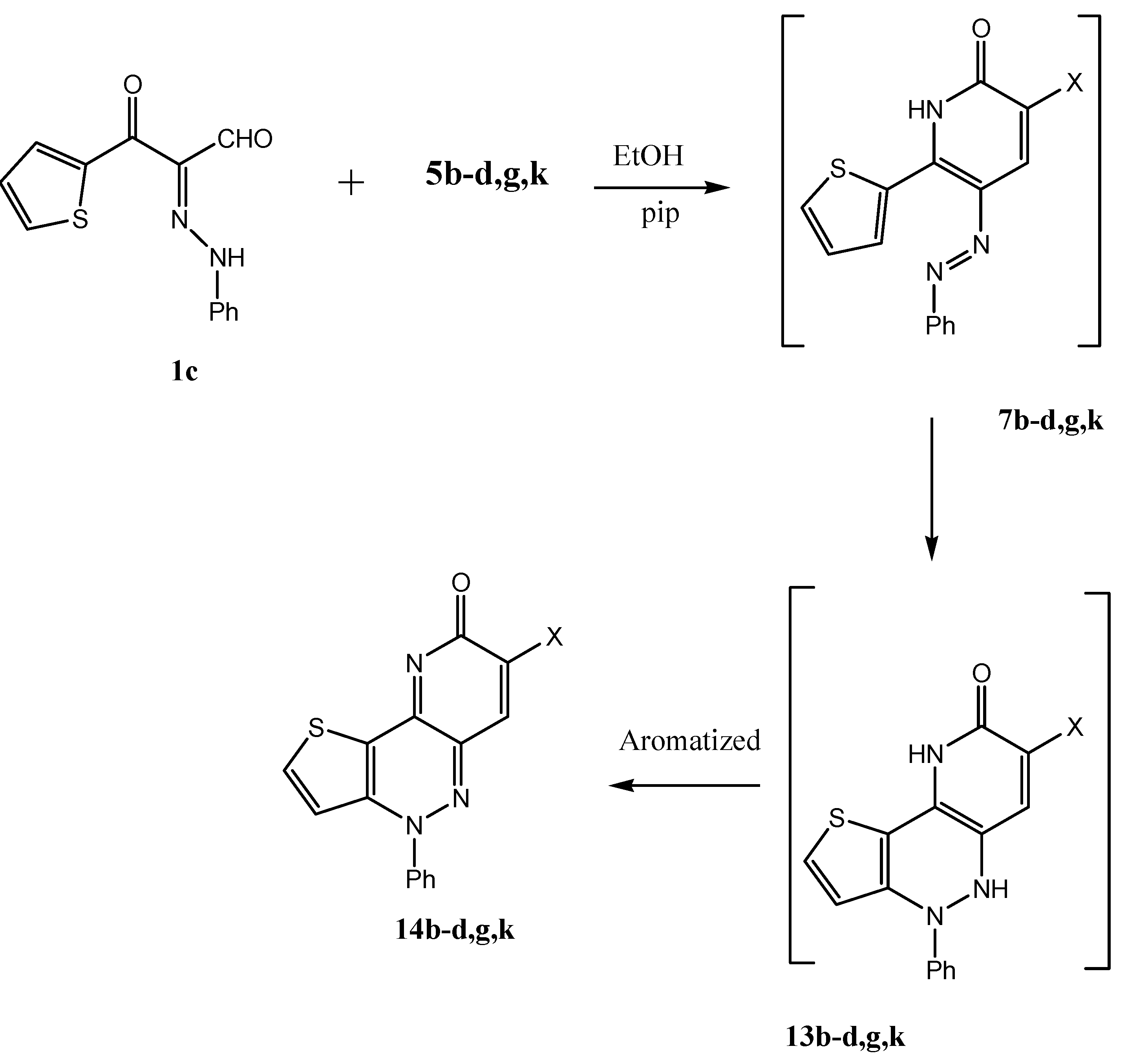
3-Benzothiazol-2-yl-5-phenylazo-6-thiophen-2-yl-1H-pyridin-2-one (7g). Yellow crystals from ethanol/AcOH, yield 98%; m.p. 242 °С; Anal. Calcd. for C22H14N4OS2 (414): C, 63.75; H, 3.40; N, 13.52. Found: C, 63.71; H, 3.31; N, 13.42; IR (KBr): υmax: 3,264 (NH), 1,680 (CO); 1H-NMR (DMSO-d6): δ = 7.2–7.3 (t, 3H, thiol-H); 7.6–8.2 (m, 9H, Ph-H); 8.6 (br, 1H, NH, D2O exchangeable); 9.0 (s, 1H, nicotine-H);13C-NMR (DMSO-d6): δ = 162.9, 156.0, 153.0, 137.7, 136.6, 133.0, 130.0, 129.0, 127.8, 126.4, 125.0, 124.0, 123.0, 122.0, 106; MS: m/z (%) 413(M+, 100), 304 (25), 111 (40), 77 (10).
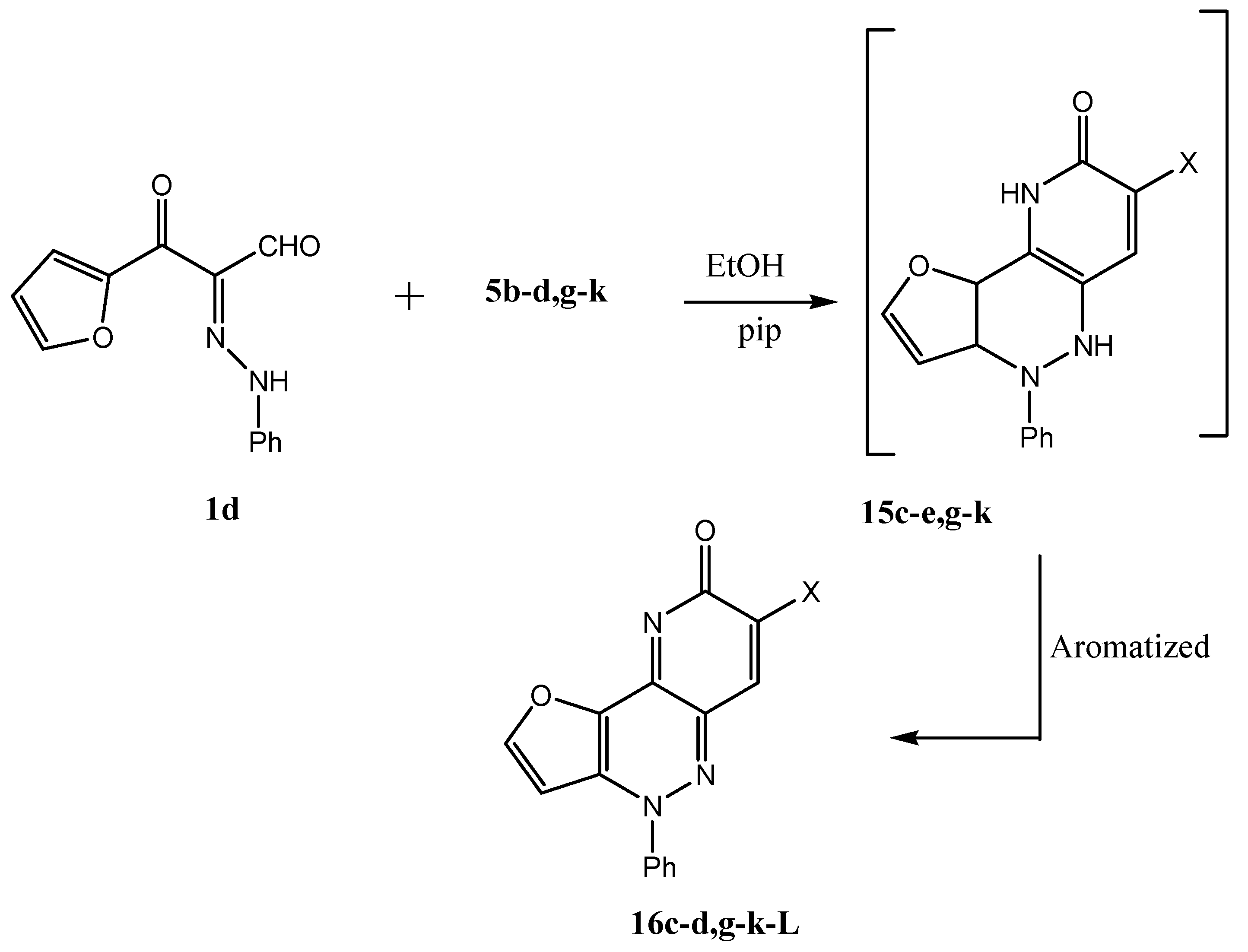
6-Furan-2-yl-2-oxo-5-phenylazo-1,2-dihydropyridine-3-carbonitrile (7h). Green crystals from ethanol, yield 95%; m.p. 214 °С; Anal. Calcd. for C16H10N4O2 (290): C, 66.20; H, 3.47; N, 19.30. Found: C, 66.19; H, 3.31; N, 19.30; IR(KBr): υmax: 3,322 (NH), 1,631 (CO); 1H-NMR (DMSO-d6): δ = 6.7–7.4 (m, 3H, furan-H); 7.6 (m, 2H, Ph-H); 8.1 (m, 2H, Ph-H); 8.1 (m, 1H, Ph-H); 13C-NMR (DMSO-d6): δ = 149.1, 148.7, 133.3, 130.4, 129.9, 126.1, 123.3, 112.8, 79.1; MS: m/z (%) 289 (M+, 100), 197 (5), 130 (5), 77 (50).
Synthesis of 5-Amino-3-(4-oxo-thiazolidin-2-yl)-6-phenyl-1H-pyridin-2-one (10a). A mixture of 7a (3.6 g, 0.1 mol) and Zn powder (2 gm) in acetic acid (20 mL) was refluxed for 2 h. then filtered while hot. The reaction mixture was cooled to room temperature and then poured onto ice-water. The solid thus formed was collected by filtration and crystallized from AcOH to give black crystals, yield 70%; m.p. up 300 °С; Anal. Calcd. for C14H13N3O2S (287): C, 58.52; H, 4.56; N, 14.62. Found: C, 58.50; H, 4.54; N, 14.53; IR (KBr): υmax: 3,432, 3,213 (NH2), 1,658 (CO); 13C-NMR (DMSO-d6): δ = 164.0, 162.9, 134.9, 128.4, 127.7, 126.2, 121.0, 108.0, 51.4, 39.0; MS: m/z (%) 287 (M+, 50), 207 (10), 93 (65), 55 (40).
Synthesis of 5-Amino-2-oxo-6-phenyl-1,2-dihydropyridine-3-carbonitrile (10b). A mixture of 7b (0.1 mol) and and Zn powder (2 gm) in acetic acid (20 mL) was refluxed for 2 h. then filtered while hot. The reaction mixture was cooled to room temperature and then poured onto ice-water. The solid thus formed was collected by filtration and crystallized from AcOH to give pale brown crystals, yield 70%; m.p. 190 °С; Anal. Calcd. for C12H9N3O (211): C, 68.24; H, 4.29; N, 19.89. Found: C, 68.20; H, 4.19; N, 19.83; IR (KBr): υmax: 3,432, 3,312, (NH2), 1,640 (CO); 13C-NMR (DMSO-d6): δ = 162.0, 156.9, 134.9, 128.4, 127.7, 126.0, 121.1, 117.2, 108.0, 106.7; MS: m/z (%) 211 (M+, 10), 129 (25), 77 (80).
6-Phenyl-3-(4-phenylthiazol-2-yl)-6H-pyrido[3,2-c]cinnolin-2-one (12a). Deep red crystals from ethanol, Yield 98%; m.p. 150 °С; Anal. Calcd. for C26H16N4OS (432): C, 72.20; H, 3.73; N, 12.95. Found: C, 72.19; H, 3.61; N, 12.92; IR (KBr): υmax: 1,614 (CN), 1,680 (CO); 1H-NMR (DMSO-d6): δ = 7.2 (s, 1H, thiazole-H); 7.3–8.1 (m, 14H, Ph-H); 8.3 (s, 1H, nicotine-H); 13C-NMR (DMSO-d6): δ = 157.2, 154.9, 149.2, 141.1, 140.6, 136.1, 134.3, 133.3, 133.2, 131.1, 130.8, 130.7, 130.1, 129.3, 128.8, 128.5, 127.0, 126.6, 121.3, 120.9; MS: m/z (%) 433 (M+, 100), 329 (10), 105 (20), 77 (15).
2-Oxo-6,8-diphenyl-2,6-dihydropyrido[3,2-c]cinnoline-3-carboxylic acid amide (12b). Red crystals from ethanol, yield 90%; m.p. 230 °С; Anal. Calcd. for C24H16N4O2 (392): C, 73.46; H, 4.11; N, 14.28. Found: C, 73.35; H, 4.00; N, 14.11; IR (KBr): υmax: 3,267, 3,189 (NH2); 13C-NMR (DMSO-d6): δ = 165.0, 150.0, 148.9, 144.2, 139.8, 136.6, 130.0, 129.4, 127.4, 119.5, 118.0, 117.0; MS: m/z (%) 393 (M+, 100), 181 (75), 77 (50).
2-Oxo-6,8-diphenyl-2,6-dihydropyrido[3,2-c]cinnoline-3-carbothioic acid amide (12c). Orange crystals from AcOH, yield 95%; m.p. 170 °С; Anal. Calcd. for C24H16N4OS (408): C, 70.57; H, 3.95; N, 13.72. Found: C, 70.49; H, 3.71; N, 13.52; IR (KBr): υmax 3,399, 3,298 (NH2), 1670 (CO); 13C-NMR (DMSO-d6): δ = 164.0, 155.0, 144.0, 143.2, 141.0, 139.8, 136.6, 130.6, 129.0, 127.0, 119.5, 118.0, 116.9; MS: m/z (%) 407 (M+, 25), 391 (50), 151 (40), 51 (50).
8-Oxo-4-phenyl-4,8-dihydro-1-thia-4,5,9-triazacyclopenta[a]naphthalene-7-carbonitrile (14b). Yellow crystals from ethanol, yield 97%; m.p. 210 °С; Anal. Calcd. for C16H8N4OS (304): C, 63.15; H, 2.65; N, 18.14. Found: C, 63.05; H, 2.52; N, 18.11. IR (KBr): υmax: 1,640 (CO); 1H-NMR (DMSO-d6): δ = 7.2–7.5 (m, 2H, thiazole-H); 7.6–7.7 (m, 5H, Ph-H); 13C-NMR (DMSO-d6): δ = 141.4, 138.7, 138.4, 136.8, 136.9, 135.8, 134.9, 132.7, 131.7, 129.7, 129.0, 128.2, 127.8, 126.1, 117.2, 79.16; MS: m/z (%) 305 (M+, 80), 195 (5), 83 (15), 77 (25).
8-Oxo-4-phenyl-4,8-dihydro-1-thia-4,5,9-triazacyclopenta[a]naphthalene-7-carboxylic acid amide (14c). Orange crystals from ethanol, yield 98%; m.p. 270 °С; Anal. Calcd. for C16H10N4O2S (322): C, 59.62; H, 3.13; N, 17.83. Found: C, 59.59; H, 3.11; N, 17.80. IR (KBr): υmax: 3,400, 3,312 (NH2), 1,615 (CN), 1,680 (CO); 1H-NMR (DMSO-d6): δ = 7.2–7.2 (t, 2H, thiol-H); 7.6–8.0 (m, 5H, Ph-H); 8.1 (S, 1H, nicotine-H); 13C-NMR (DMSO-d6): δ = 147.5, 139.3, 139.0, 138.1, 137.2, 136.2, 133.3, 133.0, 130.3, 129.9, 128.8, 128.2, 126.1, 115.0, 114.2; MS: m/z (%) 323 (M+, 100), 306 (15), 111 (90), 77 (30).
8-Oxo-4-phenyl-4,8-dihydro-1-thia-4,5,9-triazacyclopenta[a]naphthalene-7-carbothioic acid amide (14d). Brown crystal from ethanol/AcOH, yield 90%; m.p. 195 °С; Anal. Calcd. for C16H10N4OS2 (338): C, 56.79; H, 2.98; N, 16.56. Found: C, 56.65; H, 2.82; N, 16.41; IR (KBr): υmax: 1,640 (CO), 1,620 (CN); 13C-NMR (DMSO-d6): δ = 164.15, 146.7, 144.0, 141.0, 129.3, 127.0, 126.0, 118.5, 115.1; MS: m/z (%) 339 (M+, 25), 111 (75), 77 (50).
7-(1H-Benzoimidazol-2-yl)-4-phenyl-4H-1-thia-4,5,9-triazacyclopenta[a]naphthalen-8-one (14g). Yellow crystals from ethanol, yield 95%; m.p. 230 °С; Anal. Calcd. for C22H13N5OS (395): C, 66.82; H, 3.31; N, 17.71. Found: C, 66.70; H, 3.21; N, 17.68; IR (KBr): υmax: 1,670 (CO), 1,620 (CN); 1H-NMR (DMSO-d6): δ = 7.2–7.2 (t, 1H, NH, D2O exchangeable); 7.2–7.3 (m, 2H, thiol-H); 7.5–8.1 (m, 9H, Ph-H); 8.4 (s, 1H, nicotine-H); 13C-NMR (DMSO-d6): δ = 151.9, 147.0, 138.5, 136.9, 136.0, 129.1, 128.9, 128.4, 128.1, 126.5, 123.3, 121.0; MS: m/z (%) 396 (M+, 100), 286 (25), 195 (15), 111 (90), 77 (30).
4-Phenyl-7-(4-phenylthiazol-2-yl)-4H-1-thia-4,5,9-triazacyclopenta[a]naphthalen-8-one (14K). Yellow crystals from ethanol, yield 95%; m.p. 200 °С; Anal. Calcd. for C24H14N4OS2 (438): C, 65.73; H, 3.22; N, 12.78. Found: C, 65.70; H, 3.11; N, 12.68; IR (KBr): υmax: 1,680 (CO), 1,620 (CN); 1H-NMR (DMSO-d6): δ = 7.2–7.4 (t, 2H, thiol-H); 7.4–8.1 (m, 10H, Ph-H); 8.41 (s, 1H, thiazole-H); 8.6 (s, 1H, nicotine-H); 13C-NMR (DMSO-d6): δ = 154.4, 140.2, 138.5, 137.0, 136.2, 133.8, 130.7, 130.3, 129.7, 128.9, 128.3, 128.1, 126.6, 126.1, 121.0, 119.52; MS: m/z (%) 439 (M+, 100), 368 (5), 236 (10), 111 (20).
8-Oxo-4-phenyl-4,8-dihydro-1-oxa-4,5,9-triazacyclopenta[a]naphthalene-7-carboxylic acid amide (16c). Yellow crystals from ethanol, yield 95%; m.p. 288 °С; Anal. Calcd. for C16H10N4O3 (306): C, 62.74; H, 3.29; N, 18.29.;Found: C, 56.40; H, 3.44; N, 16.32; IR (KBr): υmax: 1,685 (CO), 1,620 (CN); 1H-NMR (DMSO-d6): δ = 6.7–6.7 (m, 2H, furan-H); 7.0 (s, 1H, nicotine-H); 7.5–8.4 (m, 5H, Ph-H), 8.7 (br, 2H, NH2, D2O exchangeable); 13C-NMR (DMSO-d6): δ = 162.6, 149.0, 148.9, 139.9, 130.4, 129.8, 127.2, 126.5, 123.1, 112.7; MS: m/z (%) 307 (M+, 100), 290 (15), 95 (50), 77 (25).
8-Oxo-4-phenyl-4,8-dihydro-1-oxa-4,5,9-triaza-cyclopenta[a]naphthalene-7-carbothioic acid amide (16d). Deep brown crystal from ethanol, yield 95%; m.p. 220 °С; Anal. Calcd. for C16H10N4O2S (322): C, 59.62; H, 3.13; N, 17.38. Found: C, 59.40; H, 3.0; N, 17.3. IR (KBr): υmax: 1,638 (CO), 1,620 (CN); 13C-NMR (DMSO-d6): δ = 164.0, 155.0, 146.7, 143.0, 141.0, 129.3, 118.5, 115.1, 110.0; MS: m/z (%) 323 (M+, 25), 305 (75), 289 (60), 95 (80), 51 (40).
7-(1H-Benzoimidazol-2-yl)-4-phenyl-4H-1-oxa-4,5,9-triaza-cyclopenta[a]n-aphthalen-8-one (16g). Yellow crystals from ethanol, Yield 98%; m.p. 278 °С; Anal. Calcd. for C22H13N5O2 (379): C, 69.65; H, 3.45; N, 18.46. Found: C, 69.59; H, 3.41; N, 18.42; IR (KBr): υmax: 1,614 (CN), 1,670 (CO); 13C-NMR (DMSO-d6): δ = 151.9, 149.1, 148.7, 146.9, 138.8, 129.2, 128.4, 126.5, 123.0, 120.9, 112.7; MS: m/z (%) 380 (M+, 100), 286 (15), 195 (25), 95 (50), 77 (20).
7-Benzothiazol-2-yl-4-phenyl-4H-1-oxa-4,5,9-triazacyclopenta[a]naphthalen-8-one (16h). Orange crystals from ethanol, yield 98%; m.p. 258 °С; Anal. Calcd. for C18H14N4O3 (396): C, 66.6; H, 3.05; N, 14.13. Found: C, 66.59; H, 3.00; N, 14.12; IR (KBr): υmax: 1,614 (CN), 1,680 (CO); 1H-NMR (DMSO-d6): δ = 6.7 (s, 1H, furan-H); 7.3 (s, 1H, furan-H); 7.4–7.7 (m, 9H, Ph-H); 8.6 (s, 1H, nicotine-H); 13C-NMR (DMSO-d6): δ = 158.8, 151.4, 151.4, 149.0, 148.9, 148.8, 140.1, 140.0, 137.8, 131.0, 130.4, 129.8, 126.6, 126.6, 125.7, 123.3, 123.1, 122.1, 121.6, 112.8; MS: m/z (%) 397 (M+, 100), 303 (5), 212 (10), 95 (40), 77 (10).
4-Phenyl-7-(4-phenylthiazol-2-yl)-4H-1-oxa-4,5,9-triaza-cyclopenta[a]naphthalen-8-one (16k). Yellow crystals from ethanol, yield 98%; m.p. 230 °С; Anal. Calcd. for C24H14N4O2S(422): C, 68.23; H, 3.34; N, 13.26. Found: C, 68.19; H, 3.21; N, 13.12; IR (KBr): υmax: 1,614 (CN), 1,680 (CO); 13C-NMR (DMSO-d6): δ = 164.0, 155.0, 154.0, 146.7, 143.0, 139.0, 136.2, 129.3, 128.0, 127.0, 118.0, 115.1, 114.0, 110.0; MS: m/z (%) 423 (m+, 100), 329 (5), 238 (5), 95 (25), 77 (10).
8-Oxo-4-phenyl-4,8-dihydro-1-oxa-4,5,9-triazacyclopenta[a]naphthalene-7-carboxylic acid benzylidenehydrazide (16l). Orange crystals from ethanol, yield 98%; m.p. 266 °С; Anal. Calcd. for C23H15N5O3 (409): C, 67.48; H, 3.69; N, 17.11. Found: C, 67.45; H, 3.59; N, 17.00; IR (KBr): υmax: 1,559 1,614 (CN), 1,680 (CO); 1H-NMR (DMSO-d6): δ = 6.7 (s, 1H, CH); 7.2 (s,1H, nicotine-H); 7.4–7.5 (t, 2H, furan-H); 7.7–8.3 (m, 10H, Ph-H); 13C-NMR (DMSO-d6): δ = 130.5, 128.8, 127.4, 112.8, 106.4, 55.8, 18.9; MS: m/z (%) 410 (M+, 50), 291 (10), 105 (5), 77 (10).