Enantioseparation and Absolute Configuration Determination of Angular-Type Pyranocoumarins from Peucedani Radix Using Enzymatic Hydrolysis and Chiral HPLC-MS/MS Analysis
Abstract
:1. Introduction
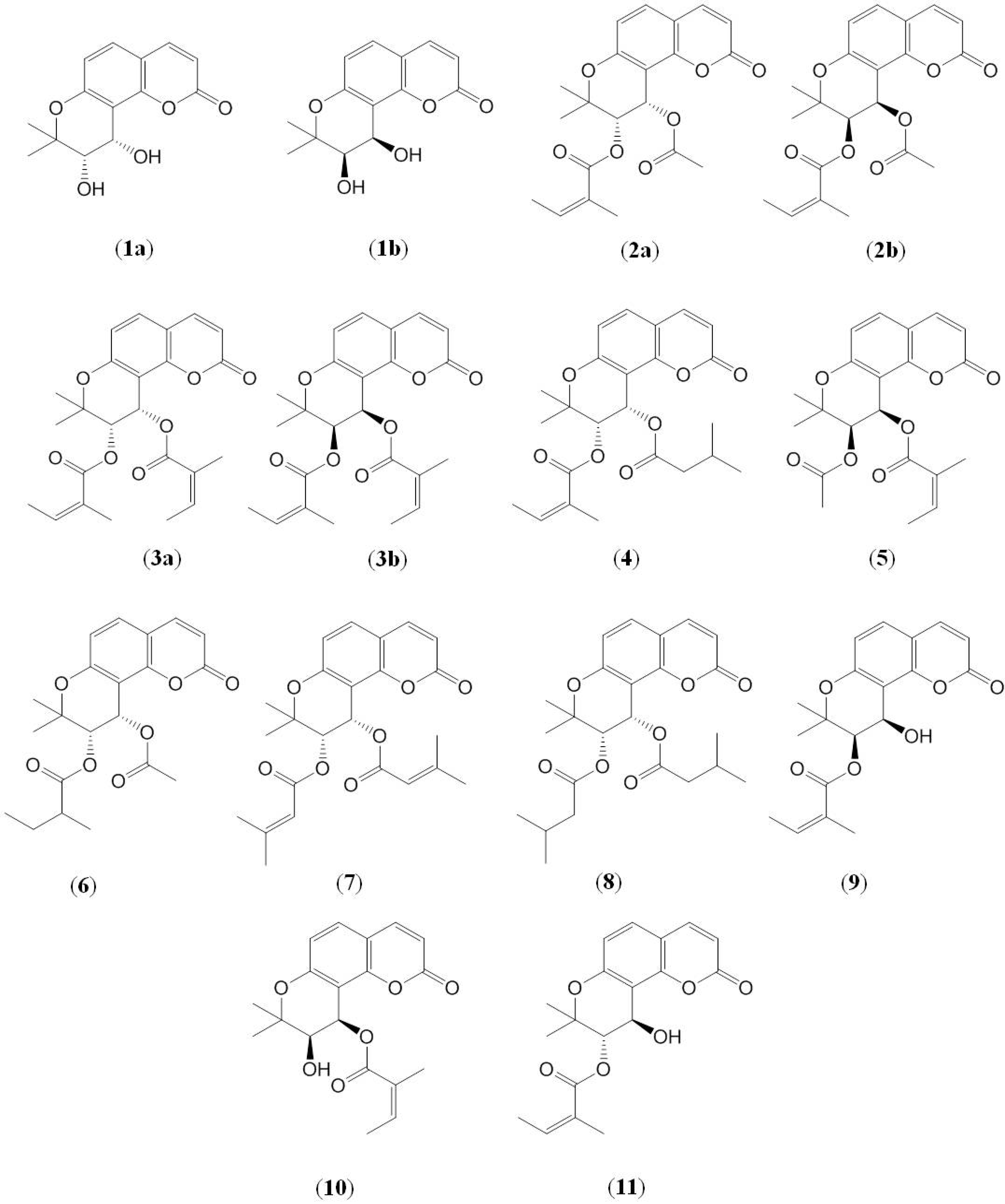
2. Results and Discussion
2.1. Identification of Pyranocoumarins from Qian-hu
2.2. Enantioseparation of Mixtures of Angular-Type Pyranocoumarins
 +57° (c, 1.0) and
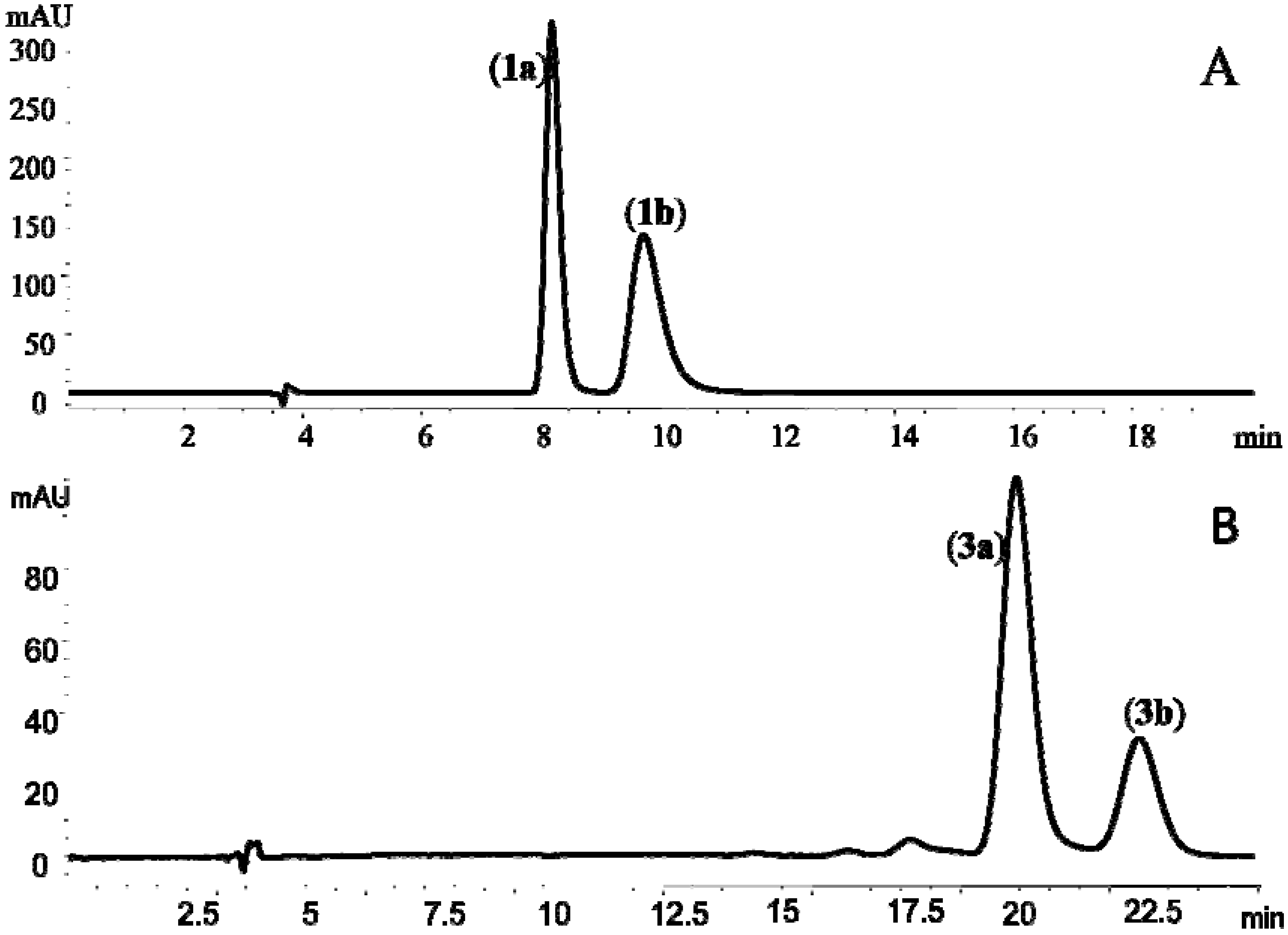
+57° (c, 1.0) and  −55° (c, 1.0), which were in good agreement with those reported previously for (+)-cis-khellactone and (−)-cis-khellactone, respectively [20]. Thus, 1a was assigned as (+)-cis-(3′S,4′S)-khellactone and 1b as (−)-cis-(3′R,4′R)-khellactone (Figure 1 and Figure 2A).
−55° (c, 1.0), which were in good agreement with those reported previously for (+)-cis-khellactone and (−)-cis-khellactone, respectively [20]. Thus, 1a was assigned as (+)-cis-(3′S,4′S)-khellactone and 1b as (−)-cis-(3′R,4′R)-khellactone (Figure 1 and Figure 2A). +37° (c, 1.0); 3b,
+37° (c, 1.0); 3b,  −36° (c, 1.0)] for the enantiomers were similar to the data reported by Okuyama and co-workers [7]. Thus the two enantiomers were identified as (+)-praeruptorin B [(+)-cis-(3′S,4′S)-3′,4′-diangeloylkhellactone, 3a] and (−)-praeruptoin B [(−)-cis-(3′R, 4′R)-3′,4′-diangeloylkhellactone, 3b] (Figure 1 and Figure 2B).
−36° (c, 1.0)] for the enantiomers were similar to the data reported by Okuyama and co-workers [7]. Thus the two enantiomers were identified as (+)-praeruptorin B [(+)-cis-(3′S,4′S)-3′,4′-diangeloylkhellactone, 3a] and (−)-praeruptoin B [(−)-cis-(3′R, 4′R)-3′,4′-diangeloylkhellactone, 3b] (Figure 1 and Figure 2B).2.3. Determination of Absolute Configuration of Angular-Type Pyranocoumarins
| compound | MS1 | MW | MS2 | Optical rotation ( c 1.0, CDCl3) | Hydrolytic metabolite(+/− cis-khellactone) | Identity |
|---|---|---|---|---|---|---|
| 1 | 263[M+H]+; 285[M+Na]+ | 262 | 245,203 | 0° | +:− = 1:1 | (±)- cis-khellactone |
| 1a | 263[M+H]+; 285[M+Na]+ | 262 | 245,203 | −55° | - | (−)- cis-(3′S, 4′S)-khellactone |
| 1b | 263[M+H]+; 285[M+Na]+ | 262 | 245,203 | +57° | + | (+)- cis-(3′R, 4′R)-khellactone |
| 2 | 404[M+NH4]+; 409[M+Na]+ | 386 | 327,245,227 | 0° | +:− = 1:1 | (±)- cis-3′-angeloyl-4′-acetylkhellactone |
| 2a | 404[M+NH4]+; 409[M+Na]+ | 386 | 327,245,227 | +59° | - | (+)- cis-(3′S, 4′S)-3′-angeloyl-4′-acetylkhellactone |
| 2b | 404[M+NH4]+; 409[M+Na]+ | 386 | 327,245,227 | −61° | + | (−)- cis-(3′R, 4′R)-3′-angeloyl-4′-acetylkhellactone |
| 3 | 444[M+NH4]+; 449[M+Na]+ | 426 | 327,245,227 | +3.4° | +:− = 2:3 | cis-3′, 4′-diangeloxylkhellactone |
| 3a | 444[M+NH4]+; 449[M+Na]+ | 426 | 327,245,227 | +36° | - | (+)- cis-(3′S, 4′S)-3′, 4′-diangeloxylkhellactone |
| 3b | 444[M+NH4]+; 449[M+Na]+ | 426 | 327,245,227 | −37° | + | (−)- cis-(3′R, 4′R)-3′, 4′-diangeloxylkhellactone |
| 4 | 446[M+NH4]+; 451[M+Na]+ | 428 | 327,245,227 | +35° | - | (+)- cis-(3′S, 4′S)-3′-angeloxyl-4′-isovalerylkhellactone |
| 5 | 404[M+NH4]+; 409[M+Na]+ | 386 | 309,245,227 | +2.9° | +:− = 7:1 | cis-3′-acetyl-4′-angeloylkhellactone |
| 5a | 404[M+NH4]+; 409[M+Na]+ | 386 | 309,245,227 | +3.5° | + | (+)- cis-(3′R, 4′R)-3′-acetyl-4′-angeloylkhellactone |
| 6 | 406[M+NH4]+; 411[M+Na]+ | 388 | 329,245,227 | +27° | +:− = 1:8 | cis-3′-isovaleryl,4′-acetylkhellactone |
| 6a | 406[M+NH4]+; 411[M+Na]+ | 388 | 329,245,227 | +33° | - | (+)- cis-(3′S, 4′S)-3′-isovaleryl,4′-acetylkhellactone |
| 7 | 444[M+NH4]+; 449[M+Na]+ | 426 | 327,245,227 | +31° | - | (+)- cis-(3′S, 4′S)-3′-angeloyl-4′-senecioylkhellactone |
| 8 | 448[M+NH4]+; 453[M+Na]+ | 430 | 329,245,227 | +35° | - | (+)- cis-(3′S, 4′S)-3′,4′-diisovalerylkhellactone |
| 9 | 367[M+Na]+; 383[M+K]+ | 344 | 267,245,227 | −57° | + | (−)- cis-(3′R, 4′R)-3′-angeloylkhellactone |
| 10 | 367[M+Na]+; 383[M+K]+ | 344 | 267,245,227 | −39° | + | (−)- cis-(3′R, 4′R)-4′-angeloylkhellactone |
| 11 | 367[M+Na]+; 383[M+K]+ | 344 | 327,245,227 | +11° | N.A. | (+)- trans-(3′S, 4′R)-3′-angeloylkhellactone |
| Position | Compound | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
| 3 | 6.27 (d, 9.5) | 6.26 (d, 9.5) | 6.23 (d, 9.5) | 6.25 (d, 9.6) | 6.24 (d, 9.6) | 6.25 (d, 9.6) | 6.23 (d, 9.5) | 6.24 (d, 9.5) | 6.27 (d, 9.6) | 6.24 (d, 9.5) | 6.27 (d, 9.5) |
| 4 | 7.67 (d, 9.5) | 7.61 (d, 9.5) | 7.60 (d, 9.5) | 7.60 (d, 9.6) | 7.60 (d, 9.6) | 7.61 (d, 9.6) | 7.59 (d, 9.5) | 7.60 (d, 9.5) | 7.65 (d, 9.6) | 7.61 (d, 9.5) | 7.67 (d, 9.5) |
| 6 | 7.34 (d, 8.7) | 7.37 (d, 8.6) | 7.37 (d, 8.6) | 7.35 (d, 8.6) | 7.37 (d, 8.6) | 7.36 (d, 8.6) | 7.36 (d, 8.6) | 7.36 (d, 8.6) | 7.36 (d, 8.5) | 7.38 (d, 8.7) | 7.35 (d, 8.6) |
| 7 | 6.81 (d, 8.7) | 6.82 (d, 8.6) | 6.82 (d, 8.6) | 6.81 (d, 8.6) | 6.82 (d, 8.6) | 6.81 (d, 8.6) | 6.81 (d, 8.6) | 6.81 (d, 8.6) | 6.81 (d, 8.5) | 6.82 (d, 8.7) | 6.83 (d, 8.6) |
| 3′ | 3.89 (d, 5.0) | 5.37 (d, 5.0) | 5.46 (d, 5.0) | 5.41 (d, 4.8) | 5.37 (d, 4.8) | 5.34 (d, 5.0) | 5.42 (d, 4.8) | 5.34 (d, 4.8) | 5.49 (d, 4.8) | 4.10 (d, 4.8) | 5.28 (d, 3.6) |
| 4′ | 5.23 (d, 5.0) | 6.65 (d, 5.0) | 6.72 (d, 5.0) | 6.63 (d, 4.8) | 6.65 (d, 4.8) | 6.57 (d, 5.0) | 6.67 (d, 4.8) | 6.57 (d, 4.8) | 5.25 (d, 4.8) | 6.51 (d, 4.8) | 5.09 (d, 3.6) |
| 5′ | 1.42 (s) | 1.45 (s) | 1.47 (s) | 1.46 (s) | 1.45 (s) | 1.42 (s) | 1.46 (s) | 1.45 (s) | 1.45 (s) | 1.45 (s) | 1.42 (s) |
| 6′ | 1.48 (s) | 1.49 (s) | 1.51 (s) | 1.49 (s) | 1.48 (s) | 1.46 (s) | 1.50 (s) | 1.47 (s) | 1.51 (s) | 1.50 (s) | 1.52 (s) |
| 2′′ | - | - | - | - | - | 2.26 (m); 2.25 (m) | - | 2.30 (m); 2.20 (m) | - | - | - |
| 3′′ | - | 6.14 (q, 7.2) | 6.13 (q, 7.2) | 6.13 (q, 7.2) | 2.11 (s) | 2.10 (m) | 6.13 (q, 7.2) | 2.15 (m) | 6.18 (q, 7.2) | - | 6.11 (q, 7.2) |
| 4′′ | - | 1.97 (d, 7.2) | 2.00 (d, 7.2) | 1.98 (d, 7.2) | - | 0.98 (d, 7.2) | 1.98 (d, 7.2) | 0.98 (d, 7.2) | 2.00 (d, 7.2) | - | 1.92 (d, 7.2) |
| 5′′ | - | 1.88 (s) | 1.87 (s) | 1.89 (s) | - | 0.98 (d, 7.2) | 1.86 (s) | 0.98 (d, 7.2) | 1.91 (s) | - | 1.85 (s) |
| 2′′′ | - | 2.12 (s) | - | 2.28 (m); 2.20 (m) | - | 2.15 (s) | 5.63 (s) | 2.30 (m); 2.20 (m) | - | - | - |
| 3′′′ | - | - | 7.25 (q, 7.2) | 2.14 (m) | 7.26 (q, 7.2) | - | - | 2.15 (m) | - | 6.13 q, 7.2 | - |
| 4′′′ | - | - | 1.98 (d, 7.2) | 0.97 (d, 7.2) | 2.01 (d, 7.2) | - | 1.89 (s) | 0.98, d, 7.2 | - | 2.02, d, 7.2 | - |
| 5′′′ | - | - | 1.85 (s) | 0.97 (d, 7.2) | 1.88 (s) | - | 2.20 (s) | 0.98, d, 7.2 | - | 1.91, s | - |
| Ref. | [6] | [6] | [6] | [8] | [23] | [24,25] | [26] | [26,27] | [14] | [14] | [20] |
 +59° (c, 1.0), 2a], (−)-praeruptorin A [
+59° (c, 1.0), 2a], (−)-praeruptorin A [  −61° (c, 1.0), 2b], (+)-praeruptorin E (4), and the two metabolites of 2b formed in rat plasma, (−)-cis-(3′R,4′R)-3′-angeloyl khellactone [
−61° (c, 1.0), 2b], (+)-praeruptorin E (4), and the two metabolites of 2b formed in rat plasma, (−)-cis-(3′R,4′R)-3′-angeloyl khellactone [  −57° (c, 1.0), 9] and (−)-cis-(3′R,4′R)-4′-angeloyl-khellactone [
−57° (c, 1.0), 9] and (−)-cis-(3′R,4′R)-4′-angeloyl-khellactone [  −39° (c, 1.0), 10], were incubated with rat liver microsomes. The chemical structures and absolute configurations of 2a, 2b, 9 and 10 have been unambiguously identified using NMR analysis and optical rotation data in our laboratory [14], while 4 was commercially available and its chemical structure was double checked by comparing its mass spectral profile and optical rotation data [
−39° (c, 1.0), 10], were incubated with rat liver microsomes. The chemical structures and absolute configurations of 2a, 2b, 9 and 10 have been unambiguously identified using NMR analysis and optical rotation data in our laboratory [14], while 4 was commercially available and its chemical structure was double checked by comparing its mass spectral profile and optical rotation data [  +35° (c, 1.0)] with the literature report [8].
+35° (c, 1.0)] with the literature report [8].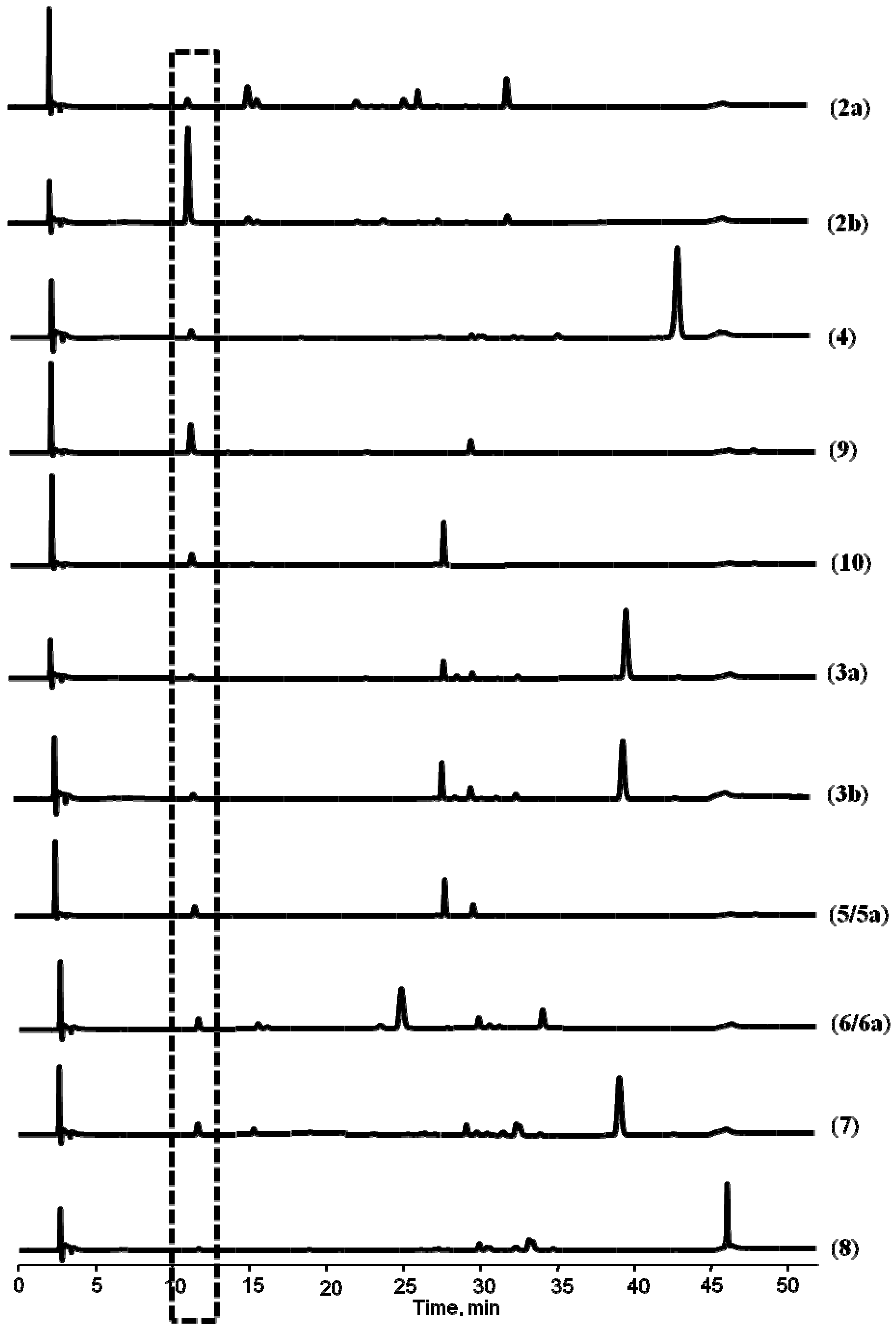
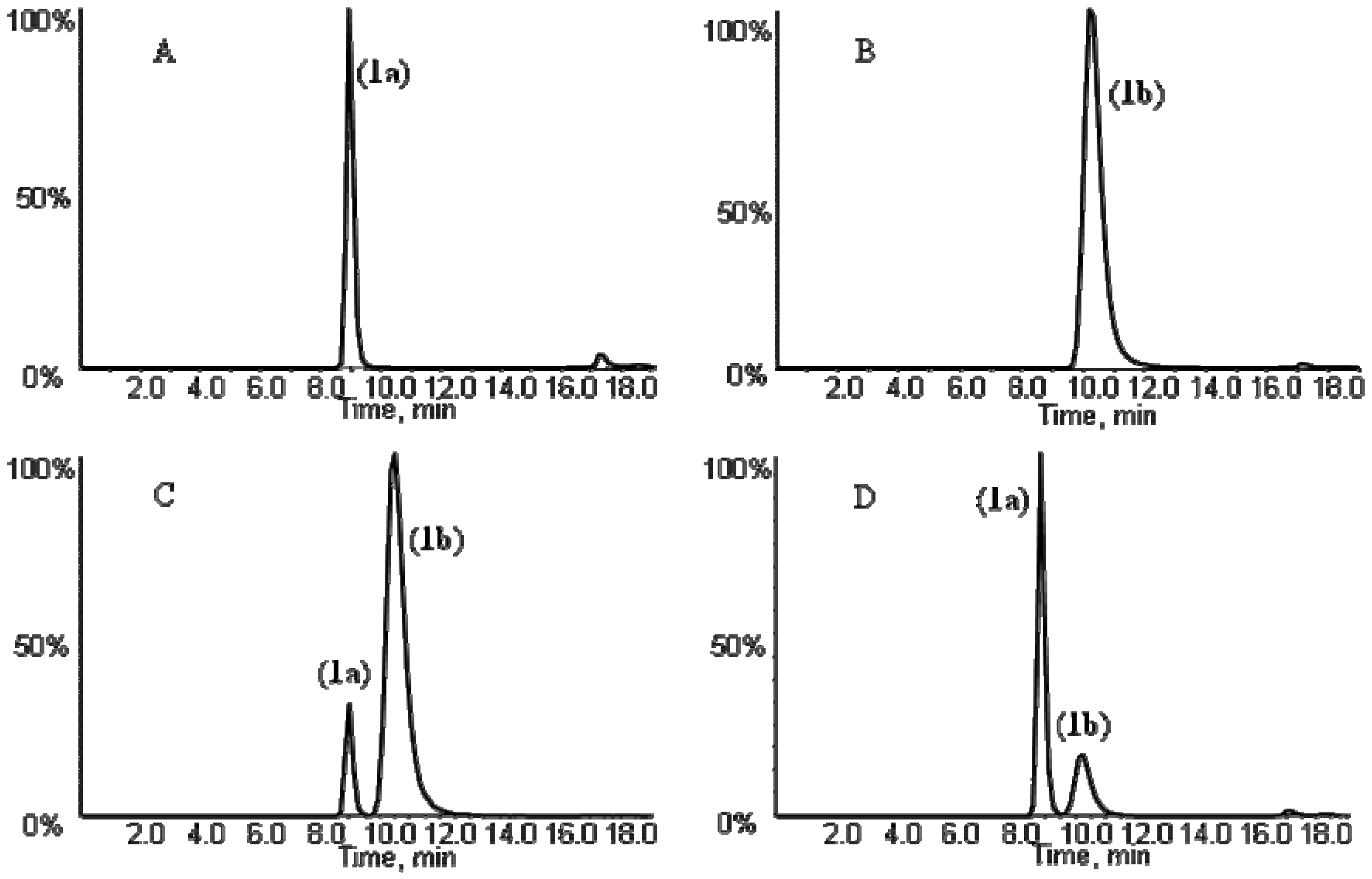
 +3.5° (c, 1.0) and the NMR data supported the structure as cis-3′-acetyl-4′-angeloylkhellactone (Table 2). These findings demonstrated that the absolute configuration of 5a predicted from the absolute configuration of its metabolite cis-khellactone corresponded well with the structure identified based on LC-MS/MS, NMR and polarimetric analysis (Table 1 and Table 2). These data of 5a were in good agreement with those of pteryxin [(3′R, 4′R)-3′-acetyl-4′-angeloylkhellactone], which has been isolated from P. praeruptorum [23], thus, compound 5a was unambiguously identified as pteryxin [(+)-cis-(3′R,4′R)-3′-acetyl-4′-angeloyl-khellactone].
+3.5° (c, 1.0) and the NMR data supported the structure as cis-3′-acetyl-4′-angeloylkhellactone (Table 2). These findings demonstrated that the absolute configuration of 5a predicted from the absolute configuration of its metabolite cis-khellactone corresponded well with the structure identified based on LC-MS/MS, NMR and polarimetric analysis (Table 1 and Table 2). These data of 5a were in good agreement with those of pteryxin [(3′R, 4′R)-3′-acetyl-4′-angeloylkhellactone], which has been isolated from P. praeruptorum [23], thus, compound 5a was unambiguously identified as pteryxin [(+)-cis-(3′R,4′R)-3′-acetyl-4′-angeloyl-khellactone]. +33° (c, 1.0)]. Compound 6a was thereby identified as (+)-cis-(3′S,4′S)-3′-isovaleryl-4′-acetylkhellactone. NMR analysis (Table 2) of compounds 7 and 8 identified their structures as cis-3′-angeloyl-4′-senecioylkhellactoneand cis-3′,4′-diisovalerylkhellactone, respectively. Their optical rotations were +31° (c, 1.0) and +35° (c, 1.0). Taken together, the structures were assigned as (+)-cis-(3′S,4′S)-3′-angeloyl-4′-senecioylkhellactone (7) and (+)-cis-(3′S, 4′S)-3′,4′-diisovalerylkhellactone (8). So far, (±)-dihydrosamidin, the racemic form of compound 6a, has been isolated from P. Turgeniifolium [24,25], while neither the racemate nor any of the single enantiomers has been reported for Qian-hu. Both 3′-angeloyl-4′-senecioylkhellactone and 3′,4′-diisovalerylkhellactone have been reported in P. japonicum,but not in P. praeruptorum (Qian-hu) [26] and their absolute configurations were not determined in the previous report [27]. Again, determination of the absolute configurations of pyranocoumarin compounds 5a, 6a, 7 and 8 based on chiral LC-MS/MS analysis of their respective hydrolytic metabolite cis-khellactone formed by RLMs were proved to be feasible and should be applicable to other pyranocoumarins that have a hidden C-3' and/or C-4' hydroxy functionality that can be released by enzymatic transformations.
+33° (c, 1.0)]. Compound 6a was thereby identified as (+)-cis-(3′S,4′S)-3′-isovaleryl-4′-acetylkhellactone. NMR analysis (Table 2) of compounds 7 and 8 identified their structures as cis-3′-angeloyl-4′-senecioylkhellactoneand cis-3′,4′-diisovalerylkhellactone, respectively. Their optical rotations were +31° (c, 1.0) and +35° (c, 1.0). Taken together, the structures were assigned as (+)-cis-(3′S,4′S)-3′-angeloyl-4′-senecioylkhellactone (7) and (+)-cis-(3′S, 4′S)-3′,4′-diisovalerylkhellactone (8). So far, (±)-dihydrosamidin, the racemic form of compound 6a, has been isolated from P. Turgeniifolium [24,25], while neither the racemate nor any of the single enantiomers has been reported for Qian-hu. Both 3′-angeloyl-4′-senecioylkhellactone and 3′,4′-diisovalerylkhellactone have been reported in P. japonicum,but not in P. praeruptorum (Qian-hu) [26] and their absolute configurations were not determined in the previous report [27]. Again, determination of the absolute configurations of pyranocoumarin compounds 5a, 6a, 7 and 8 based on chiral LC-MS/MS analysis of their respective hydrolytic metabolite cis-khellactone formed by RLMs were proved to be feasible and should be applicable to other pyranocoumarins that have a hidden C-3' and/or C-4' hydroxy functionality that can be released by enzymatic transformations.3. Experimental
3.1. Materials
3.2. Enantioseparation of Angular-type Pyranocoumarins from Qian-hu
3.3. Polarimetric and NMR Analysis
3.4. Hydrolysis of Angular-type Pyranocoumarins from Qian-hu by Rat Liver Microsomes
3.5. Achiral HPLC-UV Analysis
3.6. Chiral HPLC-MS/MS Analysis
4. Conclusions
Acknowledgments
References and Notes
- The State Pharmacopoeia Commission of P.R. China, Radix Peucedani. In Pharmacopoeia of the People’s Republic of China Beijing, 248 ed; Chemical Industry Press: Beijing, China, 2010; Volume I.
- Chang, H.M.; But, P.P.H.; Yao, S.C.; Wang, L.L.; Yeung, S.C.S. Pharmacology and Applications of Chinese Materia Medica; World Scientific Publisher: Singapore, 2001; Volume 2. [Google Scholar]
- Zhao, N.C.; Jin, W.B.; Zhang, X.H.; Guan, F.L.; Sun, Y.B.; Adachi, H.; Okuyama, T. Relaxant effects of pyranocoumarin compounds isolated from a Chinese medical plant, Bai-Hua Qian-Hu, on isolated rabbit tracheas and pulmonary arteries. Biol. Pharm. Bull. 1999, 22, 984–987. [Google Scholar] [CrossRef]
- Chang, T.H.; Adachi, H.; Okuyama, T.; Zhang, K.Y. Effects of 3'-angeloyloxy-4'-acetoxy-3',4'- dihydroseselin on myocardial dysfunction after a brief ischemia in anesthetized dogs. Acta Pharmacol. Sin. 1994, 15, 388–391. [Google Scholar]
- Fong, W.F.; Zhang, J.X.; Wu, J.Y.; Tse, K.W.; Wang, C.; Cheung, H.Y.; Yang, M.S. Pyranocoumarin(+/−)-4'-O-acetyl-3'-O-angeloyl-cis-khellactone induces mitochondrial-dependent apoptosis in HL-60 cells. Planta Med. 2004, 70, 489–495. [Google Scholar] [CrossRef]
- Chen, Z.X.; Huang, B.S.; She, Q.L.; Zeng, G.F. The chemical constituents of Bai-hua-qian-hu, the root of Peucedanum praeruptorum Dunn. (umbelliferae)—four new coumarins. Yao Xue Xue Bao 1979, 14, 486–496. [Google Scholar]
- Okuyama, T.; Shibata, S. Studies on coumarins of a Chinese drug “Qian-Hu”. Planta Med. 1981, 42, 89–96. [Google Scholar]
- Ye, J.S.; Zhang, H.Q.; Yuan, C.Q. Isolation and identification of coumarin praeruptorin E from the root of the Chinese drug Peucedanum praeruptorum DUNN (umbelliferae). Yao Xue Xue Bao 1982, 17, 431–434. [Google Scholar]
- Tao, Y.; Luo, J.; Lu, Y.; Xu, D.; Hou, Z.; Kong, L.Y. Rapid identification of two species of Peucedanum by high-performance liquid chromatography-diode array detection-electrospray ionization tandem mass spectrometry. Nat. Prod. Commun. 2009, 4, 1079–1084. [Google Scholar]
- Liang, T.G.; Yue, W.Y.; Li, Q.S. Chemopreventive effects of Peucedanum praeruptorum Dunn and its major constituents on SGC7901 gastric cancer cells. Molecules 2010, 15, 8060–8071. [Google Scholar]
- Shen, X.L.; Chen, G.Y.; Zhu, G.Y.; Fong, W.F. (±)-3′-O, 4′-O-dicynnamoyl-cis-khellactone, a derivative of (±)-praeruptorin A, resverses P-glycoprotein mediated multidrug resistance in cancer cells. Bioorg. Med. Chem. 2006, 14, 7138–7145. [Google Scholar] [CrossRef]
- Mizuno, A.; Okada, Y.; Nishino, H.; Okuyama, T. Anti-cancer activity of coumarins from Peucedanum praeruptum on second stage cancer. J. Tradit. Med. 1994, 11, 220–224. [Google Scholar]
- Xu, Z.; Wang, X.B.; Dai, Y.; Kong, L.Y.; Wang, F.Y.; Xu, H.; Lu, D.; Song, J.; Hou, Z.G. (±)-Praeruptorin A enantiomers exert distinct relaxant effects on isolated aorta rings dependent on endothelium and nitric oxide synthesis. Chem. Biol. Interact. 2010, 186, 239–246. [Google Scholar] [CrossRef]
- Song, Y.L.; Jing, W.H.; Zhao, H.Y.; Yan, R.; Li, P.T.; Wang, Y.T. Stereoselective metabolism of (±)-praeruptorin A, a calcium channel blocker from Peucedani Radix, in pooled liver microsomes of rats and humans. Xenobiotica 2011, 42, 231–247. [Google Scholar]
- Kong, L.Y.; Li, Y.; Min, Z.D.; Li, X.; Zhu, T.R. Coumarins from peucedanum praeruptorum. Phytochemistry 1996, 41, 1422–1426. [Google Scholar]
- Lou, H.X.; Sun, L.R.; Yu, W.T.; Fan, P.H.; Cui, L.; Gao, Y.H.; Ma, B.; Ren, D.M.; Ji, M. Absolute configuration determination of angular dihydrocoumarins from Peucedanum praeruptorum. J. Asian Nat. Prod. Res. 2004, 6, 177–184. [Google Scholar] [CrossRef]
- Nakanishi, K.; Berova, N.; Woody, R.W. Circular Dichroism: Principles and Applications, 1st ed; Wiley-VCH: New York, NY, USA, 1994; p. 473. [Google Scholar]
- Imai, T. Human carboxylesterase isozymes: Catalytic properties and rational drug design. Drug Metab. Pharmacokinet. 2006, 21, 173–185. [Google Scholar]
- Satoh, T.; Hosokawa, M. Structure, function and regulation of carboxylesterases. Chem. Biol. Interact. 2006, 162, 195–211. [Google Scholar]
- Wu, X.L.; Kong, L.Y.; Min, Z.D. Studies on structure modification of (+)-praeruptorin A. Yao Xue Xue Bao 2002, 377, 527–534. [Google Scholar]
- Song, Y.L.; Yan, R.; Jing, W.H.; Zhao, H.Y.; Wang, Y.T. Characterization of metabolism of (+)-praeruptorin B and (+)-praeruptorin E in human and rat liver microsomes by liquid chromatography coupled with ion trap mass spectrometry and time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2011, 25, 719–730. [Google Scholar]
- Ruan, H.; Zhang, Z.; Liang, X.F.; Fu, Y.; Su, M.Q.; Liu, Q.L.; Wang, X.M.; Zhu, X. Metabolism of dl-praeruptorin a in rat liver microsomes using HPLC-electrospray ionization tandem mass spectrometry. Arch. Pharm. Res. 2011, 34, 1311–1321. [Google Scholar]
- Takata, M.; Shibata, S.; Okuyama, T. Structures of angular pyranocoumarins of Bai-Hua Qian-Hu, the root of Peucedanum praeruptorum. Planta Med. 1990, 56, 307–311. [Google Scholar] [CrossRef]
- Rao, G.X.; Liu, Q.X.; Dai, W.S.; Sun, H.D. Chemical constituents of Peucedanum Turgeniifolium. Tianran Chanwu Yanjiu Yu Kaifa 1997, 9, 9–11. [Google Scholar]
- Sun, H.D.; Lin, Z.W.; Niu, F.D.; Ding, J.K. A study of the Chinese drugs of Umbelliferae II. New coumarins of Peucedanum Turgeniifolium Wolff. Yunnan Zhiwu Yanjiu 1981, 3, 173–180. [Google Scholar]
- Jong, T.T.; Hwang, H.C.; Jean, M.Y. An antiplatelet aggregation principle and X-ray structural analysis of cis-khellactone dister from Peucedanum japonicum. J. Nat. Prod. 1992, 55, 1396–1401. [Google Scholar] [CrossRef]
- Zhang, C.; Xiao, Y.Q.; Taniguchi, M.; Baba, K. Studies on chemical constituents in roots of Peucedanum praeruptorum. Zhongguo Zhongyao Zazhi 2005, 30, 675–677. [Google Scholar]
- Jing, W.H.; Song, Y.L.; Yan, R.; Bi, H.C.; Li, P.T.; Wang, Y.T. Transport and metabolism of (±)-praeruptorin A in Caco-2 cell monolayers. Xenobiotica 2011, 41, 71–81. [Google Scholar]
- Huang, L.; Bi, H.C.; Liu, Y.H.; Wang, Y.T.; Xue, X.P.; Huang, M. CAR-mediated up-regulation of CYP3A4 expression in LS174T cells by Chinese herbal components. Drug Metab. Pharmacokinet. 2011, 26, 331–340. [Google Scholar]
- Yang, H.; Xu, L.N.; Sui, Y.J.; Liu, X.; He, C.Y.; Fang, R.Y.; Liu, J.; Hao, F.; Ma, T.H. Stimulation of airway and intenstinal mucosal secretion by natural coumarin CFTR activators. Front. Pharmacol. 2011, 2, 52. [Google Scholar]
- Chen, G.H.; Tang, W.B.; Zhang, Z.F.; Cai, H.L.; Bai, L.Y. Activities and active components of Radix peucedani capable of inducing rice resistance to blast disease. Zhongguo Nongye Kexue 2010, 43, 1807–1814. [Google Scholar]
- Lin, G.; Tang, J.; Liu, X.Q.; Jiang, Y.; Zheng, J. Deacetylclivorine: A gender-selective metabolite of clivorine formed in female Sprague-Dawley rat liver microsomes. Drug Metab. Dispos. 2007, 35, 607–613. [Google Scholar]
- Lowry, O.H.; Rosenbrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Sample Availability: Samples of the compounds 1–11 and enantiomers of compounds 1–3 are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Song, Y.-L.; Zhang, Q.-W.; Li, Y.-P.; Yan, R.; Wang, Y.-T. Enantioseparation and Absolute Configuration Determination of Angular-Type Pyranocoumarins from Peucedani Radix Using Enzymatic Hydrolysis and Chiral HPLC-MS/MS Analysis. Molecules 2012, 17, 4236-4251. https://doi.org/10.3390/molecules17044236
Song Y-L, Zhang Q-W, Li Y-P, Yan R, Wang Y-T. Enantioseparation and Absolute Configuration Determination of Angular-Type Pyranocoumarins from Peucedani Radix Using Enzymatic Hydrolysis and Chiral HPLC-MS/MS Analysis. Molecules. 2012; 17(4):4236-4251. https://doi.org/10.3390/molecules17044236
Chicago/Turabian StyleSong, Yue-Lin, Qing-Wen Zhang, Ya-Ping Li, Ru Yan, and Yi-Tao Wang. 2012. "Enantioseparation and Absolute Configuration Determination of Angular-Type Pyranocoumarins from Peucedani Radix Using Enzymatic Hydrolysis and Chiral HPLC-MS/MS Analysis" Molecules 17, no. 4: 4236-4251. https://doi.org/10.3390/molecules17044236
APA StyleSong, Y.-L., Zhang, Q.-W., Li, Y.-P., Yan, R., & Wang, Y.-T. (2012). Enantioseparation and Absolute Configuration Determination of Angular-Type Pyranocoumarins from Peucedani Radix Using Enzymatic Hydrolysis and Chiral HPLC-MS/MS Analysis. Molecules, 17(4), 4236-4251. https://doi.org/10.3390/molecules17044236




