A Simple Halide-to-Anion Exchange Method for Heteroaromatic Salts and Ionic Liquids
Abstract
:1. Introduction
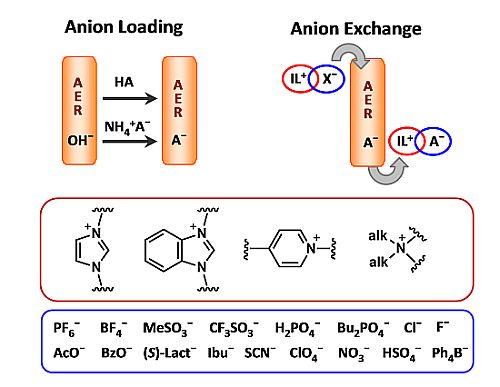
2. Results and Discussion
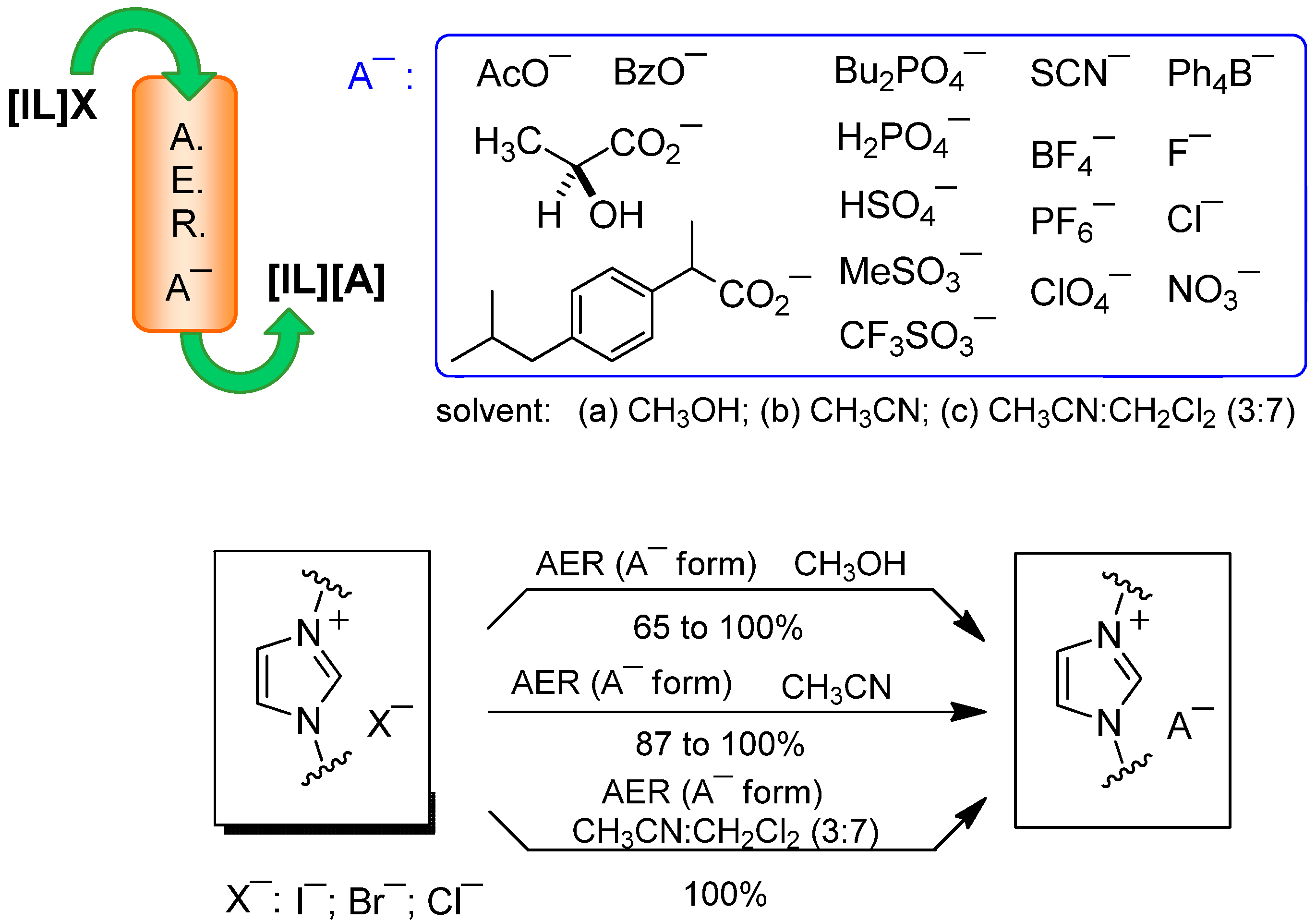
2.1. AER (A− Form) Method. Anion Loading
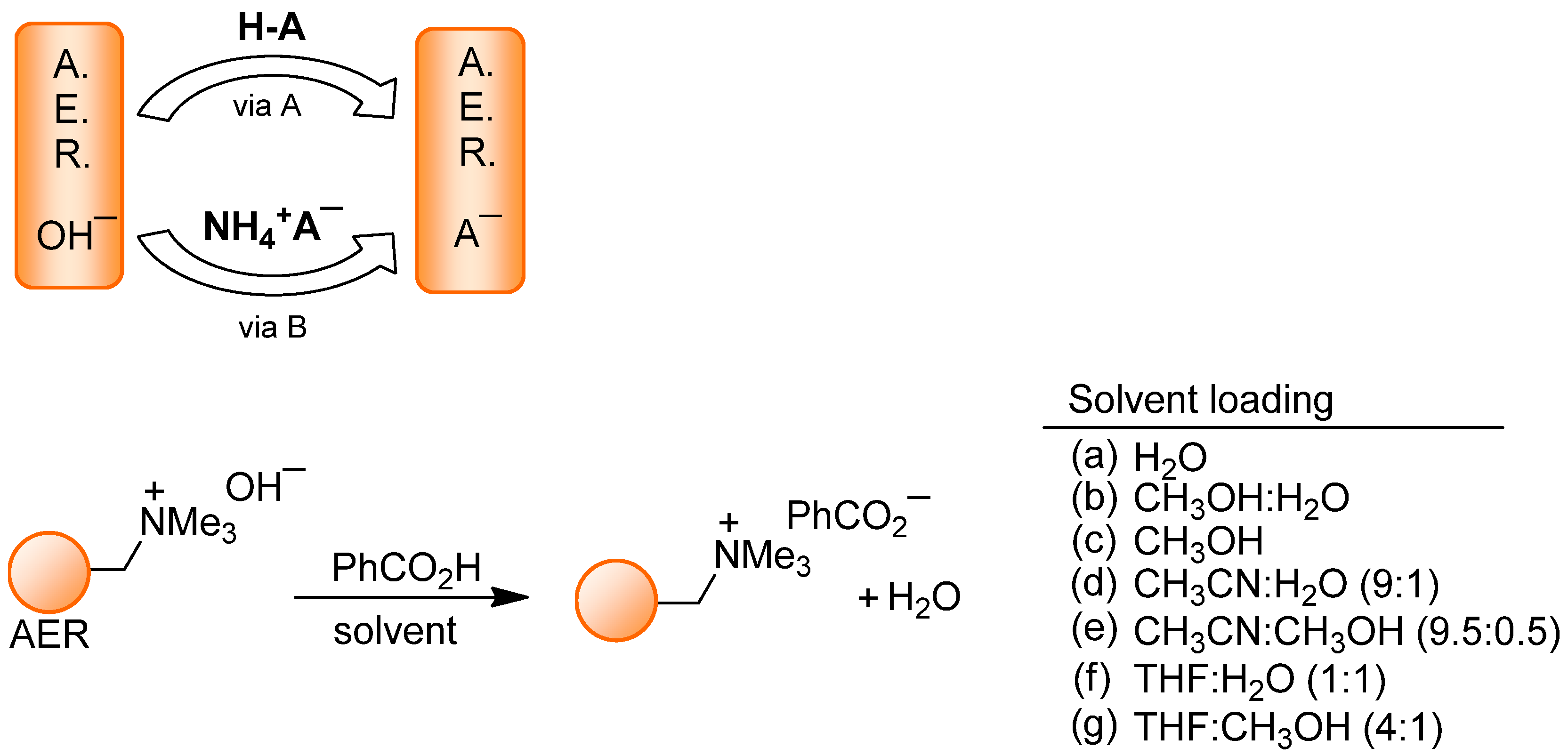
| Anion | Source | Solvent | Anion | Source | Solvent |
|---|---|---|---|---|---|
| AcO− | NH4+AcO− | (a) | AcO− | AcOH | (b) |
| Cl− | NH4+Cl− | (a) | Cl− | HCl | (a), (b) |
| PF6− | NH4+PF6− | (a) | PF6− | HPF6 | (b) |
| BF4− | NH4+BF4− | (a) | BF4− | HBF4 | (b) |
| CF3SO3− | NH4+CF3SO3− | (a) | BzO− | BzOH | (b)−(g) |
| SCN− | NH4+ SCN− | (a) | (S)-Lactate− | (S)-Lactic acid | (b) |
| F¯ | NH4+F− | (a) | MeSO3− | MeSO3H | (b) |
| H2PO4− | NH4+H2PO4− | (a) | Bu2PO4− | Bu2PO4H | (b), (c) |
| HSO4− | NH4+HSO4− | (a) | ClO4− | HClO4 | (a), (b) |
| Ph4B− | NH4+Ph4B− | (d), (e) | NO3− | HNO3 | (a), (b) |
| Ibu− | Ibuprofene | (d), (e) |
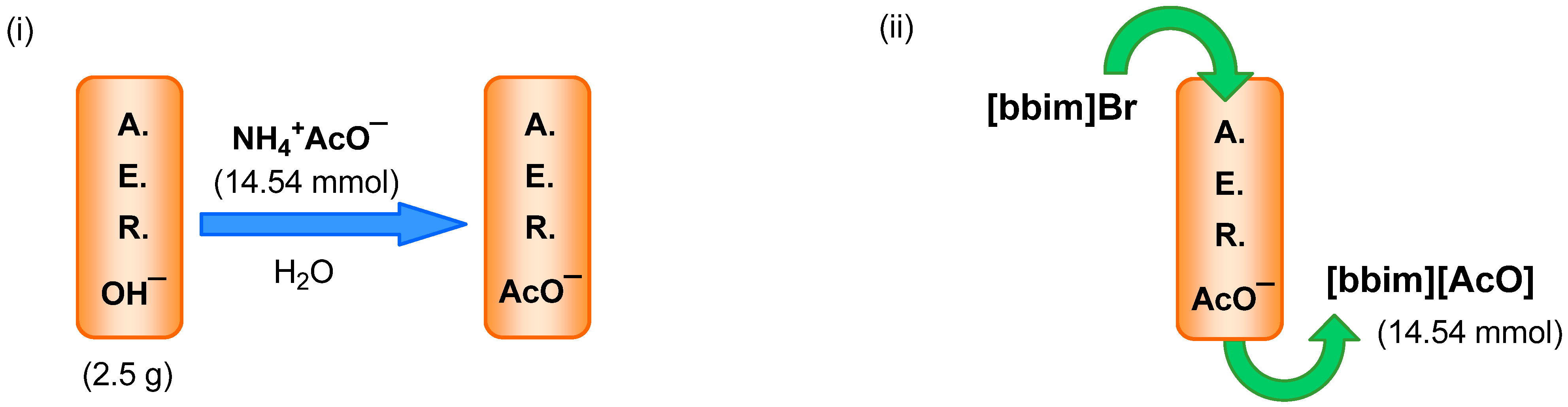
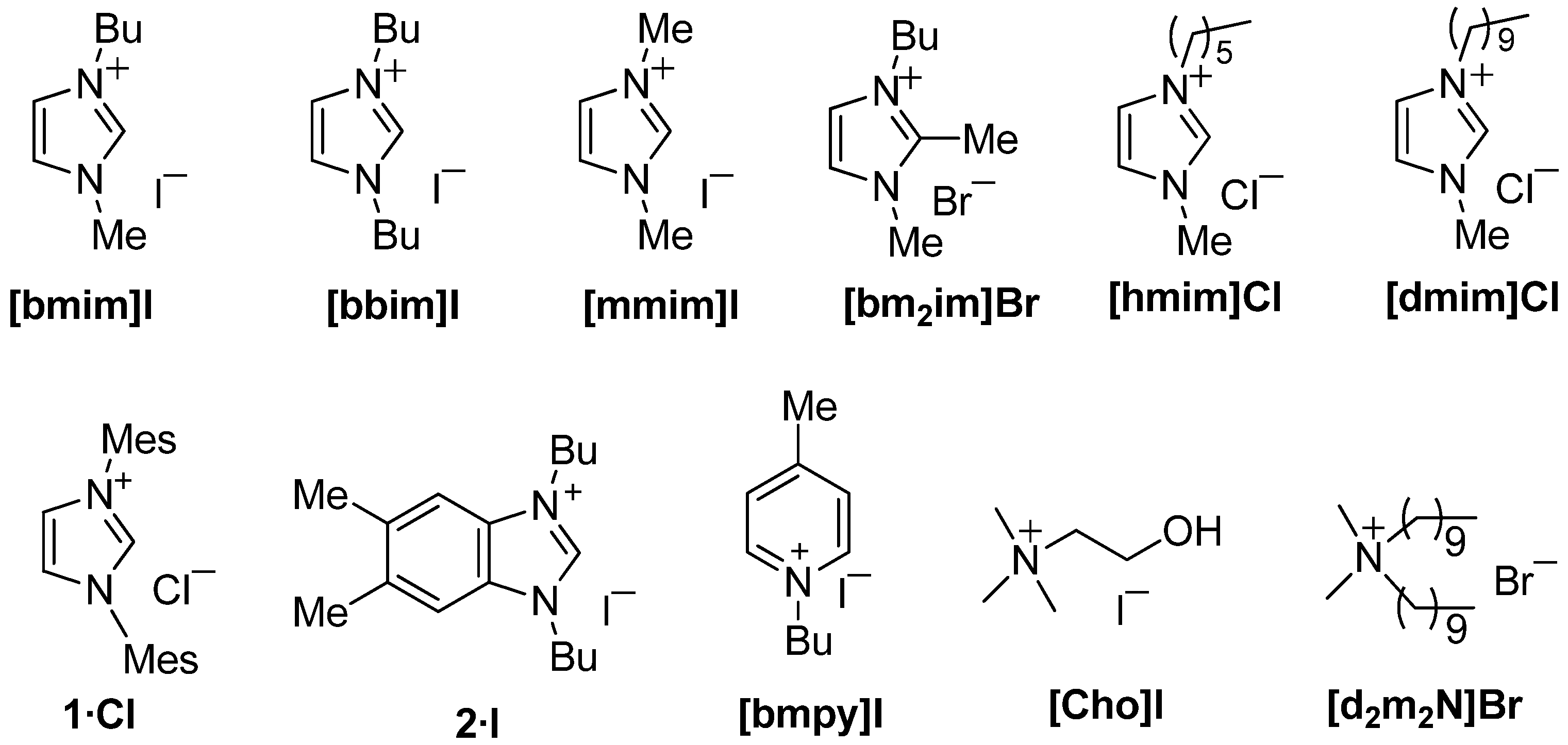
2.2. AER (A− Form) Method. Anion Exchange
| [bmim]I or Br | [bbim]I or Br | [mmim]I | [bm2im]Br | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Anion | Solvent | Yield (%) a | I− (ppm) b | Yield (%) a | I− (ppm) b | Yield (%) a | I− (ppm) b | Yield (%) a | Br− (ppm) b |
| AcO− | CH3OH | 100 | <20 | 100 | <20 | 100 | <20 | 98 | <13 |
| BzO− | CH3OH | 100 | <20 | 100 | <20 | 95 | <20 | 100 | <13 |
| (S)-Lactate− | CH3OH | 100 | 20–40 | 100 | <20 | 100 | <20 | 100 | <13 |
| MeSO3− | CH3OH | 100 | <20 | 100 | <20 | 95 | <20 | 92 | <13 |
| MeSO3− | CH3CN | ― | ― | ― | 100 | <13 | |||
| Bu2PO4− | CH3OH | 100 | <20 | 100 | <20 | 100 | <20 | 100 | <13 |
| F− | CH3OH | 82 | ND c | 100 | ND c | ― | ― | ||
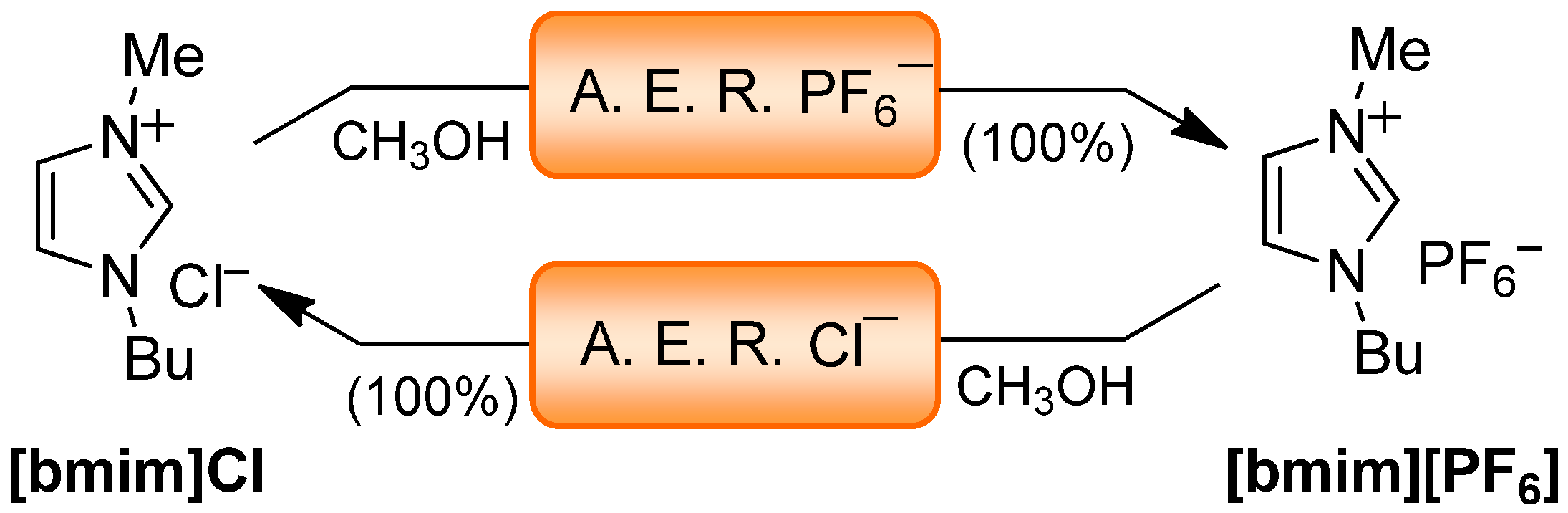
| Cl− | CH3OH | 100 | ND | 100 | ND | 100 | ND | ― | |
| PF6− | CH3OH | 100 | 20–40 | 100 | <20 | 100 | <20 | 91 | ND |
| PF6− | CH3CN | ― | ― | ― | 100 | 13–26 | |||
| NO3− | CH3OH | 100 | <20 | 100 | <20 | 100 | 20–40 | ― | |
| ClO4− | CH3OH | 100 | 100–120 | 100 | 20–40 | 100 | 20–40 | ― | |
| BF4− | CH3OH | 100 | <20 | 100 | <20 | 100 | 20–40 | 97 | 13–26 |
| H2PO4− | CH3OH | 100 | <20 | 100 | 20–40 | 100 | <20 | ― | |
| HSO4− | CH3OH | 100 | <20 | 100 | 20–40 | 100 | <20 | ― | |
| CF3SO3− | CH3OH | 100 | <20 | 100 | <20 | 100 | <20 | 100 | <13 |
| SCN− | CH3OH | 100 | ND | 100 | ND | 100 | ND | 100 | ND |
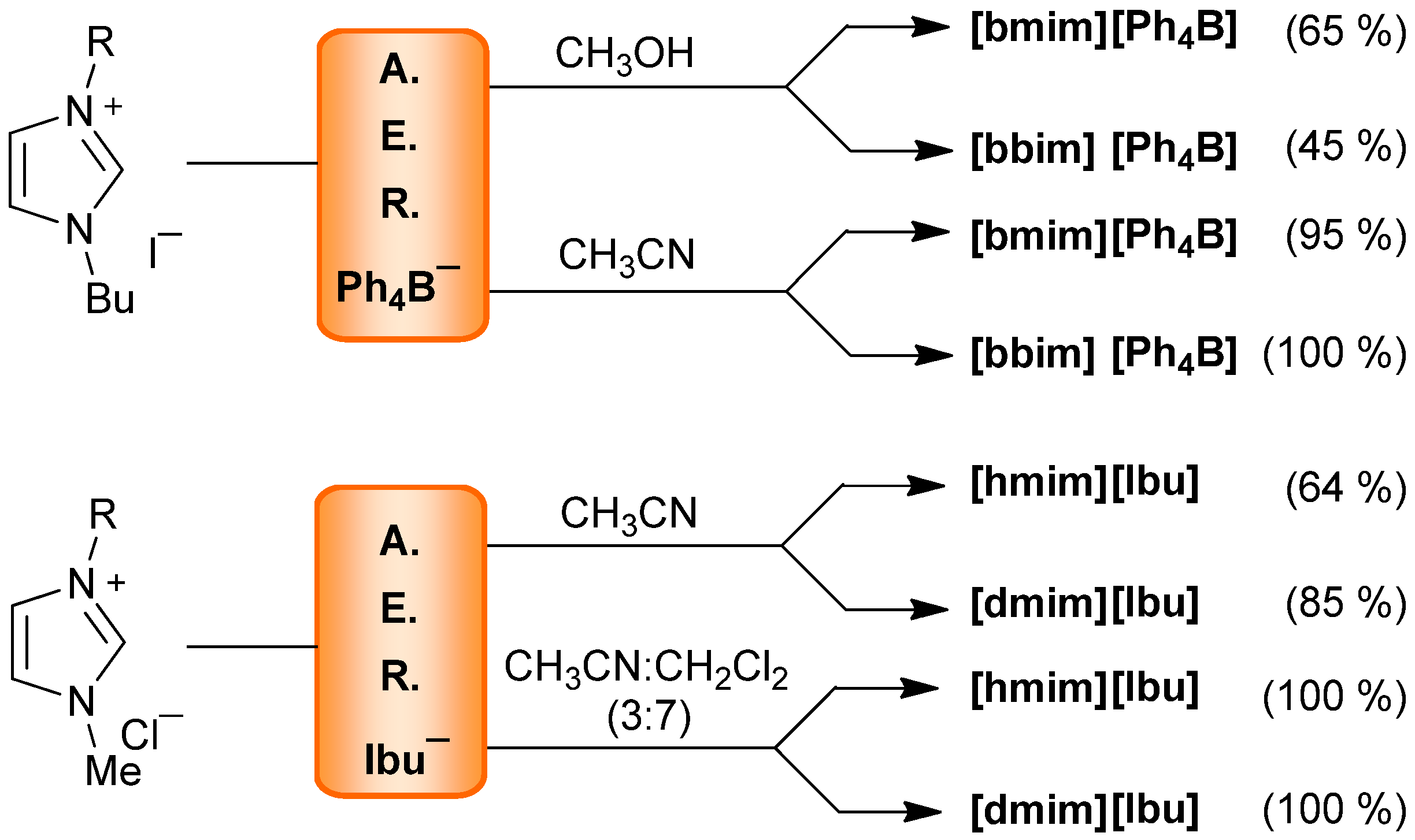
| Ph4B− | CH3OH | 65 | <20 | 45 | <20 | ― | ― | ||
| Ph4B− | CH3CN | 95 | <20 | 100 | <20 | ― | 91 | <13 | |
| Ibu− | CH3OH | 95 | <20 | ― | ― | ― | |||
| Ibu− | CH3CN | 100 | <20 | ― | ― | 96 | <13 | ||
| Cation | Anion | Solvent | Yield (%) a | Cl− (ppm) b |
|---|---|---|---|---|
| hmim | Ibu− | CH3CN | 90 | <6 |
| Ibu− | CH3CN:CH2Cl2 (3:7) | 100 | <6 | |
| dmim | Ibu− | CH3CN | 87 | <6 |
| Ibu− | CH3CN:CH2Cl2 (3:7) | 100 | <6 |
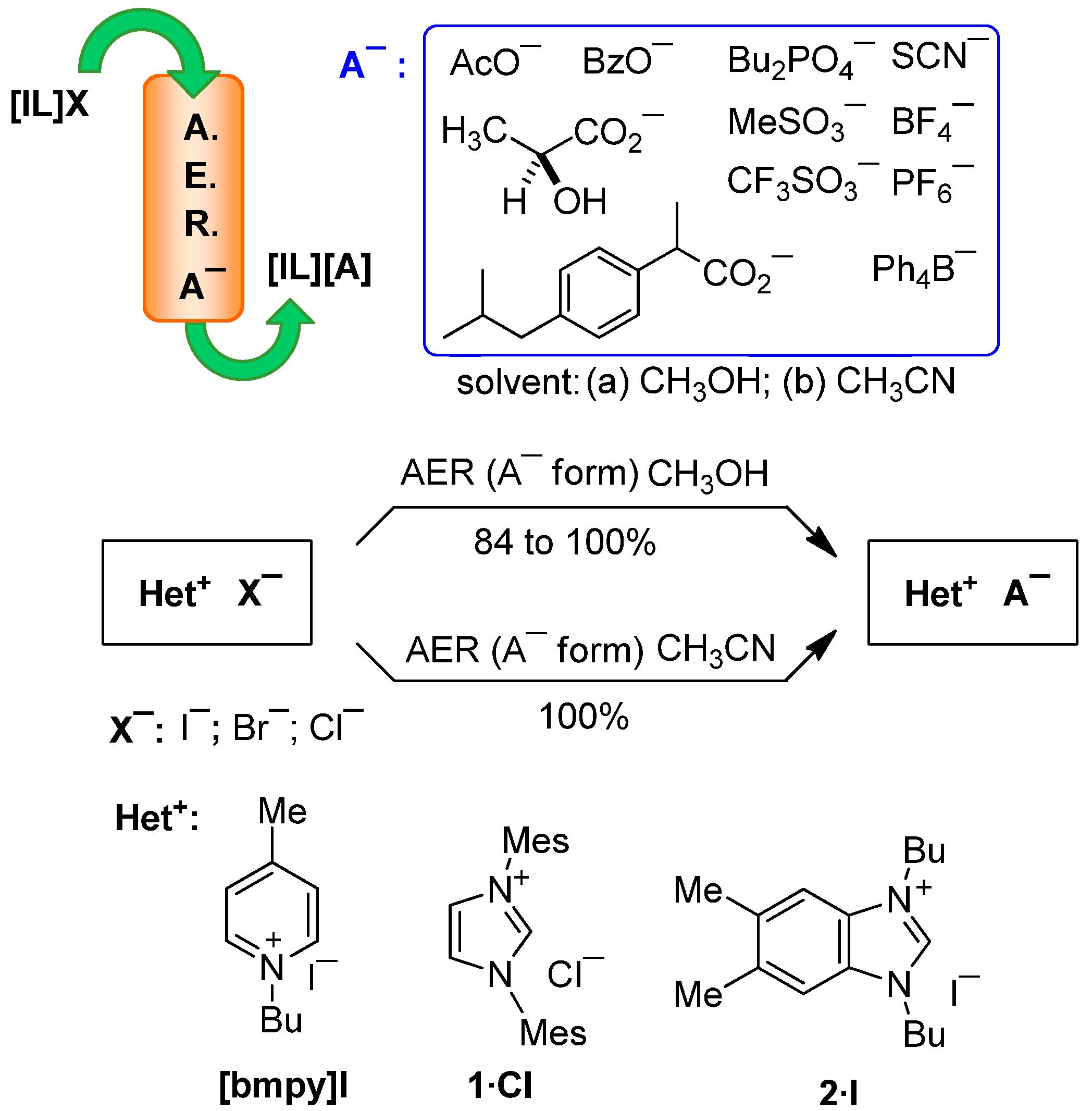
| [bmpy][I] | 1·Cl | 2·I | |||||
|---|---|---|---|---|---|---|---|
| Anion | Solvent | Yield (%) a | I− (ppm) b | Yield (%) a | Cl− (ppm) b | Yield (%) a | I− (ppm) b |
| AcO− | CH3OH | 84 | <20 | 95 | <6 | 100 | <20 |
| AcO− | CH3CN | 100 | <20 | ― | ― | ||
| BzO− | CH3OH | 100 | <20 | 92 | <6 | 95 | <20 |
| BzO− | CH3CN | ― | 100 | <6 | ― | ||
| (S)-Lactate− | CH3OH | 100 | <20 | 98 | <6 | 100 | <20 |
| MeSO3− | CH3OH | 100 | <20 | 91 | <6 | 90 | <20 |
| MeSO3− | CH3CN | ― | 100 | <6 | 100 | ||
| Bu2PO4− | CH3OH | 100 | <20 | 95 | <6 | 97 | <20 |
| PF6− | CH3OH | 100 | <20 | 100 | <6 | 100 | <20 |
| BF4− | CH3OH | 98 | <40 | 79 | <6 | 100 | <20 |
| BF4− | CH3CN | ― | 100 | <6 | ― | ||
| CF3SO3− | CH3OH | 100 | <20 | 88 | <6 | 95 | <20 |
| CF3SO3− | CH3CN | ― | 95 | <6 | ― | ||
| SCN− | CH3OH | 100 | ND | 91 | ND | 100 | ND |
| SCN− | CH3CN | ― | 97 | ND | ― | ||
| Ph4B− | CH3CN | ― | 82 | <6 | ― | ||
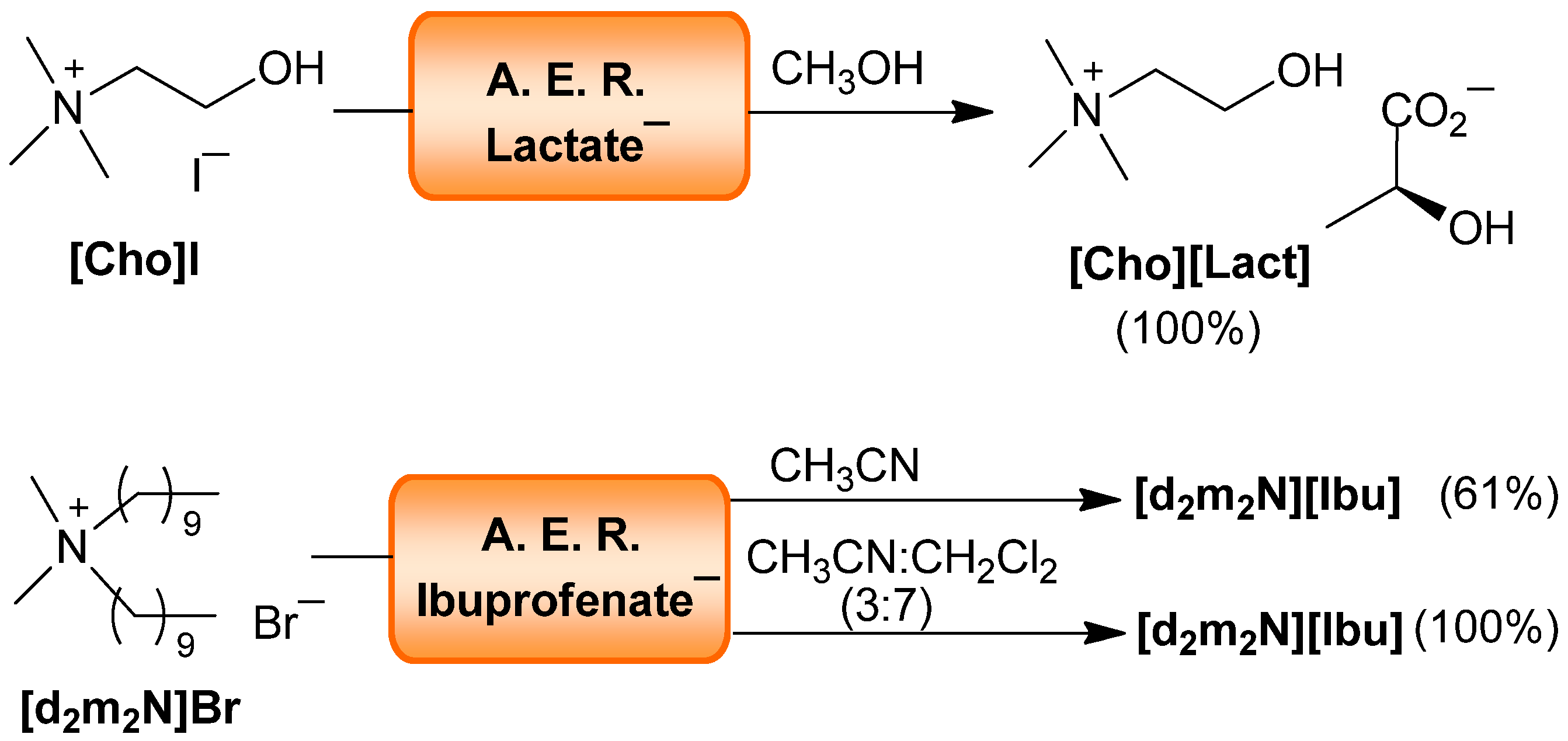
| Cation | Anion | Solvent | Yield (%) a | I− (ppm) b |
|---|---|---|---|---|
| Cho | (S)-Lactate− | CH3OH | 100 | <20 |
| d2m2N | Ibu− | CH3CN | 61 | <13 |
| Ibu− | CH3CN: CH2Cl2 (3:7) | 100 | <13 |
3. Experimental
3.1. General
3.2. Loading the AER (OH− Form) with Acids or Ammonium Salts
3.3. Anion Exchange: General Procedure
3.4. Silver Chromate Test
3.5. 1,3-Dibutyl-5,6-dimethylbenzimidazolium Iodide(2·I)
3.6. 1H-NMR Data of Compounds [bmim][A] (Table 6), [bbim][A] (Table 7), [mmim][A] (Table 8), [hmim][A] (Table 9), [dmim][A] (Table 9), [bm2im][A] (Table 10), [bmpy][A] (Table 11), 1·A (Table 12), 2·A (Table 13), [Cho][A] (Table 14) and [d2m2N][A] (Table 14)
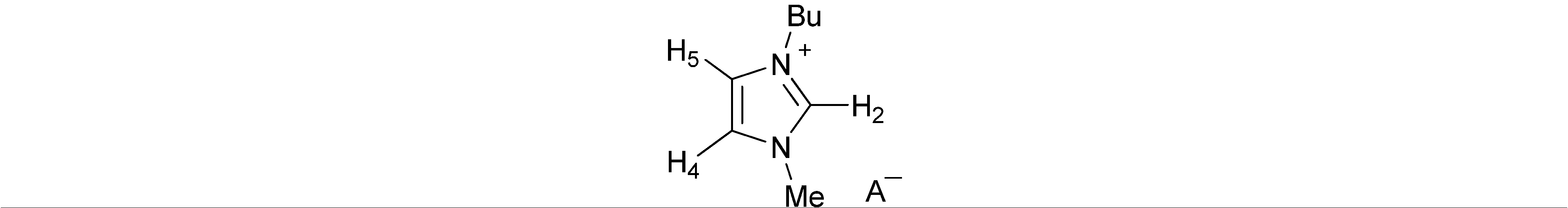
| Anion | Solvent | H-2 | H-4 | H-5 | Bu | Me | A− |
|---|---|---|---|---|---|---|---|
| AcO− | CDCl3 | 11.35 | 7.09 | 7.08 | 4.30; 1.86; 1.37; 0.96 | 4.06 | 1.99 |
| BzO− | CDCl3 | 11.00 | 7.09 | 7.09 | 4.29; 1.84; 1.33; 0.92 | 4.08 | 8.10; 7.33 |
| ( S)-Lactate− | CDCl3 | 11.19 | 7.17 | 7.17 | 4.31; 1.89; 1.38; 0.98 | 4.08 | 3.46; 1.41 |
| MeSO3− | CDCl3 | 10.21 | 7.25 | 7.20 | 4.28; 1.87; 1.38; 0.97 | 4.05 | 2.80 |
| Bu2PO4− | CDCl3 | 10.19 | 7.36 | 7.23 | 4.25; 1.80; 1.33; 0.88 | 4.00 | 3.80;1.54;1.33; 0.88 |
| I− b | CDCl3 | 10.27 | 7.52 | 7.44 | 4.35; 1.93; 1.41; 0.99 | 4.14 | |
| Br− | CDCl3 | 10.41 | 7.46 | 7.37 | 4.35; 1.91; 1.40; 0.98 | 4.13 | |
| F− | CDCl3 | (c) | 7.50 | 7.33 | 4.29; 1.87; 1.36; 0.95 | 4.06 | |
| Cl− | CDCl3 | 10.99 | 7.31 | 7.24 | 4.33; 1.91; 1.40; 0.98 | 4.13 | |
| PF6− | CDCl3 | 9.07 | 7.26 | 7.23 | 4.20; 1.88; 1.38; 0.97 | 3.98 | |
| NO3− | CDCl3 | 10.02 | 7.35 | 7.30 | 4.25; 1.88; 1.38; 0.97 | 4.02 | |
| ClO4− | CDCl3 | 9.15 | 7.30 | 7.26 | 4.23; 1.89; 1.39; 0.98 | 4.02 | |
| BF4− | CDCl3 | 8.98 | 7.28 | 7.24 | 4.21; 1.87; 1.39; 0.97 | 3.98 | |
| CF3SO3− | CDCl3 | 9.27 | 7.32 | 7.28 | 4.21; 1.88; 1.38; 0.97 | 3.99 | |
| SCN− | CDCl3 | 9.59 | 7.36 | 7.31 | 4.32; 1.92; 1.41; 0.99 | 4.11 | |
| Ibu− | CDCl3 | 9.86 | 7.10 | 7.02 | 4.02; 1.66; 1.24; 0.87 | 3.71 | 7.26; 6.95; 3.53; 2.35; 1.75; 1.39; 0.82 |
| AcO− | CD3CN | 9.25 | 7.35 | 7.32 | 4.14; 1.80; 1.31; 0.93 | 3.84 | 1.66 |
| BzO− | CD3CN | 9.43 | 7.29 | 7.28 | 4.19; 1.80; 1.30; 0.92 | 3.86 | 7.93; 7.27 |
| MeSO3− | CD3CN | 8.63 | 7.37 | 7.34 | 4.16; 1.80; 1.31; 0.94 | 3.83 | 2.43 |
| I− | CD3CN | 8.56 | 7.39 | 7.35 | 4.14; 1.81; 1.31; 0.94 | 3.83 | |
| Cl− | CD3CN | 9.04 | 7.39 | 7.36 | 4.15; 1.80; 1.31; 0.93 | 3.84 | |
| PF6− | CD3CN | 8.42 | 7.35 | 7.31 | 4.11; 1.79; 1.30; 0.93 | 3.80 | |
| NO3− | CD3CN | 8.58 | 7.37 | 7.34 | 4.13; 1.81; 1.31; 0.94 | 3.82 | |
| ClO4− | CD3CN | 8.43 | 7.37 | 7.35 | 4.12; 1.81; 1.32; 0.94 | 3.82 | |
| BF4− | CD3CN | 8.43 | 7.36 | 7.33 | 4.12; 1.82; 1.32; 0.94 | 3.81 | |
| CF3SO3− | CD3CN | 8.43 | 7.36 | 7.33 | 4.12; 1.80; 1.32; 0.94 | 3.81 | |
| SCN− | CD3CN | 8.49 | 7.37 | 7.34 | 4.13; 1.80; 1.30; 0.94 | 3.82 | |
| Ph4B− | CDCl3 | 4.54 | 6.01 | 5.84 | 3.16; 1.33; 1.13; 0.89 | 2.76 | 7.52; 6.97; 6.78 |
| Ph4B− | CD3CN | 8.19 | 7.27 d | 7.27 d | 4.05; 1.77; 1.30; 0.93 | 3.74 | 7.27; 6.99; 6.84 |
| Ph4B− | DMSO-d6 | 9.06 | 7.74 | 7.67 | 4.13; 1.75; 1.24; 0.89 | 3.82 | 7.16; 6.91; 6.78 |
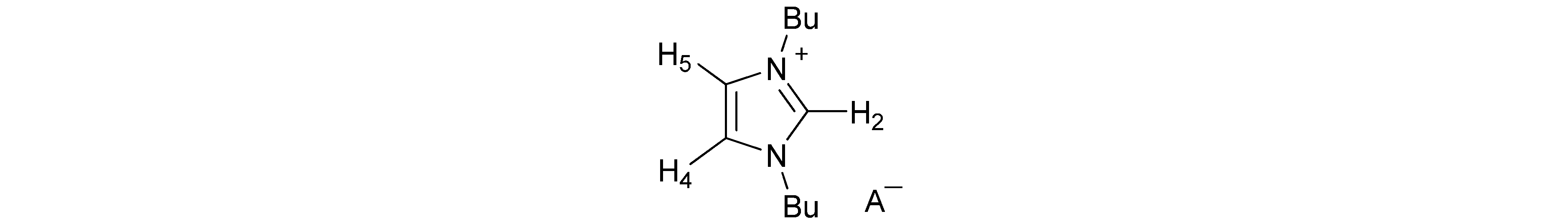
| Anion | Solvent | H-2 | H-4,5 | Bu | A− |
|---|---|---|---|---|---|
| AcO− | CDCl3 | 11.32 | 7.14 | 4.35; 1.86; 1.39; 0.97 | 2.01 |
| BzO− | CDCl3 | 11.40 | 7.16 | 4.34; 1.87; 1.35; 0.93 | 8.10; 7.32 |
| (S)-Lactate− | CDCl3 | 11.29 | 7.14 | 4.33; 1.87; 1.37; 0.96 | 4.02; 1.39 |
| MeSO3− | CDCl3 | 9.73 | 7.51 | 4.30; 1.88; 1.37; 0.96 | 2.75 |
| Bu2PO4− | CDCl3 | 11.05 | 7.11 | 4.37; 1.88; 1.40; 0.94 | 3.87; 1.62; 1.40; 0.94 |
| I− | CDCl3 | 10.34 | 7.38 | 4.38; 1.95; 1.42; 0.99 | |
| Br− | CDCl3 | 10.58 | 7.42 | 4.36; 1.90; 1.37; 0.95 | |
| F− | CDCl3 | (b) | 7.17 | 4.30; 1.89; 1.40; 0.98 | |
| Cl− | CDCl3 | 11.05 | 7.23 | 4.38; 1.92; 1.41; 0.98 | |
| PF6− | CDCl3 | 9.05 | 7.23 | 4.24; 1.88; 1.39; 0.98 | |
| NO3− | CDCl3 | 9.89 | 7.39 | 4.25; 1.86; 1.33; 0.94 | |
| ClO4− | CDCl3 | 9.24 | 7.38 | 4.26; 1.88; 1.37. 0.96 | |
| BF4− | CDCl3 | 9.12 | 7.36 | 4.23; 1.87; 1.36; 0.95 | |
| H2PO4− | CDCl3 | 10.59 | 7.31 | 4.40; 1.84; 1.34; 0.92 | |
| HSO4− | CD3CN | 10.84 | 7.40 | 4.39; 1.84; 1.34; 0.91 | |
| CF3SO3− | CDCl3 | 9.49 | 7.28 | 4.26; 1.88; 1.38; 0.98 | |
| SCN− | CDCl3 | 9.18 | 7.34 | 4.25; 1.88; 1.38; 0.97 | |
| Ph4B− | CDCl3 | (b) | 5.81 | 3.10; 1.30; 1.13; 0.89 | 7.50; 6.98; 6.82 |
| Ph4B− | DMSO-d6 | 9.19 | 7.79 | 4.15; 1.77; 1.26; 0.90 | 7.18; 6.92; 6.78 |
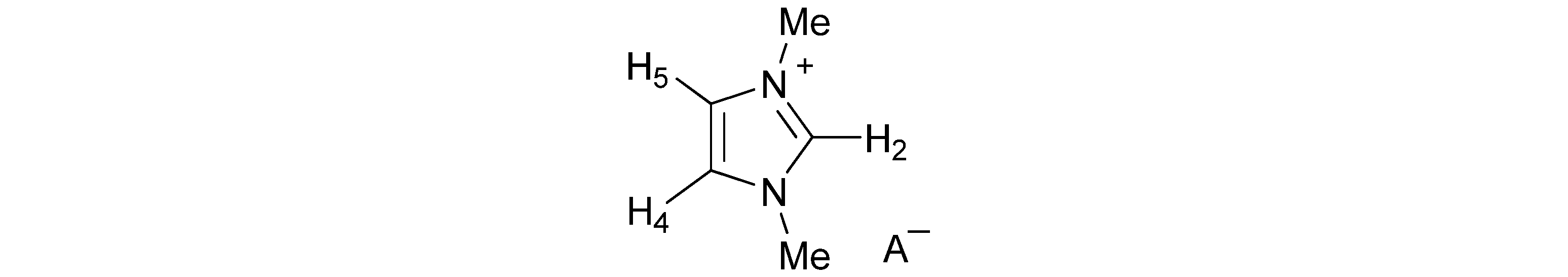
| Anion | Solvent | H-2 | H-4,5 | Me | A− |
|---|---|---|---|---|---|
| AcO− | CD3CN | 9.05 | 7.32 | 3.83 | 1.69 |
| BzO− | CD3CN | 9.29 | 7.33 | 3.85 | 7.93; 7.28 |
| (S)-Lactate− | CDCl3 | 11.04 | 7.15 | 4.03 | 3.80; 1.38 |
| MeSO3− | CD3CN | 8.58 | 7.33 | 3.83 | 2.43 |
| Bu2PO4− | CDCl3 | 10.88 | 7.15 | 4.04 | 3.86; 1.61; 1.39; 0.90 |
| I− | CD3CN | 8.48 | 7.34 | 3.83 | |
| Cl− | CD3CN | 8.57 | 7.34 | 3.83 | |
| PF6− | CD3CN | 8.38 | 7.32 | 3.82 | |
| NO3− | CD3CN | 8.57 | 7.34 | 3.83 | |
| ClO4− | CD3CN | 8.45 | 7.33 | 3.82 | |
| BF4− | CD3CN | 8.43 | 7.33 | 3.82 | |
| H2PO4− | CDCl3 | 10.26 | 7.30 | 4.09 | |
| HSO4− | CDCl3 | 10.19 | 7.34 | 4.09 | |
| CF3SO3− | CD3CN | 8.45 | 7.33 | 3.82 | |
| SCN− | CD3CN | 8.44 | 7.33 | 3.83 |
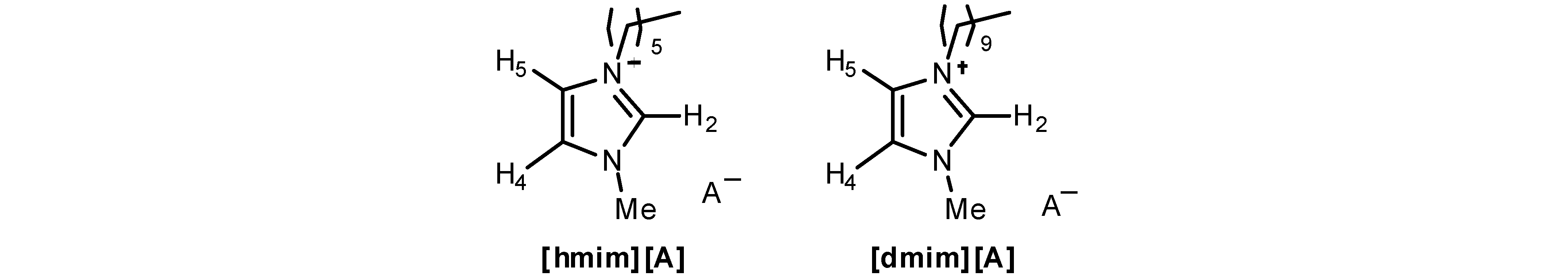
| Cation | Anion | H-2 | H-4 | H-5 | CnHn+1 | Me | A− |
|---|---|---|---|---|---|---|---|
| hmim | Cl− | 10.80 | 7.44 | 7.31 | 4.30; 1.89; 1.30; 0.86 | 4.11 | – |
| Ibu− | 9.72 | 7.08 | 7.01 | 4.05; 1.74; 1.26; 0.86 | 3.75 | 7.28; 7.01; 3.54;2.37; 1.78; 1.41; 0.86 | |
| dmim | Cl− | 10.82 | 7.38 | 7.27 | 4.32; 1.89; 1.27; 0.86 | 4.12 | – |
| Ibu− | 10.58 | 7.01 | 6.99 | 4.11; 1.78; 1.25; 0.87 | 3.81 | 7.31; 6.98; 3.60;2.39; 1.79; 1.46; 0.87 |
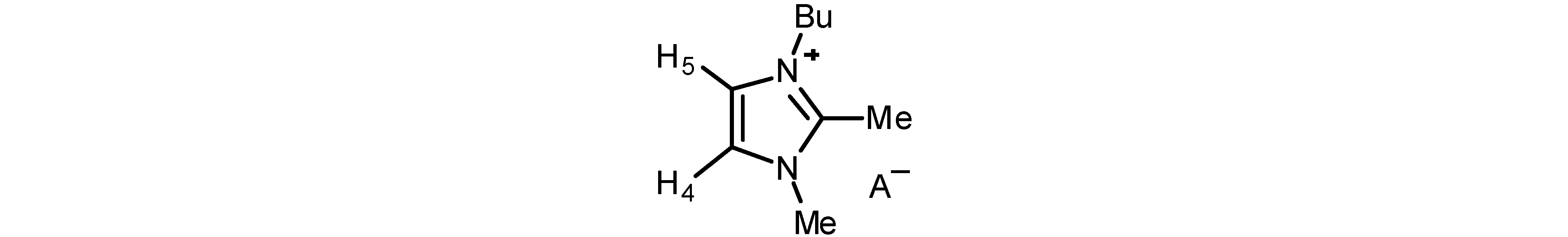
| Anion | H-4 | H-5 | Me-2 | Me-3 | Bu | A− |
|---|---|---|---|---|---|---|
| AcO− | 7.58 | 7.36 | 2.59 | 3.82 | 4.06; 1.67; 1.26; 0.86 | 1.72 |
| BzO− | 7.54 | 7.27 | 2.50 | 3.71 | 3.90; 1.58; 1.23; 0.85 | 7.97; 7.27 |
| (S)-Lactate− | 7.49 | 7.26 | 2.70 | 3.92 | 4.12; 1.79; 1.40; 0.98 | 3.87; 1.30 |
| MeSO3− | 7.47 | 7.27 | 2.69 | 3.94 | 4.14; 1.80; 1.38; 0.98 | 2.74 |
| Bu2PO4− | 7.55 | 7.27 | 2.68 | 3.92 | 4.13; 1.76; 1.37; 0.96 | 3.77; 1.56; 1.37; 0.89 |
| Br−b | 7.76 | 7.56 | 2.83 | 4.04 | 4.24; 1.81; 1.40; 0.98 | |
| I− | 7.60 | 7.46 | 2.80 | 3.98 | 4.18; 1.80; 1.39; 0.94 | |
| PF6− | 7.46 | 7.30 | 2.70 | 3.90 | 4.11; 1.79; 1.40; 0.96 | |
| BF4− | 7.40 | 7.27 | 2.68 | 3.88 | 4.10; 1.79; 1.40; 0.97 | |
| CF3SO3− | 7.32 | 7.22 | 2.66 | 3.86 | 4.09; 1.80; 1.40; 0.97 | |
| NCS− | 7.43 | 7.32 | 2.77 | 3.96 | 4.17; 1.83; 1.43; 0.98 | |
| Ph4B− | 6.38 | 6.28 | 2.39 | 2.98 | 3.36; 1.52; 1.25; 0.92 | 7.46; 6.99; 6.83 |
| Ph4B−c | 7.63 | 7.60 | 2.56 | 3.73 | 4.09; 1.68; 1.29; 0.90 | 7.17; 6.92; 6.78 |
| Ibu− | 7.30 | 7.07 | 2.37 | 3.57 | 3.88; 1.56; 1.22; 0.85 | 7.23; 6.94; 3.45; 2.33; 1.73; 1.33; 0.81 |
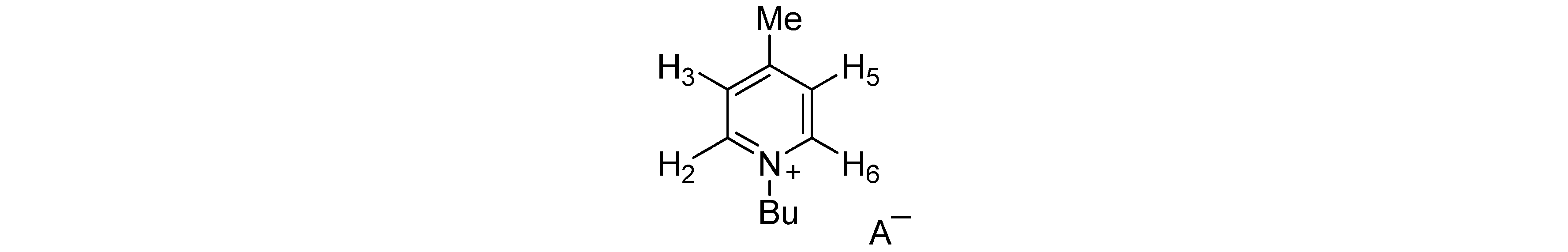
| Anion | H-2,6 | H-3,5 | Me | Bu | A− |
|---|---|---|---|---|---|
| AcO− | 9.35 | 7.82 | 2.62 | 4.82; 1.96; 1.35; 0.94 | 1.96 |
| BzO− | 8.94 | 7.70 | 2.47 | 4.67; 1.82; 1.25; 0.83 | 8.00; 7.31 |
| (S)-Lactate− | 9.05 | 7.81 | 2.57 | 4.65; 1.88; 1.35; 0.87 | 3.89; 1.26 |
| MeSO3− | 9.09 | 7.83 | 2.57 | 4.65; 1.91; 1.32; 0.87 | 2.68 |
| Bu2PO4− | 9.36 | 7.83 | 2.53 | 4.72; 1.89; 1.30; 0.83 | 3.78; 1.50; 1.30; 0.83 |
| I− | 9.24 | 7.90 | 2.66 | 4.84; 2.00; 1.41; 0.95 | |
| PF6− | 8.60 | 7.80 | 2.66 | 4.54; 1.95; 1.39; 0.95 | |
| BF4− | 8.73 | 7.82 | 2.66 | 4.60; 1.95; 1.39; 0.95 | |
| CF3SO3− | 8.80 | 7.82 | 2.65 | 4.60; 1.94; 1.38; 0.94 | |
| NCS− | 8.94 | 7.91 | 2.70 | 4.77; 2.03; 1.44; 0.99 |
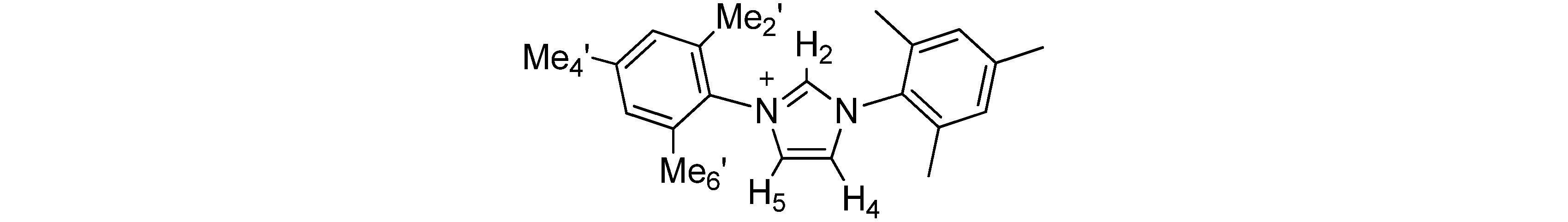
| Anion | H-2 | H-4,5 | Me-2',6' | Me-4' | H-3' | A− | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AcO− | 11.54 | 7.46 | 2.20 | 2.35 | 7.04 | 2.16 | ||||||
| BzO− | 11.03 | 7.44 | 2.07 | 2.25 | 6.87 | 7.63; 7.14 | ||||||
| (S)-lactate− | 10.31 | 7.56 | 2.10 | 2.32 | 7.00 | 3.65; 1.04 | ||||||
| MeSO3− | 9.83 | 7.63 | 2.09 | 2.31 | 6.98 | 2.31 | ||||||
| Bu2PO4− | 10.76 | 7.67 | 2.12 | 2.30 | 6.97 | 3.43; 1.32; 1.20; 0.79 | ||||||
| Cl− | 10.98 | 7.57 | 2.20 | 2.34 | 7.03 | |||||||
| PF6− | 8.77 | 7.57 | 2.14 | 2.37 | 7.07 | |||||||
| BF4− | 9.19 | 7.57 | 2.09 | 2.32 | 6.99 | |||||||
| CF3SO3− | 9.29 | 7.57 | 2.09 | 2.34 | 7.01 | |||||||
| SCN− | 9.70 | 7.63 | 2.19 | 2.37 | 7.08 | |||||||
| Ph4B− | 6.32 | 7.06 | 2.02 | 2.20 | 6.77 | 7.30; 6.88; 6.77 | ||||||
| Ph4B−b | 9.64 | 8.25 | 2.11 | 2.35 | 7.20 | 7.18; 6.92; 6.78 | ||||||
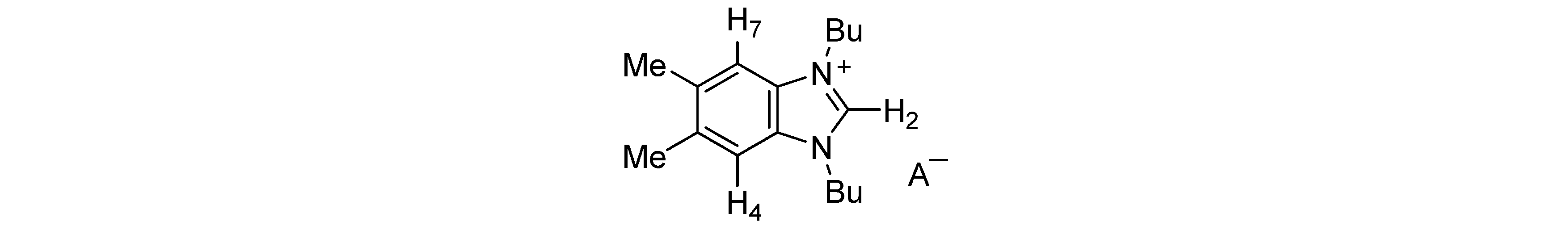
| Anion | H-2 | H-4,7 | Me | Bu | A− |
|---|---|---|---|---|---|
| AcO− | 11.86 | 7.37 | 2.46 | 4.55; 1.96; 1.42; 0.97 | 2.03 |
| BzO− | 11.91 | 7.37 | 2.45 | 4.56; 2.00; 1.41; 0.93 | 8.11; 7.34 |
| (S)-lactate− | 11.39 | 7.36 | 2.43 | 4.49; 1.92; 1.37; 0.93 | 4.03; 1.37 |
| MeSO3− | 10.63 | 7.40 | 2.47 | 4.53; 1.98; 1.44; 0.99 | 2.84 |
| Bu2PO4− | 11.52 | 7.36 | 2.45 | 4.57; 1.96; 1.41; 0.97 | 3.90; 1.62; 1.41; 0.90 |
| I− | 10.98 | 7.43 | 2.46 | 4.55; 2.02; 1.46; 0.99 | |
| PF6− | 9.25 | 7.43 | 2.48 | 4.41; 1.97; 1.43; 0.99 | |
| BF4− | 9.33 | 7.48 | 2.45 | 4.43; 1.94; 1.40; 0.94 | |
| CF3SO3− | 9.86 | 7.42 | 2.47 | 4.48; 1.97; 1.43; 0.98 | |
| SCN− | 10.13 | 7.43 | 2.48 | 4.53; 2.02; 1.47; 1.00 |
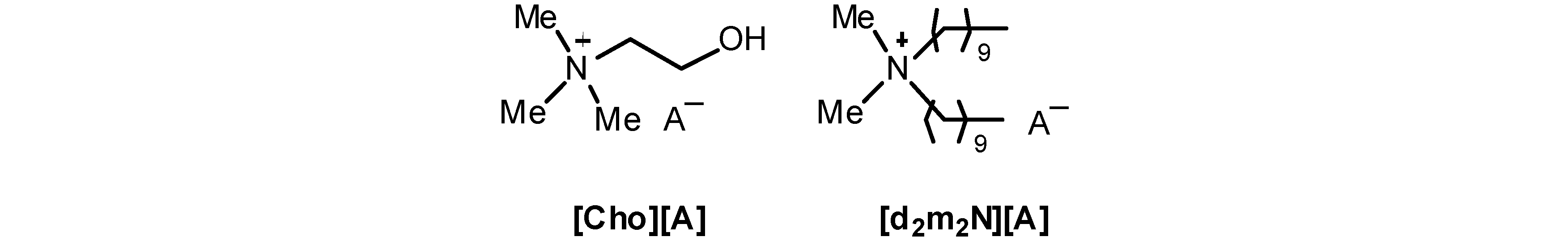
| Cation | Anion | Solvent | Me | N+-CH2-CH2-OH | A− |
|---|---|---|---|---|---|
| Cho | I− | CD3CN | 3.12 | 3.95; 3.41; 3.59(OH) | – |
| ( S)-Lactate− | CD3CN | 3.13 | 3.95; 3.43; 3.67(OH) | 3.78; 1.19 | |
| N+-CnHn+1 | |||||
| d2m2N | Br− | CDCl3 | 3.41 | 3.51; 1.65; 1.30; 0.88 | – |
| Ibu− | CDCl3 | 3.01 | 3.10; 1.52; 1.26; 0.88 | 7.30; 7.00; 3.57;2.39; 1.81; 1.42; 0.88 |
4. Conclusions
Acknowledgments
References and Notes
- Sowmiah, S.; Srinivasadesikan, V.; Tseng, M.-C.; Chu, Y.-H. On the chemical stabilities of ionic liquids. Molecules 2009, 14, 3780–3813. [Google Scholar]
- MacFarlane, D.R.; Pringle, J.M.; Howlett, P.C.; Forsyth, M. Ionic liquids and reactions at the electrochemical interface. Phys. Chem. Chem. Phys. 2010, 12, 1659–1669. [Google Scholar]
- Dupont, J.; Scholten, J.D. On the structural and surface properties of transition-metal nanopartícules in ionic liquids. Chem. Soc. Rev. 2010, 39, 1780–1804. [Google Scholar]
- Hallett, J.P.; Welton, T. Room-temperature ionic liquids: Solvents for synthesis and catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef]
- le Bideau, J.; Viau, L.; Vioux, A. Ionogels, ionic liquid based hybrid materials. Chem. Soc. Rev. 2011, 40, 907–925. [Google Scholar]
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar]
- Stoimenovski, J.; MacFarlane, D.R.; Bica, K.; Rogers, R.D. Crystalline vs. ionic liquid salt forms of active pharmaceutical ingredients: A position paper. Pharm. Res. 2010, 27, 521–526. [Google Scholar] [CrossRef]
- Deetlefs, M.; Seddon, K.R. Assessing the greenness of some typical laboratory ionic liquid preparations. Green Chem. 2010, 12, 17–30. [Google Scholar]
- Yau, H.M.; Chan, S.J.; George, S.R.D.; Hook, J.M.; Croft, A.K.; Harper, J.B. Ionic liquids: Just molten salts after all? Molecules 2009, 14, 2521–2534. [Google Scholar] [CrossRef]
- Petkovic, M.; Seddon, K.R.; Rebelo, L.P.N.; Pereira, C.S. Ionic liquids: A pathway to environmental acceptability. Chem. Soc. Rev. 2011, 40, 1383–1403. [Google Scholar]
- Sun, N.; Rodríguez, H.; Rahmana, M.; Rogers, R.D. Where are ionic liquid strategies most suited in the pursuit of chemicals and energy from lignocellulosic biomass? Chem. Commun. 2011, 47, 1405–1421. [Google Scholar]
- Alcalde, E.; Dinarès, I.; Mesquida, N. Imidazolium-based receptors. Top. Heterocycl. Chem. 2010, 24, 267–300. [Google Scholar]
- Clare, B.; Sirwardana, A.; MacFarlane, D.R. Synthesis and purification of ionic liquids. Top. Curr. Chem. 2009, 290, 1–40. [Google Scholar]
- Olivier-Bourbigou, H.; Magna, L.; Morvan, D. Ionic liquids and catalysis: Recent progress from knowledge to applications. Appl. Catal. A 2010, 373, 1–56. [Google Scholar]
- Smiglak, M.; Hines, C.C.; Rogers, R.D. New hydrogen carbonate precursors for efficient and byproduct-free syntheses of ionic liquids based on 1,2,3-trimethylimidazolium and N,N-dimethylpyrrolidinium cores. Green Chem. 2010, 12, 491–501. [Google Scholar] [CrossRef]
- Nockemann, P.; Thijs, B.; Driesen, K.; Janssen, C.R.; van Hecke, K.; van Meervelt, L.; Kossmann, S.; Kirchner, B.; Binnemans, K. Choline saccharinate and choline acesulfamate: Ionic liquids with low toxicities. J. Phys. Chem. B 2007, 111, 5254–5263. [Google Scholar]
- Alcalde, E.; Dinarès, I.; Fayet, J.-P.; Vertut, M.-C.; Elguero, J. Synthesis of highly dipolar betaines: Pyridinium azolates. J. Chem. Soc. Chem. Commun. 1986, 734–735. [Google Scholar]
- Alcalde, E.; Alemany, M.; Pérez-García, L.; Rodríguez, M.L. Non-classical [14]metaheterophanes containing betaine units. Synthesis, NMR spectroscopy, and X-ray crystallography. J. Chem. Soc. Chem. Commun. 1995, 1239–1240. [Google Scholar]
- Alcalde, E.; Alemany, M.; Gisbert, M. A direct synthetic approach to novel quadrupolar [14] azolophanes. Tetrahedron 1996, 52, 15171–15188. [Google Scholar]
- Alcalde, E.; Alvarez-Rúa, C.; García-Granda, S.; García-Rodriguez, E.; Mesquida, N.; Pérez-García, L. Hydrogen bonded driven anion binding by dicationic [14] imidazoliophanes. Chem. Commun. 1999, 295–296. [Google Scholar]
- Alcalde, E.; Mesquida, N.; Vilaseca, M.; Alvarez-Rúa, C.; García-Granda, S. Imidazolium-based dicationic cyclophanes. Solid-state aggregates with unconventional (C-H)+···Cl− hydrogen bonding revealed by X-ray diffraction. Supramol. Chem. 2007, 19, 501–509. [Google Scholar] [CrossRef]
- Dinarès, I.; de Miguel, C.G.; Mesquida, N.; Alcalde, E. Bis(imidazolium)-calix[4]arene receptors for anion binding. J. Org. Chem. 2009, 74, 482–485. [Google Scholar]
- Ogihara, W.; Yoshizawa, M.; Ohno, H. Novel ionic liquids composed of only azole ions. Chem. Lett. 2004, 33, 1022–1023. [Google Scholar]
- Fukumoto, K.; Yoshizawa, M.; Ohno, H. Room temperature ionic liquids from 20 natural amino acids. J. Am. Chem. Soc. 2005, 127, 2398–2399. [Google Scholar]
- Fukaya, Y.; Iizuka, Y.; Sekikawa, K.; Ohno, H. Bio ionic liquids: Room temperature ionic liquids composed wholly of biomaterials. Green Chem. 2007, 9, 1155–1157. [Google Scholar]
- Ou, G.; Zhu, M.; Shea, J.; Yuan, Y. Ionic liquid buffers: A new class of chemicals with potential for controlling pH in non-aqueous media. Chem. Commun. 2006, 4626–4628. [Google Scholar]
- Lall, S.I.; Mancheno, D.; Castro, S.; Behaj, V.; Cohen, J.I.; Engel, R. Polycations. Part X. LIPs, a new category of room temperature ionic liquid based on polyammonium salts. Chem. Commun. 2000, 2413–2414. [Google Scholar]
- Golding, J.; Forsyth, S.; MacFarlane, D.R.; Forsyth, M.; Deacon, G.B. Methanesulfonate and p-toluenesulfonate salts of the N-methyl-N-alkylpyrrolidinium and quaternary ammonium cations: Novel low cost ionic liquids. Green Chem. 2002, 4, 223–229. [Google Scholar] [CrossRef]
- Machado, M.Y.; Dorta, R. Synthesis and characterization of chiral imidazolium salts. Synthesis 2005, 2473–2475. [Google Scholar]
- Nobuoka, K.; Kitaoka, S.; Kunimitsu, K.; Iio, M.; Harran, T.; Wakisaka, A.; Ishikawa, Y. Camphor ionic liquid: Correlation between stereoselectivity and cation-anion interaction. J. Org. Chem. 2005, 70, 10106–10108. [Google Scholar]
- Ruiz-Toral, A.; de los Ríos, A.P.; Hernández, F.J.; Janssen, M.H.A.; Schoevaart, R.; van Rantwijk, F.; Sheldon, R.A. Cross-linked Candida antarctica lipase B is active in denaturing ionic liquids. Enzyme Microb. Technol. 2007, 40, 1095–1099. [Google Scholar]
- Dinarès, I.; de Miguel, C.G.; Ibáñez, A.; Mesquida, N.; Alcalde, E. Imidazolium ionic liquids: A simple anion exchange protocol. Green Chem. 2009, 11, 1507–1510. [Google Scholar]
- Alcalde, E.; Dinarès, I.; Ibáñez, A.; Mesquida, N. A general halide-to-anion switch for imidazolium-based ionic liquids and oligocationic systems using anion exchange resins (A− form). Chem. Commun. 2011, 47, 3266–3268. [Google Scholar]
- Viau, L.; Tourné-Péteilh, C.; Devoisselle, J.-M.; Vioux, A. Ionogels as drug delivery system: One-step sol-gel synthesis using imidazolium ibuprofenate ionic liquid. Chem. Commun. 2010, 46, 228–230. [Google Scholar]
- Dupont, J.; Suarez, P.A.Z.; de Souza, R.F.; Burrow, R.A.; Kintzinger, J.-P. C-H—π interactions in 1-n-Butyl-3-methylimidazolium Tetraphenylborate molten salt: Solid and solution structures. Chem.-Eur. J. 2000, 6, 2377–2381. [Google Scholar]
- Yong, L.; Yao, M.-L.; Green, J.F.; Kelly, H.; Kabalka, G.W. Syntheses and characterization of polymer-supported organotrifluoroborates: Applications in radioiodination reactions. Chem. Commun. 2010, 46, 2623–2625. [Google Scholar]
- MacFarlane, D.R.; Pringle, J.M.; Johansson, K.M.; Forsyth, S.A.; Forsyth, M. Lewis base ionic liquids. Chem. Commun. 2006, 1905–1917. [Google Scholar]
- Rodríguez, H.; Gurau, G.; Holbreya, J.D.; Rogers, R.D. Reaction of elemental chalcogens with imidazolium acetates to yield imidazole-2-chalcogenones: Direct evidence for ionic liquids as proto-carbenes. Chem. Commun. 2011, 47, 3222–3224. [Google Scholar]
- Lee, K.M.; Lee, C.K.; Lin, I.J.B. First example of interdigitated U-shape benzimidazolium ionic liquid crystals. Chem. Commun. 1997, 899–900. [Google Scholar]
- Wang, K.F.; Jian, F.F.; Zhuang, R.R.; Xiao, H.L. New ionic liquids of N,N'-dialkylbenzimidazolium salt comprising copper(II) ions. Cryst. Growth Des. 2009, 9, 3934–3940. [Google Scholar] [CrossRef]
- Wang, K.F.; Jian, F.F.; Zhuang, R.R. A new ionic liquid comprising lanthanum(III) bulk-modified carbon paste electrode: Preparation, electrochemistry and electrocatalysis. Dalton Trans. 2009, 4532–4537. [Google Scholar]
- Zhuang, R.R.; Jian, F.F.; Wang, K.F. A new binuclear Cd(II)-containing ionic liquid: Preparation and electrocatalytic activities. J. Organomet. Chem. 2009, 694, 3614–3618. [Google Scholar]
- Arduengo, A.J., III. Looking for stable carbenes: The difficulty in starting anew. Acc. Chem. Res. 1999, 32, 913–921. [Google Scholar]
- Vijayaraghavan, R.; Thompson, B.C.; MacFarlane, D.R.; Kumar, R.; Surianarayanan, M.; Aishwarya, S.; Sehgal, P.K. Biocompatibility of choline salts as crosslinking agents for collagen based biomaterials. Chem. Commun. 2010, 46, 294–296. [Google Scholar]
- Hough, W.L.; Smiglak, M.; Rodríguez, H.; Swatloski, R.P.; Spear, S.K.; Daly, D.T.; Pernak, J.; Grisel, J.E.; Carliss, R.D.; Soutullo, M.D.; et al. The third evolution of ionic liquids: Active pharmaceutical ingredients. New J. Chem. 2007, 31, 1429–1436. [Google Scholar] [CrossRef]
- Clare, B.R.; Bayley, P.M.; Best, A.S.; Forsyth, M.; MacFarlane, D.R. Purification or contamination? The effect of sorbents on ionic liquids. Chem. Commun. 2008, 2689–2691. [Google Scholar]
- Burrell, A.K.; del Sesto, R.E.; Baker, S.N.; McCleskey, T.M.; Baker, G.A. The large scale synthesis of pure imidazolium and pyrrolidinium ionic liquids. Green Chem. 2007, 9, 449–454. [Google Scholar]
- Wang, J.; Cao, W.-F.; Su, J.-H.; Tian, H.; Huang, Y.-H.; Sun, Z.-R. Syntheses and nonlinear absorption of novel unsymmetrical cyanines. Dyes Pigm. 2003, 57, 171–179. [Google Scholar]
- Arduengo, A.J., III; Krafczyk, R.; Schmutzler, R. Imidazolylidenes, imidazolinylidenes and imidazolidines. Tetrahedron 1999, 55, 14523–l4534. [Google Scholar] [CrossRef]
- Sample Availability: Samples of all compounds are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Alcalde, E.; Dinarès, I.; Ibáñez, A.; Mesquida, N. A Simple Halide-to-Anion Exchange Method for Heteroaromatic Salts and Ionic Liquids. Molecules 2012, 17, 4007-4027. https://doi.org/10.3390/molecules17044007
Alcalde E, Dinarès I, Ibáñez A, Mesquida N. A Simple Halide-to-Anion Exchange Method for Heteroaromatic Salts and Ionic Liquids. Molecules. 2012; 17(4):4007-4027. https://doi.org/10.3390/molecules17044007
Chicago/Turabian StyleAlcalde, Ermitas, Immaculada Dinarès, Anna Ibáñez, and Neus Mesquida. 2012. "A Simple Halide-to-Anion Exchange Method for Heteroaromatic Salts and Ionic Liquids" Molecules 17, no. 4: 4007-4027. https://doi.org/10.3390/molecules17044007
APA StyleAlcalde, E., Dinarès, I., Ibáñez, A., & Mesquida, N. (2012). A Simple Halide-to-Anion Exchange Method for Heteroaromatic Salts and Ionic Liquids. Molecules, 17(4), 4007-4027. https://doi.org/10.3390/molecules17044007