18F-labeled Pyrazolo[1,5-a]pyrimidine Derivatives: Synthesis from 2,4-Dinitrobenzamide and Tosylate Precursors and Comparative Biological Evaluation for Tumor Imaging with Positron Emission Tomography
Abstract
:1. Introduction

2. Results and discussion
2.1. Chemistry

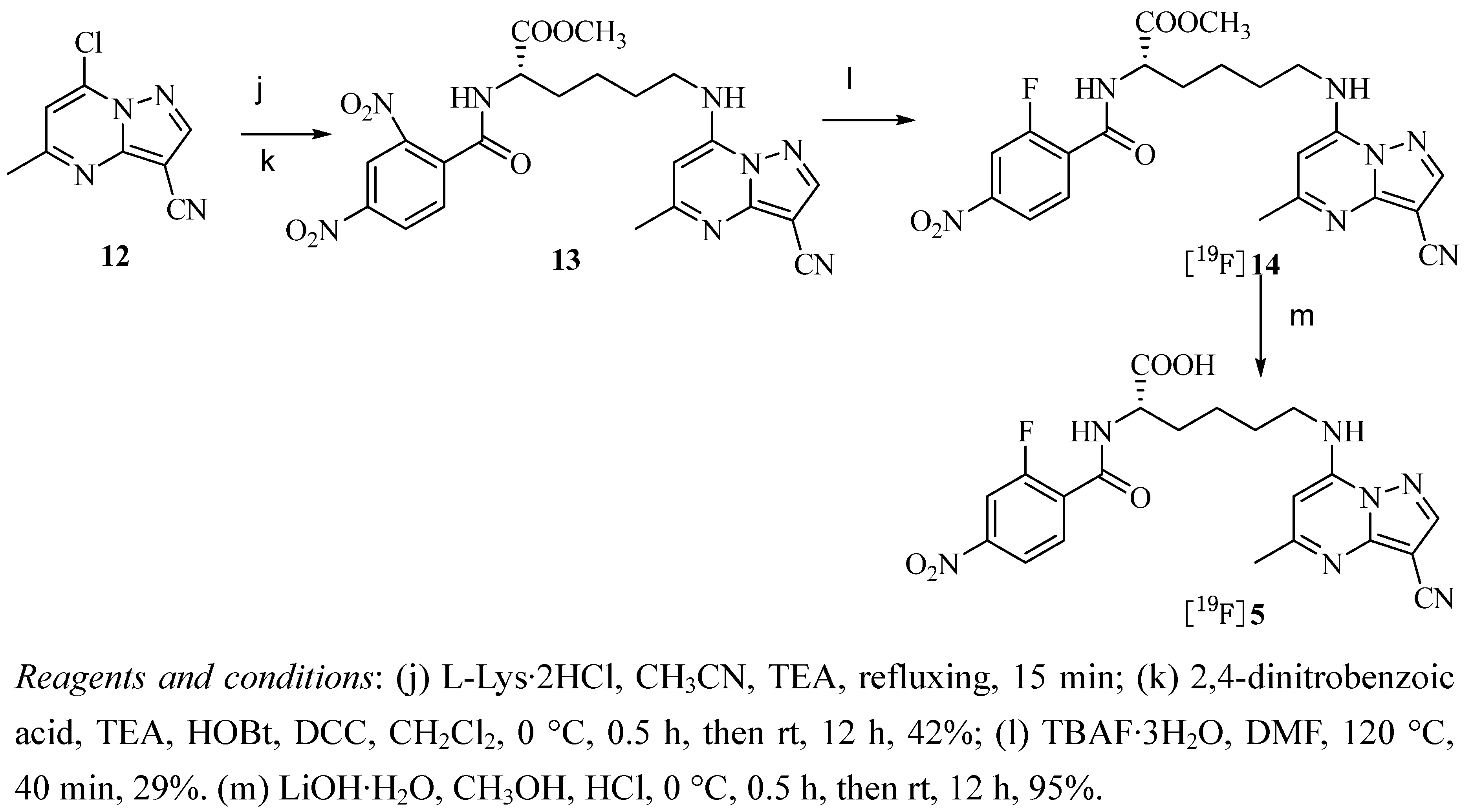
2.2. Radiochemistry
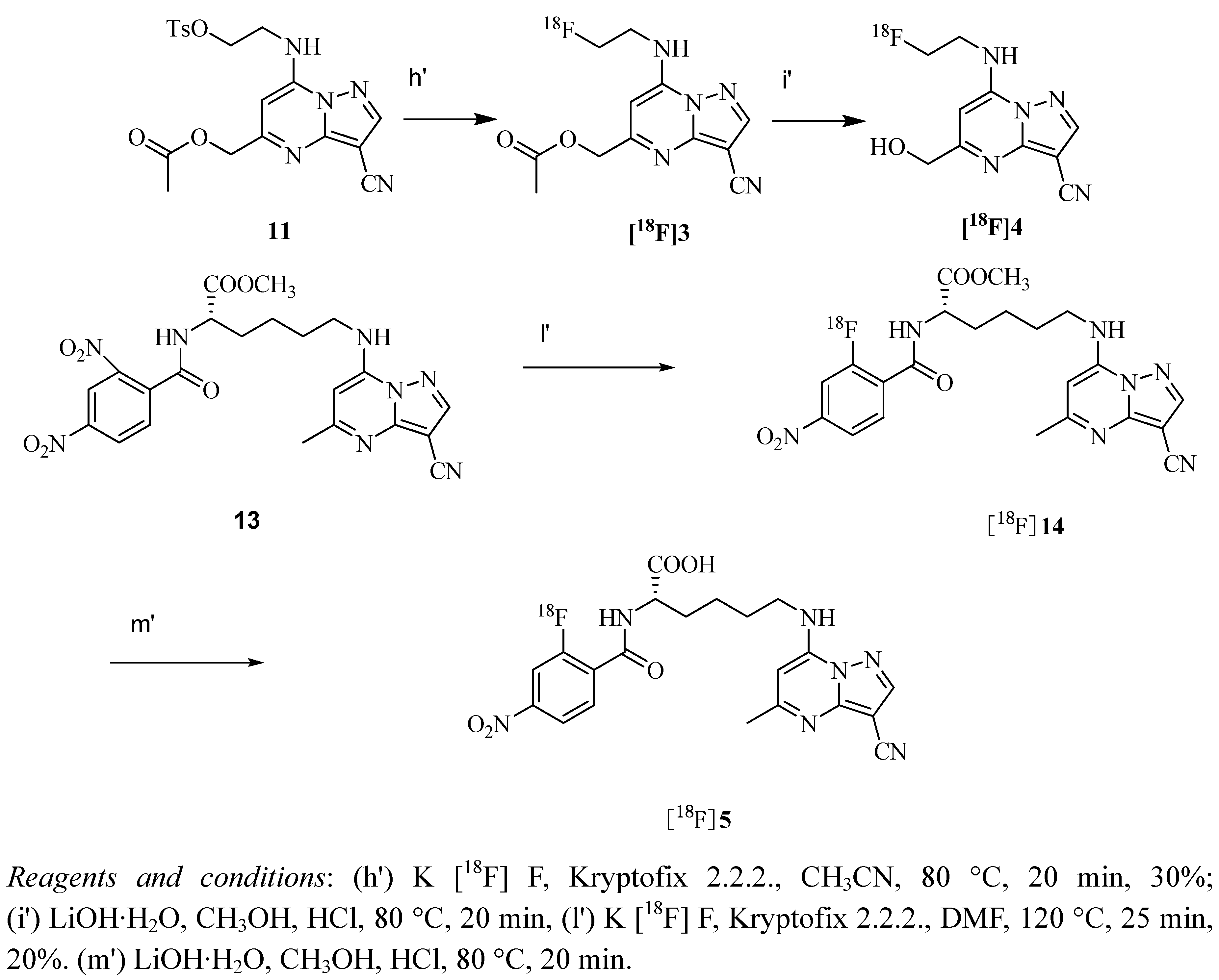
2.3. Measurement of Partition Coefficient
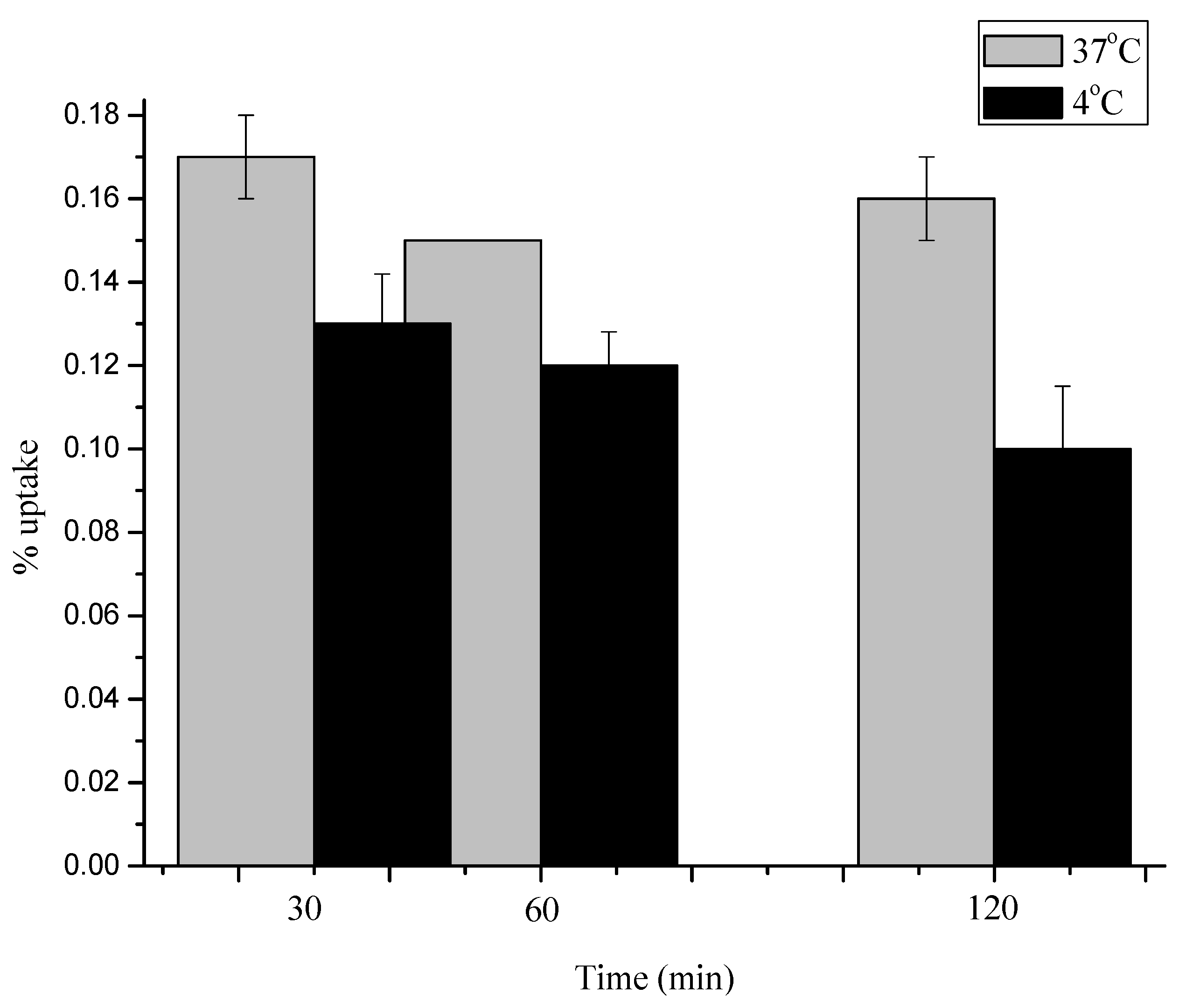
2.4. Stability of [18F]3, [18F]4 and [18F]5
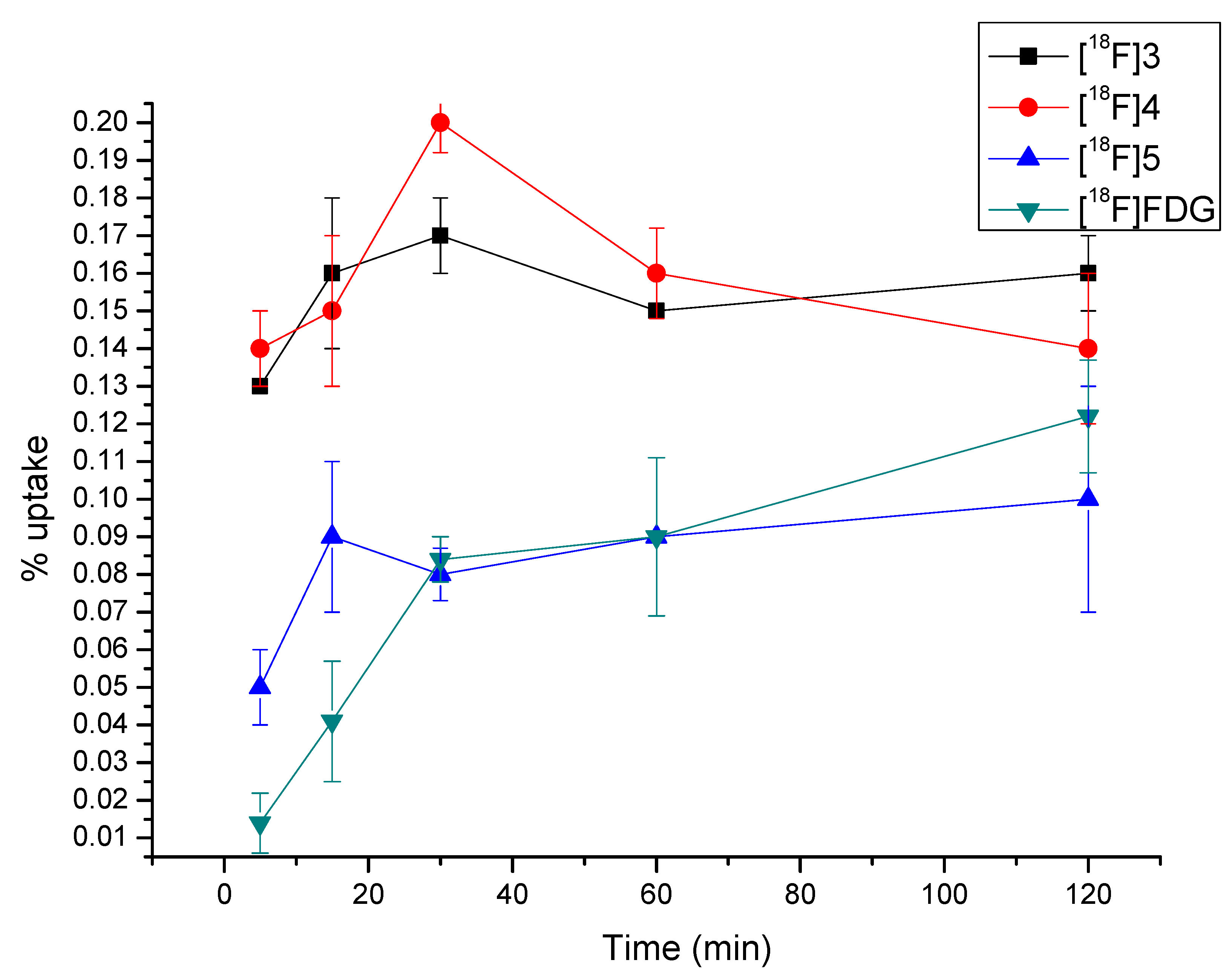
2.5. Cellular Accumulation of [18F]3, [18F]4 and [18F]5
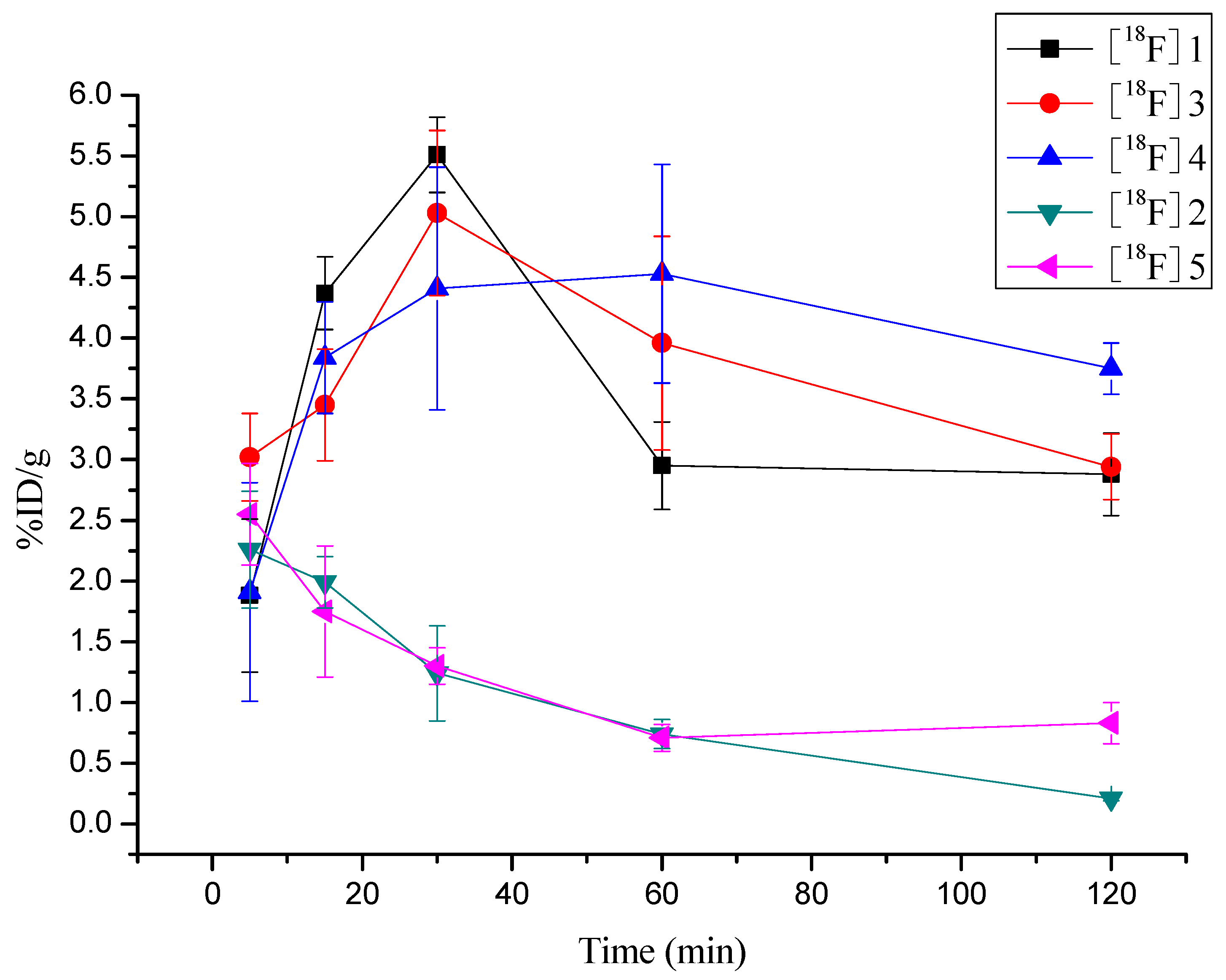
2.6. Biodistribution of [18F]3, [18F]4 and [18F]5 in S180 Bearing Mice
| Organs | Time (min) | ||||
|---|---|---|---|---|---|
| 5 | 15 | 30 | 60 | 120 | |
| Heart | 6.97 ± 0.47 | 5.08 ± 1.50 | 4.78 ± 0.36 | 3.63 ± 0.65 | 2.50 ± 0.67 |
| Liver | 10.3 ± 0.44 | 6.03 ± 0.48 | 4.72 ± 0.81 | 2.97 ± 0.41 | 1.82 ± 0.079 |
| Spleen | 6.92 ± 0.95 | 4.02 ± 0.90 | 3.14 ± 0.096 | 3.09 ± 0.69 | 1.66 ± 0.080 |
| Lung | 6.21 ± 1.12 | 4.96 ± 1.08 | 4.02 ± 0.48 | 3.38 ± 0.38 | 1.88 ± 0.32 |
| Kidney | 9.36 ± 0.25 | 4.97 ± 0.66 | 4.26 ± 0.29 | 2.94 ± 0.43 | 2.08 ± 0.26 |
| Brain | 2.38 ± 0.17 | 2.30 ± 0.62 | 2.27 ± 0.69 | 2.18 ± 0.29 | 1.47 ± 0.14 |
| Muscle | 6.64 ± 0.91 | 4.02 ± 1.03 | 3.79 ± 0.31 | 3.59 ± 0.43 | 2.12 ± 0.69 |
| Blood | 6.09 ± 0.25 | 4.46 ± 0.18 | 4.43 ± 0.30 | 3.78 ± 0.56 | 2.59 ± 0.14 |
| Tumor | 3.02 ± 0.36 | 3.45 ± 0.46 | 5.03 ± 0.68 | 3.96 ± 1.10 | 2.94 ± 0.27 |
| Tumor/Brain | 1.27 | 1.50 | 2.21 | 1.82 | 2.00 |
| Tumor/Muscle | 0.45 | 0.86 | 1.33 | 1.10 | 1.39 |
| Tumor/Blood | 0.49 | 0.77 | 1.14 | 1.05 | 1.14 |
| Organs | Time (min) | ||||
|---|---|---|---|---|---|
| 5 | 15 | 30 | 60 | 120 | |
| Heart | 6.67 ± 0.92 | 4.20 ± 1.0 | 5.07 ± 0.96 | 4.09 ± 0.59 | 3.61 ± 1.1 |
| Liver | 10.7 ± 0.65 | 6.05 ± 0.56 | 4.61 ± 1.0 | 3.07 ± 0.35 | 2.32 ± 0.18 |
| Spleen | 6.97 ± 0.34 | 3.87 ± 0.13 | 4.33 ± 1.3 | 2.99 ± 0.66 | 2.34 ± 0.69 |
| Lung | 6.33 ± 0.87 | 4.06 ± 0.41 | 4.07 ± 0.18 | 3.24 ± 0.73 | 2.58 ± 0.30 |
| Kidney | 11.1 ± 1.4 | 5.86 ± 0.14 | 4.2 ± 0.52 | 3.03 ± 0.55 | 2.58 ± 0.48 |
| Brain | 1.75 ± 0.18 | 2.33 ± 0.26 | 2.95 ± 0.56 | 2.26 ± 0.49 | 1.6 ± 0.16 |
| Muscle | 6.48 ± 1.0 | 3.75 ± 0.32 | 4.19 ± 0.65 | 3.28 ± 0.71 | 1.54 ± 0.39 |
| Blood | 6.33 ± 0.45 | 4.91 ± 0.46 | 4.57 ± 0.59 | 3.64 ± 0.40 | 3.30 ± 0.22 |
| Tumor | 1.91 ± 1.2 | 3.84 ± 0.46 | 4.41 ± 1.3 | 4.53 ± 0.90 | 3.75 ± 0.21 |
| Tumor/Brain | 1.09 | 1.65 | 1.5 | 2.01 | 2.35 |
| Tumor/Muscle | 0.29 | 1.03 | 1.05 | 1.38 | 2.44 |
| Tumor/Blood | 0.30 | 0.78 | 0.96 | 1.25 | 1.14 |
| Organs | Time (min) | ||||
|---|---|---|---|---|---|
| 5 | 15 | 30 | 60 | 120 | |
| Heart | 3.02 ± 0.51 | 1.19 ± 0.21 | 0.97 ± 0.19 | 0.42 ± 0.07 | 0.52 ± 0.05 |
| Liver | 10.39 ± 1.86 | 2.90 ± 0.86 | 2.29 ± 0.38 | 1.79 ± 0.38 | 1.63 ± 0.22 |
| Spleen | 3.10 ± 0.12 | 2.76 ± 0.11 | 0.54 ± 0.07 | 0.55 ± 0.19 | 0.53 ± 0.02 |
| Lung | 16.34 ± 1.80 | 15.13 ±1.57 | 6.35 ± 1.28 | 2.45 ± 0.55 | 4.09 ± 0.19 |
| Kidney | 12.70 ± 0.90 | 3.71 ± 0.69 | 2.21 ± 0.29 | 0.94 ± 0.28 | 0.58 ± 0.01 |
| Brain | 0.22 ± 0.00 | 0.14 ± 0.00 | 0.15 ± 0.02 | 0.15 ± 0.00 | 0.14 ± 0.00 |
| Muscle | 2.15 ± 0.66 | 1.07 ± 0.35 | 0.49 ± 0.09 | 0.54 ± 0.00 | 0.18 ± 0.04 |
| Blood | 6.68 ± 0.05 | 2.24 ± 0.04 | 0.95 ± 0.10 | 0.45 ± 0.09 | 0.35 ± 0.02 |
| Tumor | 2.55 ± 0.42 | 1.75 ± 0.54 | 1.30 ± 0.15 | 0.71 ± 0.11 | 0.83 ± 0.17 |
| Tumor/Brain | 11.59 | 12.5 | 8.67 | 4.73 | 5.93 |
| Tumor/Muscle | 1.19 | 1.64 | 2.65 | 1.31 | 4.61 |
| Tumor/Blood | 0.38 | 0.78 | 1.37 | 1.58 | 2.37 |
2.7. MicroPET Imagings of [18F]3 in S180 Bearing Mouse

3. Experimental
3.1. General
3.2. Synthesis
3.3. Octanol/Water Partition Coefficient
3.4. Quality Control of Purified Radiotracers
3.5. Stability Study
3.6. Cellular Accumulation Studies
3.7. Biodistribution and MicroPET Imaging Studies in S180 Tumor Bearing Mice
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
References and Notes
- Yu, W.; McConathy, J.; Williams, L.; Camp, V.M.; Malveaux, E.J.; Zhang, Z.; Olson, J.J.; Goodman, M.M. Synthesis, Radiolabeling, and Biological Evaluation of (R)- and (S)-2-Amino-3-[18F]Fluoro-2-Methylpropanoic Acid (FAMP) and (R)- and (S)-3-[18F]Fluoro-2-Methyl-2-N-(Methylamino)propanoic Acid (NMeFAMP) as Potential PET Radioligands for Imaging Brain Tumors. J. Med. Chem. 2010, 53, 876–886. [Google Scholar]
- Wood, K.A.; Hoskin, P.J.; Saunders, M.I. Positron Emission Tomography in Oncology: A Review. Clin. Oncol. 2007, 19, 237–255. [Google Scholar]
- George, C.F.P. Pyrazolopyrimidines. Lancet 2001, 357, 1623–1626. [Google Scholar]
- Stefano, A.; Anna, A.; Maurizio, B.; Alessandra, T.Z.; Francisco, O.; Francesco, O.; Silvia, S.; Chiara, B.; Matilde, Y. Hit Identification and Biological Evaluation of Anticancer Pyrazolopyrimidines Endowed with Anti-inflammatory Activity. Chem. Med. Chem. 2010, 5, 1242–1246. [Google Scholar]
- Curran, K.J.; Verheijen, J.C.; Kaplan, J.; Richard, D.J.; Toral-Barza, L.; Hollander, I.; Lucas, J.; Ayral-Kaloustian, S.; Yu, K.; Zask, A. Pyrazolopyrimidines as highly potent and selective, ATP-competitive inhibitors of the mammalian target of rapamycin (mTOR): Optimization of the 1-substituent. Bioorg. Med. Chem. Lett. 2010, 20, 1440–1444. [Google Scholar]
- Zask, A.; Verheijen, J.C.; Curran, K.; Kaplan, J.; Richard, D.J.; Nowak, P.; Malwitz, D.J.; Brooijmans, N.; Bard, J.; Svenson, K.; et al. ATP-Competitive Inhibitors of the Mammalian Target of Rapamycin: Design and Synthesis of Highly Potent and Selective Pyrazolopyrimidines. J. Med. Chem. 2009, 52, 5013–5016. [Google Scholar]
- Manetti, F.; Santucci, A.; Locatelli, G.A.; Maga, G.; Spreafico, A.; Serchi, T.; Orlandini, M.; Bernardini, G.; Caradonna, N.P.; Spallarossa, A.; et al. Identification of a Novel Pyrazolo[3,4-d]pyrimidine Able To Inhibit Cell Proliferation of a Human Osteogenic Sarcoma in Vitro and in a Xenograft Model in Mice. J. Med. Chem. 2007, 50, 5579–5588. [Google Scholar]
- Schenone, S.; Bruno, O.; Ranise, A.; Bondavalli, F.; Brullo, C.; Fossa, P.; Mosti, L.; Menozzi, G.; Carraro, F.; Naldini, A.; et al. New pyrazolo[3,4-d]pyrimidines endowed with A431 antiproliferative activity and inhibitory properties of Src phosphorylation. Bioorg. Med. Chem. Lett. 2004, 14, 2511–2517. [Google Scholar]
- Traxler, P.; Bold, G.; Frei, J.; Lang, M.; Lydon, N.; Mett, H.; Buchdunger, E.; Meyer, T.; Mueller, M.; Furet, P. Use of a Pharmacophore Model for the Design of EGF-R Tyrosine Kinase Inhibitors: 4-(Phenylamino)pyrazolo[3,4-d]pyrimidines. J. Med. Chem. 1997, 40, 3601–3616. [Google Scholar]
- Ballell, L.; Field, R.A.; Chungc, G.A.C.; Youngc, R.J. New thiopyrazolo[3,4-d]pyrimidine derivatives as anti-mycobacterial agents. Bioorg. Med. Chem. Lett. 2007, 17, 1736–1740. [Google Scholar]
- Gudmundsson, K.S.; Johns, B.A.; Weatherhead, J. Pyrazolopyrimidines and pyrazolotriazines with potent activity against herpesviruses. Bioorg. Med. Chem. Lett. 2009, 19, 5689–5692. [Google Scholar]
- Rashad, A.E.; Hegab, M.I.; Abdel-Megeid, R.E.; Micky, J.A.; Abdel-Megeid, F.M.E. Synthesis and antiviral evaluation of some new pyrazole and fused pyrazolopyrimidine derivatives. Bioorg. Med. Chem. 2008, 16, 7102–7106. [Google Scholar]
- Frizzo, C.P.; Scapin, E.; Campos, P.T.; Moreira, D.N.; Martins, M.A.P. Molecular structure of pyrazolo[1,5-a]pyrimidines: X-ray diffractometry and theoretical study. J. Mol. Struct. 2009, 933, 142–147. [Google Scholar]
- Novinson, T.; Bhooshan, B.; Okabe, T.; Revankar, G.R.; Wilson, H.R.; Robins, R.K.; Senga, K. Novel heterocyclic nitrofurfural hydrazones. In Vivo antitrypanosomal activity. J. Med. Chem. 1976, 19, 512–516. [Google Scholar]
- Senga, K.; Novinson, T.; Wilson, H.R.; Robins, R.K. Synthesis and antischistosomal activity of certain pyrazolo[1,5-a]pyrimidines. J. Med. Chem. 1981, 24, 610–613. [Google Scholar]
- Di Grandi, M.J.; Berger, D.M.; Hopper, D.W.; Zhang, C.; Dutia, M.; Dunnick, A.L.; Torres, N.; Levin, J.I.; Diamantidis, G.; Zapf, C.W.; et al. Novel pyrazolopyrimidines as highly potent B-Raf inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 6957–6961. [Google Scholar]
- Ahmeda, O.M.; Mohamedc, M.A.; Ahmedb, R.R.; Ahmedd, S.A. Synthesis and anti-tumor activities of some new pyridines and pyrazolo[1,5-a]pyrimidines. Eur. J. Med. Chem. 2009, 44, 3519–3523. [Google Scholar]
- Heathcote, D.A.; Patel, H.; Kroll, S.H.B.; Hazel, P.; Periyasamy, M.; Alikian, M.; Kanneganti, S.K.; Jogalekar, A.S.; Scheiper, B.; Barbazanges, M.; et al. A Novel Pyrazolo[1,5-a]pyrimidine Is a Potent Inhibitor of Cyclin-Dependent Protein Kinases 1, 2, and 9, Which Demonstrates Antitumor Effects in Human Tumor Xenografts Following Oral Administration. J. Med. Chem. 2010, 53, 8508–8522. [Google Scholar]
- Fraley, M.E.; Rubino, R.S.; Hoffman, W.F.; Hambaugh, S.R.; Arrington, K.L.; Hungate, R.W.; Bilodeau, M.T.; Tebben, A.J.; Rutledge, R.Z.; Kendall, R.L.; et al. Optimization of a pyrazolo[1,5-a]pyrimidine class of KDR kinase inhibitors: Improvements in physical properties enhance cellular activity and pharmacokinetics. Bioorg. Med. Chem. Lett. 2002, 12, 3537–3541. [Google Scholar]
- Powell, D.; Gopalsamy, A.; Wang, Y.D.; Zhang, N.; Miranda, M.; McGinnisb, J.P.; Rabindranb, S.K. Pyrazolo[1,5-a]pyrimidin-7-yl phenyl amides as novel antiproliferative agents: Exploration of core and headpiece structure–activity relationships. Bioorg. Med. Chem. Lett. 2007, 17, 1641–1645. [Google Scholar]
- Gopalsamy, A.; Yang, H.; Ellingboe, J.W.; Tsou, H.-R.; Zhang, N.; Honores, E.; Powell, D.; Miranda, M.; McGinnisb, J.P.; Rabindranb, S.K. Pyrazolo[1,5-a]pyrimidin-7-yl phenyl amides as novel anti-proliferative agents: parallel synthesis for lead optimization of amide region. Bioorg. Med. Chem. Lett. 2005, 15, 1591–1594. [Google Scholar]
- Bejot, R.; Kersemans, V.; Kelley, C.; Carroll, L.; King, R.C.; Gouverneur, V. Pre-clinical evaluation of a 3-nitro-1,2,4-triazole analogue of [18F]FMISO as hypoxia-selective tracer for PET. Nucl. Med. Biol. 2010, 37, 565–575. [Google Scholar]
- Wang, W.; Guo, Q.; You, Q.; Zhang, K.; Yang, Y.; Yu, J.; Liu, W.; Zhao, L.; Gu, H.; Hu, Y.; et al. The Anticancer Activities of Wogonin in Murine Sarcoma S180 both in Vitro and in Vivo. Biol. Pharm. Bull. 2006, 29, 1132–1137. [Google Scholar]
- Du, H.; Cui, C.; Wang, L.; Liu, H.; Cui, G. Novel Tetrapeptide, RGDF, Mediated Tumor Specific Liposomal Doxorubicin (DOX) Preparations. Mol. Pharm. 2011, 8, 1224–1232. [Google Scholar]
- Sun, X.; Chu, T.; Wang, X. Preliminary studies of 99mTc-BnAO and its analogues: synthesis, radiolabeling and in Vitro cell uptake. Nucl. Med. Biol. 2010, 37, 117–123. [Google Scholar] [CrossRef]
- Wu, D.; Gao, Y.; Chen, L.; Qi, Y.; Kang, Q.; Wang, H.; Zhu, L.; Ye, Y.; Zhai, M. Anti-tumor effects of a novel chimeric peptide on S180 and H22 xenografts bearing nude mice. Peptides 2010, 31, 850–854. [Google Scholar]
- Xu, J.; Liu, H.; Li, G.; He, Y.; Ding, R.; Wang, X.; Feng, M.; Zhang, S.; Chen, Y.; Li, S.; et al. Synthesis and biological evaluation of novel F-18 labeled pyrazolo[1,5-a]pyrimidine derivatives: Potential PET imaging agents for tumor detection. Bioorg. Med. Chem. Lett. 2011, 21, 4736–4741. [Google Scholar]
- Evensa, N.; Mucciolib, G.G.; Houbrechtsa, N.; Lambertb, D.M.; Verbruggena, A.M.; Laerec, K.V.; Bormansa, G.M. Synthesis and biological evaluation of carbon-11- and fluorine-18-labeled 2-oxoquinoline derivatives for type 2 cannabinoid receptor positron emission tomography imaging. Nucl. Med. Biol. 2009, 36, 455–465. [Google Scholar]
- Li, J.; Zhao, Y.; Zhao, X.; Yuan, X.; Gong, P. Synthesis and Anti-tumor Activities of Novel Pyrazolo[1,5-a]pyrimidines. Arch. Pharm. Chem. Life Sci. 2006, 339, 593–597. [Google Scholar]
- Qiao, Y.; He, Y.; Zhang, S.; Li, G.; Liu, H.; Xu, J.; Wang, X.; Qi, C.; Peng, C. Synthesis and evaluation of novel F-18 labeled fluoroarylvaline derivatives: Potential PET imaging agents for tumor detection. Bioorg. Med. Chem. Lett. 2009, 19, 4873–4877. [Google Scholar]
- Dearling, J.L.J.; Lewis, J.S.; Mullen, G.E.D.; Rae, M.T.; Zweit, J.; Blower, P.J. Design of hypoxia-targeting radiopharmaceuticals: Selective uptake of copper-64 complexes in hypoxic cells in Vitro. Eur. J. Nucl. Med. 1998, 25, 788–792. [Google Scholar]
- Sample Availability: Samples of the compounds [19F]1-5 are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xu, J.; Liu, H.; Li, G.; He, Y.; Ding, R.; Wang, X.; Feng, M.; Zhang, S.; Chen, Y.; Li, S.; et al. 18F-labeled Pyrazolo[1,5-a]pyrimidine Derivatives: Synthesis from 2,4-Dinitrobenzamide and Tosylate Precursors and Comparative Biological Evaluation for Tumor Imaging with Positron Emission Tomography. Molecules 2012, 17, 3774-3793. https://doi.org/10.3390/molecules17043774
Xu J, Liu H, Li G, He Y, Ding R, Wang X, Feng M, Zhang S, Chen Y, Li S, et al. 18F-labeled Pyrazolo[1,5-a]pyrimidine Derivatives: Synthesis from 2,4-Dinitrobenzamide and Tosylate Precursors and Comparative Biological Evaluation for Tumor Imaging with Positron Emission Tomography. Molecules. 2012; 17(4):3774-3793. https://doi.org/10.3390/molecules17043774
Chicago/Turabian StyleXu, Jingli, Hang Liu, Guixia Li, Yong He, Rui Ding, Xiao Wang, Man Feng, Shuting Zhang, Yurong Chen, Shilei Li, and et al. 2012. "18F-labeled Pyrazolo[1,5-a]pyrimidine Derivatives: Synthesis from 2,4-Dinitrobenzamide and Tosylate Precursors and Comparative Biological Evaluation for Tumor Imaging with Positron Emission Tomography" Molecules 17, no. 4: 3774-3793. https://doi.org/10.3390/molecules17043774
APA StyleXu, J., Liu, H., Li, G., He, Y., Ding, R., Wang, X., Feng, M., Zhang, S., Chen, Y., Li, S., Zhao, M., Li, Y., Qi, C., & Dang, Y. (2012). 18F-labeled Pyrazolo[1,5-a]pyrimidine Derivatives: Synthesis from 2,4-Dinitrobenzamide and Tosylate Precursors and Comparative Biological Evaluation for Tumor Imaging with Positron Emission Tomography. Molecules, 17(4), 3774-3793. https://doi.org/10.3390/molecules17043774




