Synthesis and Antiradical/Antioxidant Activities of Caffeic Acid Phenethyl Ester and Its Related Propionic, Acetic, and Benzoic Acid Analoguesc
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
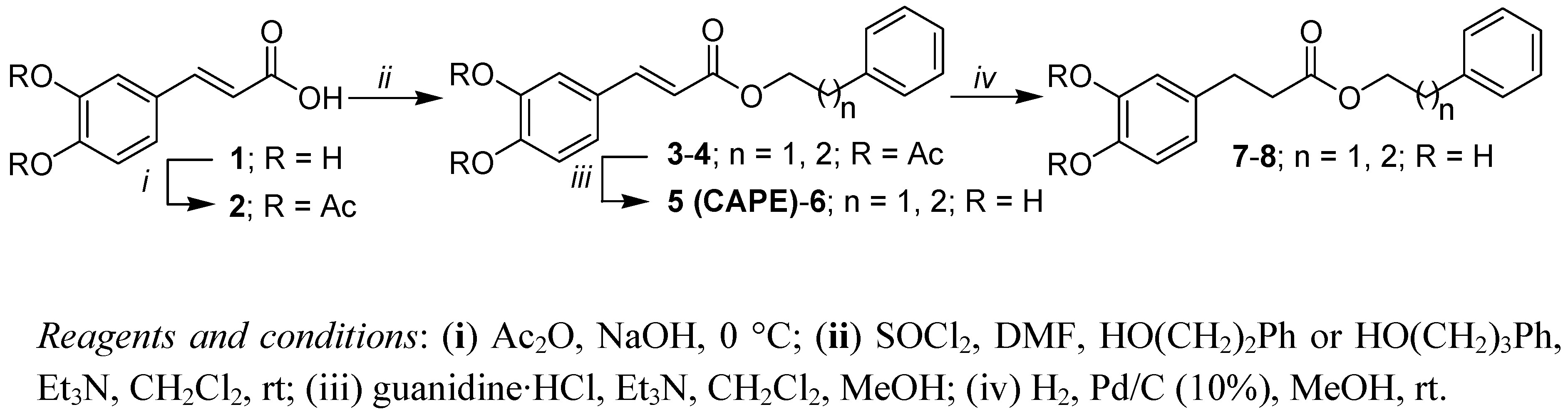


2.2. Free Radical Scavenging Activity
| Compound | IC50 (μM) | Compound | IC50 (μM) |
|---|---|---|---|
| 1 | 15.3 | 10 | 7.1 |
| 5 | 16.5 | 14 | 18.1 |
| 6 | 11.9 | 15 | 20.3 |
| 9 | 7.8 | 16 | 43.7 |
| 7 | 24.6 | 20 | 20.4 |
| 8 | 23 | 21 | 9.6 |
2.3. Antioxidant Activity
| Compound | IC50 (μM) | Compound | IC50 (μM) |
|---|---|---|---|
| 1 | 2.01 | 10 | 3.29 |
| 5 | 1.09 | 14 | 3.00 |
| 6 | 3.94 | 15 | 3.80 |
| 9 | 2.10 | 16 | 9.10 |
| 7 | 0.70 | 20 | 1.20 |
| 8 | 2.19 | 21 | 1.83 |
3. Experimental
3.1. General
3.2. Synthesis
3.3. In Vitro Antioxidant Activity
3.3.1. AAPH Assay
3.3.2. DPPH Assay
3.3.3. Data Analysis
4. Conclusions
Acknowledgments
References
- Newman, D.J. Natural Products as Sources of New Drugs over the Period 1981–2002. J. Nat. Prod. 2003, 66, 1022–1037. [Google Scholar] [CrossRef]
- Deshpande, S.S.; Sathe, S.K.; Salunkhe, D.K. Chemistry and safety of plant polyphenols. Adv. Exp. Med. Biol. 1984, 177, 457–495. [Google Scholar] [CrossRef]
- Harris, P.J.; Hartley, R.D. Detection of bound ferulic acid in cell walls of the gramineae by ultraviolet fluorescent microscopy. Nature 1976, 259, 508–510. [Google Scholar] [CrossRef]
- Hartley, R.D.; Morrison, W.H.; Himmelsbach, D.S.; Borneman, N.S. Cross-linking of cell wall phenolics to arabinoxylans in graminaceous plants. Phytochemistry 1990, 29, 3701–3709. [Google Scholar]
- Cuvelier, M.E.; Richard, H.; Berset, C. Comparison of the antioxidant activity of some acid phenols:structure-activity relationship. Biosci. Biotechnol. Biochem. 1992, 56, 324–325. [Google Scholar] [CrossRef]
- Maillard, M.N.; Soum, M.H.; Boivia, P.; Berset, C. Antioxidant activity of barley and malt: Relationship with phenolic content. Lebensm.-Wiss.-Technol. 1996, 3, 238–244. [Google Scholar]
- Friedman, M. Chemistry, biochemistry and dietary role of potato polyphenols: A review. J. Agric. Food Chem. 1997, 45, 1523–1540. [Google Scholar] [CrossRef]
- Michaluart, P.; Masferrer, J.L.; Carothers, A.M.; Subbaramaiah, K.; Zweifel, B.S.; Koboldt, C.; Mestre, J.R.; Grunberger, D.; Sacks, P.G.; Tanabe, T.; et al. Inhibitory Effects of Caffeic Acid Phenethyl Ester on the Activity and Expression of Cyclooxygenase-2 in Human Oral Epithelial Cells and in a Rat Model of Inflammation. Cancer Res. 1999, 59, 2347–2352. [Google Scholar]
- Liao, H.F.; Chen, Y.Y.; Liu, J.J.; Hsu, M.L.; Shieh, H.J.; Liao, H.J.; Shieh, C.J.; Shiao, M.S.; Chen, Y.J. Inhibitory effect of caffeic acid phenethyl ester on angiogenesis, tumor invasion and metastasis. J. Agric. Food Chem. 2003, 51, 7907–7912. [Google Scholar] [CrossRef]
- Baranowski, J.D.; Davidson, P.M.; Nagel, C.W.; Brannen, R.L. Inhibition of Saccharomyces cerevisiae by naturally occurring hydroxy cinnamates. J. Food Sci. 1980, 45, 592–594. [Google Scholar] [CrossRef]
- Natarajan, K.; Singh, S.; Burke, T.R., Jr.; Grunberger, D.; Aggarwal, B.B. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kB. Proc. Natl. Acad. Sci. USA 1996, 93, 9090–9095. [Google Scholar]
- Sugiura, M.; Naito, Y.; Yamaura, Y.; Fukaya, C.; Yokohama, K. Inhibitory activities and inhibition specificities of caffeic acid derivatives and related compounds toward 5-lipoxygenase. Chem. Pharm. Bull. 1989, 37, 1039–1043. [Google Scholar] [CrossRef]
- Toyoda, T.; Tsukamoto, T.; Takasu, S.; Shi, L.; Hirano, N.; Ban, H.; Kumagai, T.; Tatematsu, M. Anti-inflammatory effects of caffeic acid phenethyl ester (CAPE), a nuclear factor-kappaB inhibitor, on Helicobacter pylori-induced gastritis in Mongolian gerbils. Int. J. Cancer 2009, 125, 1786–1795. [Google Scholar] [CrossRef]
- Son, S.; Lewis, B.A. Free radical scavenging and antioxydative activity of caffeic acid amide and ester analogues: Structure-activity relationship. J. Food Agric. Chem. 2002, 50, 468–472. [Google Scholar] [CrossRef]
- Göçer, H.; Gülçin, I. Caffeic acid phenethyl ester (CAPE): Correlation of structure and antioxidant properties. Int. J. Food Sci. Nutr. 2011, 62, 821–825. [Google Scholar] [CrossRef]
- Jayaprakasam, B.; Vanisree, M.; Zhang, Y.; Dewitt, D.L.; Nair, M.G. Impact of alkyl esters of caffeic acid and ferulic acids on tumor cell proliferation, cyclooxygenase enzyme and lipid peroxidation. J. Agric. Food Chem. 2006, 54, 5375–5381. [Google Scholar] [CrossRef]
- Silva, F.A.M.; Borges, F.; Guimaraes, C.; Lima, J.L.F.C.; Matos, C.; Reis, S. Phenolic acids and derivatives: Studies on the relationship among structure, radical scavenging activity and physicochemical properties. J. Agric. Food Chem. 2000, 48, 2122–2126. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiao, B.; Chen, Q.; Lian, X.Y. Synthesis and biological evaluation of caffeic acid 3,4-dihydroxyphenethyl ester. J. Nat. Prod. 2010, 73, 252–254. [Google Scholar] [CrossRef]
- Tazaki, H.; Taguchi, D.; Hayashida, T.; Nabeta, K. Stable isotope-labeling studies on the oxidative coupling of caffeic acid via o-quinone. Biosci. Biotechnol. Biochem. 2001, 65, 2613–2621. [Google Scholar] [CrossRef]
- Saito, S.; Kurakane, S.; Seki, M.; Takai, E.; Kasai, T.; Kawabata, J. Radical scavenging activity of dicaffeoyloxycyclohexanes: Contribution of an intramolecular interaction of two caffeoyl residues. Bioorg. Med. Chem. 2005, 13, 4191–4199. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.L.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Akita, H.; Nozawa, M.; Mitsuda, A.; Ohsawa, H. A convenient synthesis of (+)-albicanol based on enzymatic function: total syntheses of (+)-albicanyl acetate, (−)-albicanyl 3,4-dihydroxycinnamate, (−)-drimenol, (−)-drimenin and (−)-ambrox. Tetrahedron: Asymmetry 2000, 11, 1375–1388. [Google Scholar]
- Touaibia, M.; Guay, M. Natural Product Total Synthesis in the Organic Laboratory: Total Synthesis of Caffeic Acid Phenethyl Ester (CAPE), A Potent 5-Lipoxygenase Inhibitor from Honeybee Hives. J. Chem. Educ. 2011, 88, 473–475. [Google Scholar] [CrossRef]
- Bruckner, R. Advanced Organic Chemistry: Reaction Mechanisms; Harcourt/Academic Press: San Diego, CA, USA, 2002; pp. 238–240. [Google Scholar]
- Magalhaes, L.M.; Segundo, M.A.; Reis, S.; Lima, J.F.L.C. Methodological aspects about in vitro evaluation of antioxidant properties. Anal. Chim. Acta 2008, 613, 1–19. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxydant activity of food constituents. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef]
- Boudreau, L.H.; Maillet, J.; LeBlanc, L.M.; Jean-François, J.; Touaibia, M.; Flamand, N.; Surette, M.E. Caffeic acid phenethyl ester and its amide analogue are potent inhibitors of leukotriene biosynthesis in human polymorphonuclear leukocytes. PLoS One 2012, 7, e31833. [Google Scholar]
- Lee, Y.; Shin, D.; Kim, J.H.; Hong, S.; Choi, D.; Kim, Y.J.; Kwak, M.K.; Jung, Y. Caffeic acid phenethyl ester-mediated Nfr-2 activation and IκB kinase inhibition are involved in NFκB inhibitory effect: structural analysis of NFκB inhibition. Eur. J. Pharmacol. 2010, 643, 21–28. [Google Scholar] [CrossRef]
- Liégeois, C.; Lermusieau, G.; Collin, C. Measuring antioxidant efficiency of wort, malt and hop against 2, 2'-azobis(2-amidopropane)-dihydrochloride-induced oxidation of an aqueous dispersion of linoleic acid. J. Agric. Food Chem. 2000, 48, 1129–1134. [Google Scholar] [CrossRef]
- Lin, C.F.; Chang, T.C.; Chiang, C.C.; Tsai, H.J.; Hsu, L.Y. Synthesis of selenium containing polyphenolic acid esters an evaluation of their effects on antioxydation and 5-lipoxygenase inhibition. Chem. Pharm. Bull. 2005, 53, 1402–1407. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 5–8, 14, 15, 20, 21 are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
LeBlanc, L.M.; Paré, A.F.; Jean-François, J.; Hébert, M.J.G.; Surette, M.E.; Touaibia, M. Synthesis and Antiradical/Antioxidant Activities of Caffeic Acid Phenethyl Ester and Its Related Propionic, Acetic, and Benzoic Acid Analoguesc. Molecules 2012, 17, 14637-14650. https://doi.org/10.3390/molecules171214637
LeBlanc LM, Paré AF, Jean-François J, Hébert MJG, Surette ME, Touaibia M. Synthesis and Antiradical/Antioxidant Activities of Caffeic Acid Phenethyl Ester and Its Related Propionic, Acetic, and Benzoic Acid Analoguesc. Molecules. 2012; 17(12):14637-14650. https://doi.org/10.3390/molecules171214637
Chicago/Turabian StyleLeBlanc, Luc M., Aurélie F. Paré, Jacques Jean-François, Martin J. G. Hébert, Marc E. Surette, and Mohamed Touaibia. 2012. "Synthesis and Antiradical/Antioxidant Activities of Caffeic Acid Phenethyl Ester and Its Related Propionic, Acetic, and Benzoic Acid Analoguesc" Molecules 17, no. 12: 14637-14650. https://doi.org/10.3390/molecules171214637
APA StyleLeBlanc, L. M., Paré, A. F., Jean-François, J., Hébert, M. J. G., Surette, M. E., & Touaibia, M. (2012). Synthesis and Antiradical/Antioxidant Activities of Caffeic Acid Phenethyl Ester and Its Related Propionic, Acetic, and Benzoic Acid Analoguesc. Molecules, 17(12), 14637-14650. https://doi.org/10.3390/molecules171214637






