Inhibitory Effects of Resveratrol Analogs on Mushroom Tyrosinase Activity
Abstract
:1. Introduction
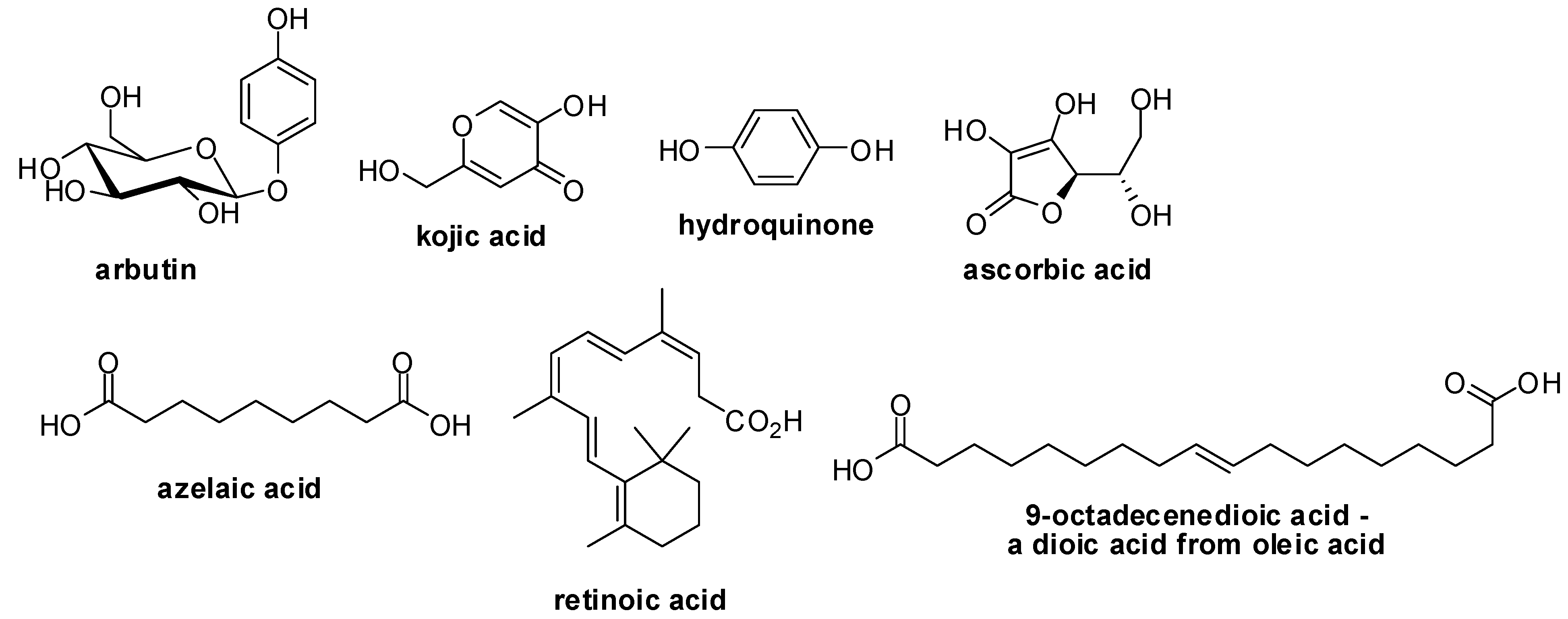
2. Results and Discussions
| Analogs (n = 6) | Chemical Structure | IC 60 min (%) | IC 120 min (%) | IC50 (µg/mL) |
|---|---|---|---|---|
| A |  | 46.43 a | 38.81 f | 44.89 ij |
| B |  | 46.69 a | 44.6 fg | 72.58 ij |
| C |  | 36.23 b | 31.20 f | 160.1 k |
| D |  | 78.16 c | 71.97 h | 28.66 ijl |
| E |  | 71.66 c | 68.49 h | 49.47 i |
| F |  | 59.88 d | 51.59 gh | 147.96 k |
| Kojic acid |  | 90.12 e | 75.92 h | 5.27 l |
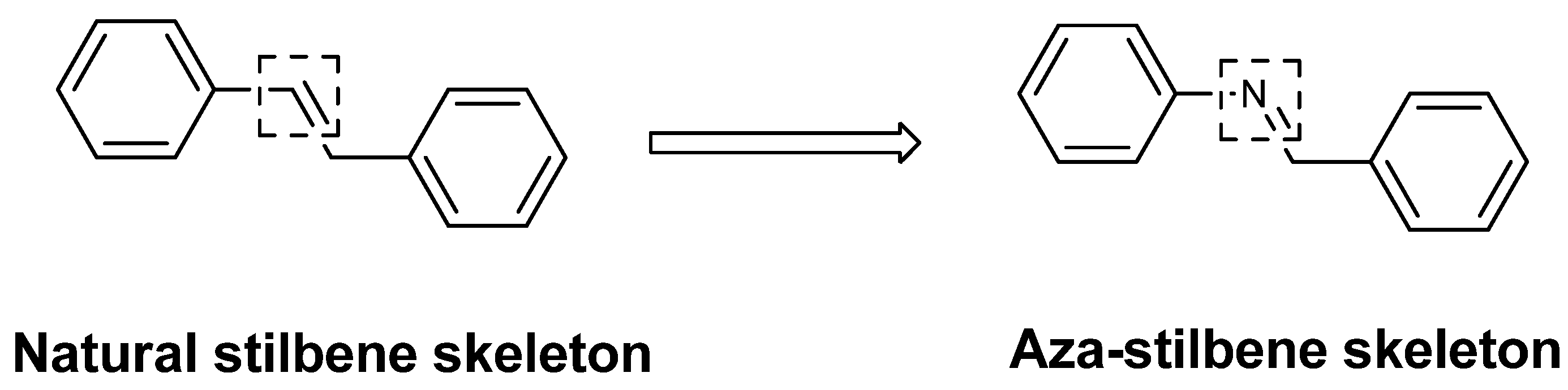
3. Experimental
3.1. Samples
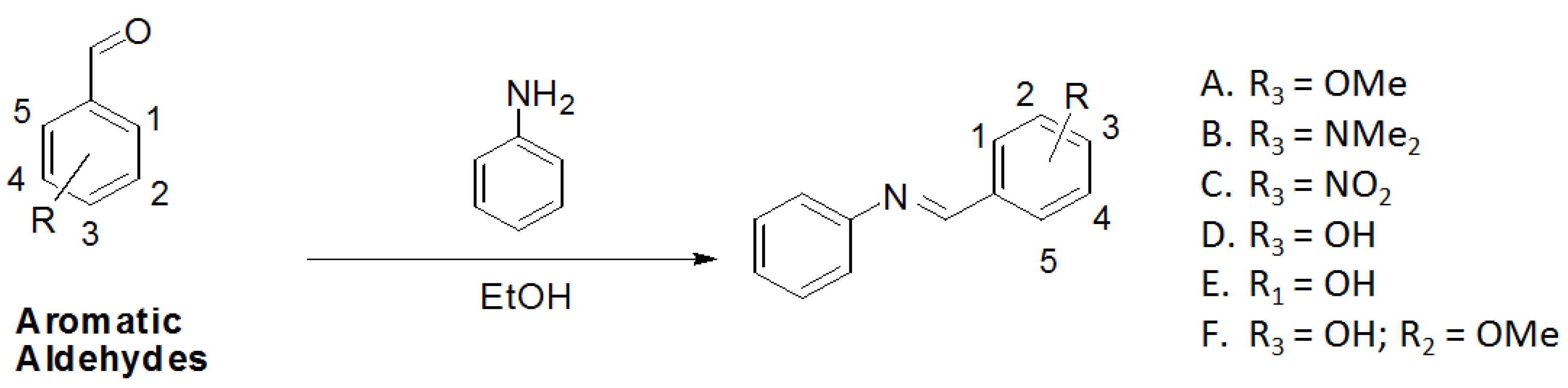
| Compounds | δ CH=N | δ C=N |  C=N C=N | Melting Point (°C) | Yield (%) |
|---|---|---|---|---|---|
| A | 8.51 | 159.8 | 1602 | 61.4–62.1 | 65.0 |
| B | 8.39 | 159.9 | 1600 | 96.8–97.3 | 72.0 |
| C | 8.80 | 158.8 | 1600 | 89.6–90.7 | 75.0 |
| D | 8.44 | 160.0 | 1602 | 89.2–90.7 | 74.0 |
| E | 8.96 | 163.5 | 1614 | 50.7–51.4 | 63.0 |
| F | 8.43 | 160.2 | 1622 | 53.1–54.2 | 63.0 |
3.2. Preparation of Samples
3.3. Test for Tyrosinase Inhibitory Activity
3.3.1. Tyrosinase Inhibition Qualitative Enzymatic Reaction Screening
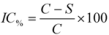
3.3.2. Tyrosinase Inhibition Quantitative Enzymatic Reaction Assay
3.4. Statistical Analysis
4. Conclusions
Acknowledgments
- Sample Availability: The samples of the compounds A-B are available from the authors.
References
- Adhikari, A.; Devkota, H.P.; Masuda, K.; Nakane, T.; Basnet, P.; Skalko-Basnet, N. Screening of Nepalese crude drugs traditionally used to treat hyperpigmentation: In vitro tyrosinase inhibition. Int. J. Cosmet. Sci. 2008, 30, 353–360. [Google Scholar] [CrossRef]
- Ding, H.Y.; Chang, T.S.; Shen, H.C.; Tai, S.S.K. Murine tyrosinase inhibitors from Cynanchum bungei and evaluation of in vitro and in vivo depigmenting activity. Exp. Dermatol. 2011, 20, 720–724. [Google Scholar] [CrossRef]
- Miot, L.D.B.; Silva, M.G.S.; Mior, H.A.; Marques, M.E.A. Fisiopatologia do melasma. An. Bras. Dermatol. 2009, 6, 623–635. [Google Scholar]
- Plensdorf, S.; Martinez, J. Common pigmentation Disorders. Am. Fam. Physician 2009, 79, 109–116. [Google Scholar]
- Fistarol, S.K.; Itin, P.H. Disorders of pigmentation. J. Dtsch. Dermatol. Ges. 2009, 8, 187–202. [Google Scholar]
- Scheinfeld, N.S. Melasma. Skinmed 2007, 6, 35–37. [Google Scholar] [CrossRef]
- Alchorne, M.M.A.; Abreu, M.A.M.M. Dermatologia na pele negra. An. Bras. Dermatol. 2008, 1, 7–20. [Google Scholar] [CrossRef]
- Urasaki, M.B.M. Skin physiological alterations perceived by pregnant women attended at public health services. Acta Pau. Enferm. 2010, 23, 519–525. [Google Scholar] [CrossRef]
- Alves, G.F.; Varella, T.C.N.; Nogueira, L.S.C. Dermatologia e Gestação. An. Bras. Dermatol. 2005, 80, 179–186. [Google Scholar] [CrossRef]
- Costa, A.; Cordero, T.; Marmirori, J.; Moiséis, T.A.; Alves, C.R.T. Associação de emblica, licorice e belides como alternativa à hidroquinona no tratamento clínico do melasma. An. Bras. Dermatol. 2010, 85, 613–620. [Google Scholar] [CrossRef]
- Sanches, J.A., Jr.; Brandt, H.R.C.; Moure, E.R.D.; Pereira, G.L.S.; Criado, P.R. Reações tegumentares adversas relacionadas aos agentes antineoplásicos—Parte I. An. Bras. Dermatol. 2010, 85, 425–437. [Google Scholar] [CrossRef]
- Baurin, N.; Arnoult, E.; Scior, T.; Do, Q.T.; Bernard, P. Preliminary screening of some tropical plants for anti-tyrosinase activity. J. Ethnopharmacol. 2002, 82, 155–158. [Google Scholar] [CrossRef]
- Bernard, P.; Berthon, J.Y. Resveratrol: An original mechanism on tyrosinase inhibition. Int. J. Cosmet. Sci. 2000, 22, 219–226. [Google Scholar] [CrossRef]
- Tirado-Sánchez, A.; Santamaría-Román, A.; Ponce-Olivera, R.M. Efficacy of dioic acid compared with hydroquinone in the treatment of melasma. Int. J. Dermatol. 2009, 48, 893–895. [Google Scholar] [CrossRef]
- Sato, M.E.O.; Gomara, F.; Pontarolo, R.; Andreazza, I.F.; Zaroni, M. Permeação cutânea in vitro do ácido kójico. Rev. Bras. Cienc. Farm. 2007, 43, 195–203. [Google Scholar] [CrossRef]
- Calaça, G.N.; Stets, S.; Nagata, N. Determinação simultânea de ácido kójico e hidroquinona por espectrofotometria visível e calibração multivariada. Quim. Nova 2011, 34, 630–635. [Google Scholar] [CrossRef]
- Sheth, V.M.; Pandya, A.G. Melasma: A comprehensive update—Part II. J. Am. Acad. Dermatol. 2011, 65, 699–714. [Google Scholar] [CrossRef]
- Cerqueira, F.M.; Medeiros, M.H.G.; Augusto, O. Antioxidantes dietéticos: Controvérsias e perspectivas. Quim. Nova 2007, 30, 441–449. [Google Scholar] [CrossRef]
- Chodurek, E.; Orchel, A.; Kurkiewicz, S.; Gawlik, N.; Dzierzewicz, Z.; Stepien, K. Evaluation of melanogenesis in A-375 cells in the presence of DMSO and analysis of pyrolitic profile of isolated melanin. ScientificWorldJournal 2012, 2012. [Google Scholar] [CrossRef]
- Holthoff, J.H.; Woodling, K.A.; Doerge, D.R.; Burns, S.T.; Hinson, J.A.; Mayeux, P.R. Resveratrol, a dietary polyphenolic phytoalexin, is a functional scavenger of peroxynitrite. Biochem. Pharmacol. 2010, 80, 1260–1265. [Google Scholar]
- Calil, O.N.; Carvalho, G.S.G.; Franco, D.C.Z.; Silva, A.D.; Raposo, N.B.R. Antioxidant activity of Resveratrol Analogs. Lett. Drug Des. Discov. 2012, 9, 8–11. [Google Scholar] [CrossRef]
- Mendes, J.B.E.; Riekes, M.R.; Oliveira, V.M.; Michel, M.D.; Stulzer, H.K.; Zawadzki, S.F.; Mainardes, R.M.; Farago, P.V. PHBV/PCL Microparticles for Controlled Release of Resveratrol: Physicochemical characterization, antioxidant potential, and effect on hemolysis of human erythrocytes. ScientificWorldJournal 2012, 2012. [Google Scholar] [CrossRef]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug. Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef]
- Fulda, S. Modulation of apoptosis by natural products for cancer therapy. Planta Med. 2010, 76, 1075–1079. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.J.; Lee, K.W. Naturally occurring phytochemicals for the prevention of Alzheimer’s disease. J. Neurochem. 2010, 112, 1415–1430. [Google Scholar] [CrossRef]
- Wang, Y.; Romigh, T.; He, X.; Orloff, M.S.; Silverman, R.H.; Heston, W.D.; Eng, C. Resveratrol regulates the PTEN/AKT pathway through androgen receptor-dependent and independent mechanisms in prostate cancer cell lines. Hum. Mol. Genet. 2010, 19, 4319–4329. [Google Scholar] [CrossRef]
- Kim, Y.M.; Yun, J.; Lee, C.K.; Lee, H.; Min, K.R.; Kim, Y. Oxyresveratrol and Hydroxystilbene Compounds: Inhibitory effect on tyrosinase and mechanism of action. J. Biol. Chem. 2002, 277, 16340–16344. [Google Scholar]
- Satooka, H.; Kubo, I. Resveratrol as a kcat type inhibitor for tyrosinase: Potentiated melanogenesis inhibitor. Bioorg. Med. Chem. 2012, 20, 1090–1099. [Google Scholar] [CrossRef]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef]
- Patani, G.A.; Lavoie, E.J. Bioisosterism: A rational approach in drug design. Chem. Rev. 1996, 96, 3147–3176. [Google Scholar] [CrossRef]
- Pavan, F.R.; de Carvalho, G.S.G.; da Silva, A.D.; Leite, C.Q.F. Synthesis and Anti-Mycobacterium tuberculosis Evaluation of Aza-Stilbene Derivatives. ScientificWorldJournal 2011, 11, 1113–1119. [Google Scholar] [CrossRef]
- Calil, N.O.; de Carvalho, G.S.G.; da Silva, A.F.; da Silva, A.D.; Raposo, N. R.B. Antioxidant Activity of Synthetic Resveratrol Analogs: A Structure-Activity Insight. Lett. Drug Des. Discov. 2012, 9, 676–679. [Google Scholar] [CrossRef]
- Rayne, S.; Goss, C.D.; Forest, K.; Friesen, K.J. Quantitative structure-activity relationship for estimating the aryl hydrocarbon receptors binding of resveratrol derivates and the antioxidant activities hydroxystilbenes. Med. Chem. Res. 2010, 19, 864–901. [Google Scholar] [CrossRef]
- Pannala, A.S.; Chan, S.T.; O’Brien, J.P.; Rice-Evans, A.C. Flavonoid B-ring chemistry and antioxidant activity: Fast reaction kinetics. Biochem. Biophys. Res. Commun. 2001, 282, 1161–1168. [Google Scholar] [CrossRef]
- Cheng, Z.; Ren, J.; Yuanzong, L.; Chang, W.; Chen, Z. Study on the multiples mechanisms underlying the reaction between hydroxyl radical and phenolic compounds by qualitative structure and activity relationship. Bioorg. Med. Chem. 2002, 10, 4067–4073. [Google Scholar] [CrossRef]
- Soares, S.E. Ácidos fenólicos como antioxidantes. Rev. Nutr. 2002, 15, 71–81. [Google Scholar]
- Scotti, L.; Scotti, M.T.; Cardoso, C.; Pauletti, P.; Castro-Gamboa, I.; Bolzani, V.S.; Velasco, M.V.R.; Menezes, C.M.S.; Ferreira, E.I. Modelagem molecular aplicada ao desenvolvimento de moléculas com atividade antioxidante visando ao uso cosmético. Rev. Bras. Cienc. Farm. 2007, 43, 153–166. [Google Scholar]
- Ramalho, V.C.; Jorge, N. Antioxidantes utilizados em óleos, gorduras e alimentos gordurosos. Quim. Nova 2006, 29, 755–760. [Google Scholar]
- Picardo, M.; Carrera, M. New and experimental treatments of chloasma and other hypermelanoses. Dermatol. Clin. 2007, 25, 353–362. [Google Scholar]
- Aspinall, H.C.; Greeves, N.; Hin, S.L. A new Yb3+-catalyzed pinacol and imine-coupling reaction. Tetrahedron Lett. 2010, 51, 1558–1561. [Google Scholar] [CrossRef]
- Cordes, E.H.; Jencks, W.P. Nucleophilic Catalysis of Semicarbazone Formation by Anilines. J. Am. Chem. Soc. 1962, 84, 826–831. [Google Scholar] [CrossRef]
- Ebara, N. Benzylideneaniline. III. Anils of Substituted Benzaldehydes. Bull. Chem. Soc. Jpn. 1961, 34, 1151–1158. [Google Scholar] [CrossRef]
- Stevens, J.B.; Pandit, U.K. NAD(P)H models-XVII: Metal ion catalyzed reduction of imines by 3,5-diethoxycarbonyl 2,6-dimethyl-1,4-dihydropyridine (Hantzsch ester). Tetrahedron 1983, 39, 1395–1400. [Google Scholar] [CrossRef]
- Rani, N.; Sharma, J.R.; Manrao, M.R. Synthesis and Comparative Fungitoxicity of Benzalbenzylamines and Benzalanilines. Pest. Res. J. 2006, 18, 129–132. [Google Scholar]
- Sekiya, M.; Morimoto, T. Decarboxylation Reactions. IV. Reaction of Schiff Bases with Trichloroacetic Anhydride. Chem. Pharm. Bull. 1975, 23, 2353–2357. [Google Scholar] [CrossRef]
- Macrini, D.J.; Suffredini, I.B.; Varella, A.D.; Younes, R.N.; Ohara, M.T. Extracts from Amazonian plants have inhibitory activity against tyrosinase: An in vitro evaluation. Braz. J. Pharm. Sci. 2009, 45, 715–721. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zimmermann Franco, D.C.; Gonçalves de Carvalho, G.S.; Rocha, P.R.; Da Silva Teixeira, R.; Da Silva, A.D.; Barbosa Raposo, N.R. Inhibitory Effects of Resveratrol Analogs on Mushroom Tyrosinase Activity. Molecules 2012, 17, 11816-11825. https://doi.org/10.3390/molecules171011816
Zimmermann Franco DC, Gonçalves de Carvalho GS, Rocha PR, Da Silva Teixeira R, Da Silva AD, Barbosa Raposo NR. Inhibitory Effects of Resveratrol Analogs on Mushroom Tyrosinase Activity. Molecules. 2012; 17(10):11816-11825. https://doi.org/10.3390/molecules171011816
Chicago/Turabian StyleZimmermann Franco, Danielle Cristina, Gustavo Senra Gonçalves de Carvalho, Paula Rafaela Rocha, Raquel Da Silva Teixeira, Adilson David Da Silva, and Nádia Rezende Barbosa Raposo. 2012. "Inhibitory Effects of Resveratrol Analogs on Mushroom Tyrosinase Activity" Molecules 17, no. 10: 11816-11825. https://doi.org/10.3390/molecules171011816





