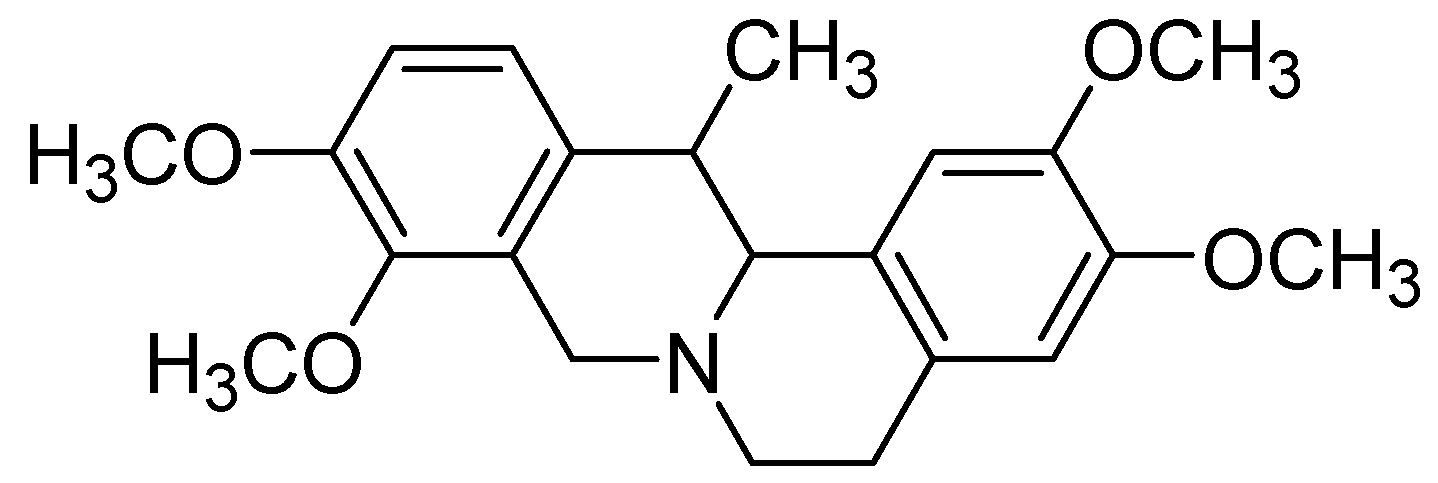
Corydaline Inhibits Multiple Cytochrome P450 and UDP-Glucuronosyltransferase Enzyme Activities in Human Liver Microsomes
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental
3.1. Materials and Reagents
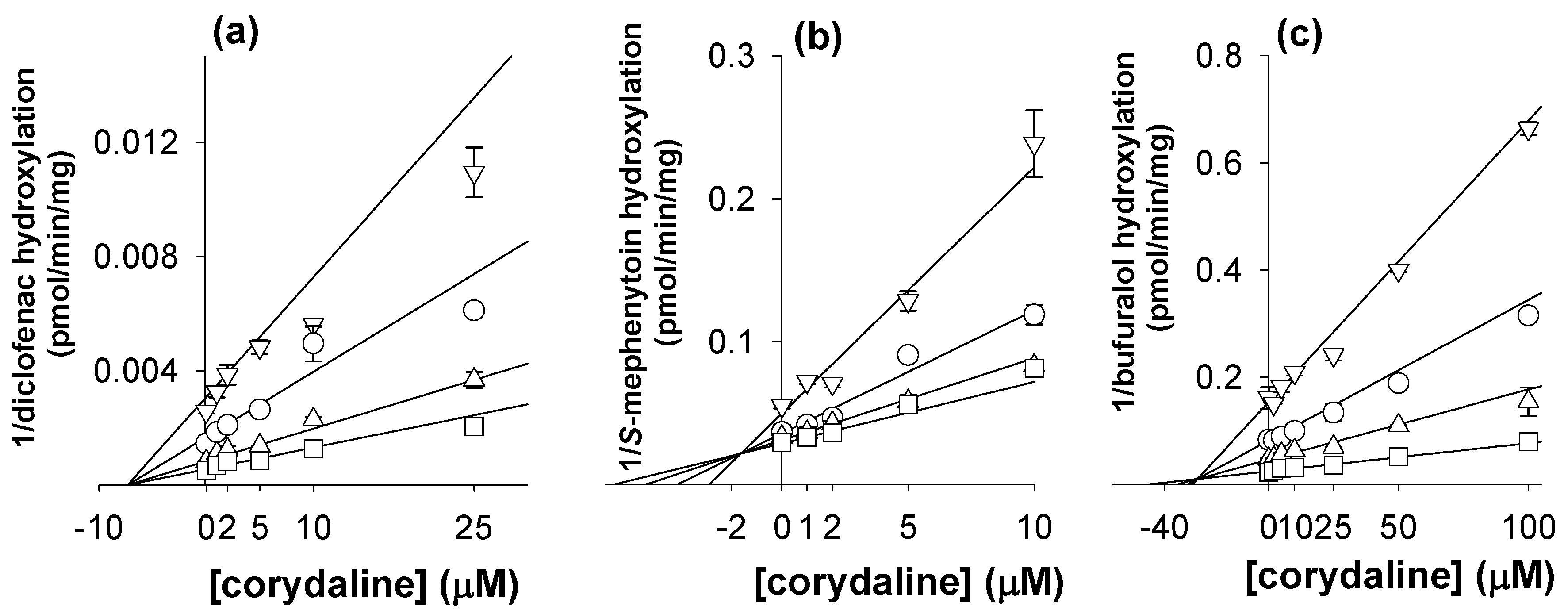
3.2. Inhibitory Effects of Corydaline on Seven Major CYP Activities in Human Liver Microsomes
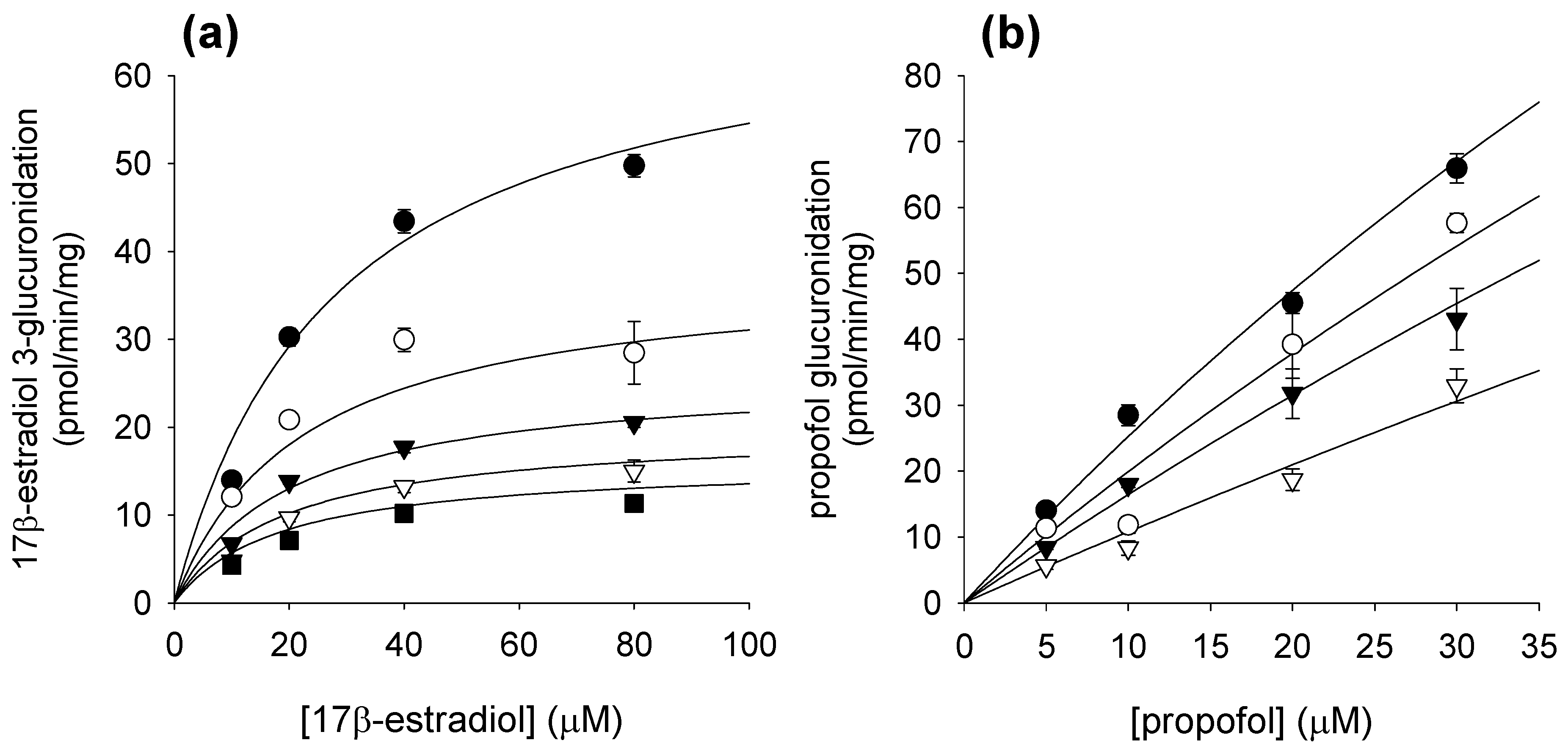
3.3. Inhibitory Effects of Corydaline on Four UGT Activities in Human Liver Microsomes
3.4. Kinetic Analysis
3.5. Data Analysis
4. Conclusions
Conflict of interest
Acknowledgements
References and Notes
- Matsuda, H.; Tokuoka, K.; Wu, J.; Tanaka, T.; Kubo, M. Inhibitory effects of methanolic extract from corydalis tuber against types I-IV allergic models. Biol. Pharm. Bull. 1995, 18, 963–967. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.S.; Mehendale, S.R.; Wang, C.Z.; Aung, H.H.; Jiang, T.; Guan, X.; Shoyama, Y. Effects of Corydalis yanhusuo and Angelicae dahuricae on cold pressor-induced pain in humans: A controlled trial. J. Clin. Pharmacol. 2004, 44, 1323–1327. [Google Scholar] [CrossRef] [PubMed]
- Kubo, M.; Matsuda, H.; Tokuoka, K.; Ma, S.; Shiomoto, H. Anti-inflammatory activities of methanolic extract and alkaloidal components from Corydalis tuber. Biol. Pharm. Bull. 1994, 17, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Adsersen, A.; Kjolbye, A.; Dall, O.; Jager, A.K. Acetylcholinesterase and butyrylcholinesterase inhibitory compounds from Corydalis cava Schweigg. & Kort. J. Ethnopharmacol. 2007, 113, 179–182. [Google Scholar] [PubMed]
- Berkov, S.; Bastida, J.; Nikolova, M.; Viladomat, F.; Codina, C. Rapid TLC/GC-MS identification of acetylcholinesterase inhibitors in alkaloid extracts. Phytochem. Anal. 2008, 19, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.T.; Peng, J.; Liang, Y.; Yang, J.; Bai, X.; Hao, X.Y.; Yang, F.M.; Sun, Q.Y. Acetylcholinesterase inhibitors from Corydalis yanhusuo. Nat. Prod. Res. 2010, 1–5. [Google Scholar]
- Saito, S.Y.; Tanaka, M.; Matsunaga, K.; Li, Y.; Ohizumi, Y. The combination of rat mast cell and rabbit aortic smooth muscle is the simple bioassay for the screening of anti-allergic ingredient from methanolic extract of Corydalis tuber. Biol. Pharm. Bull. 2004, 27, 1270–1274. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, S.; Fan, G.; Zou, H. Screening of antinociceptive components in Corydalis yanhusuo W.T. Wang by comprehensive two-dimensional liquid chromatography/tandem mass spectrometry. Anal. Bioanal. Chem. 2010, 396, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Son, M.; Kim, S.Y. Effects of corydaline from Corydalis tuber on gastric motor function in an animal model. Biol. Pharm. Bull. 2010, 33, 958–962. [Google Scholar] [CrossRef] [PubMed]
- Chang, J. Medicinal herbs: Drugs or dietary supplements? Biochem. Pharmacol. 2000, 59, 211–219. [Google Scholar] [CrossRef]
- Zhou, S.F.; Zhou, Z.W.; Li, C.G.; Chen, X.; Yu, X.; Xue, C.C.; Herington, A. Identification of drugs that interact with herbs in drug development. Drug Discov. Today 2007, 12, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.W.; Lin, L.C.; Tsai, T.H. Drug-drug interactions of silymarin on the perspective of pharmacokinetics. J. Ethnopharmacol. 2009, 121, 185–193. [Google Scholar] [CrossRef] [PubMed]
- He, S.M.; Yang, A.K.; Li, X.T.; Du, Y.M.; Zhou, S.F. Effects of herbal products on the metabolism and transport of anticancer agents. Expert Opin. Drug Metab. Toxicol. 2010, 6, 1195–1213. [Google Scholar] [CrossRef] [PubMed]
- He, S.M.; Chan, E.; Zhou, S.F. ADME properties of herbal medicines in humans: Evidence, challenges and strategies. Curr. Pharm. Des. 2011, 17, 357–407. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, K.B.; Ku, H.Y.; Park, S.J.; Choi, H.; Moon, J.K.; Park, B.S.; Kim, J.H.; Yea, S.S.; Lee, C.H.; et al. Identification and characterization of potent CYP2B6 inhibitors in Woohwangcheongsimwon suspension, an herbal preparation used in the treatment and prevention of apoplexy in Korea and China. Drug Metab. Dispos. 2008, 36, 1010–1015. [Google Scholar] [CrossRef] [PubMed]
- Kiang, T.K.; Ensom, M.H.; Chang, T.K. UDP-glucuronosyltransferases and clinical drug-drug interactions. Pharmacol. Ther. 2005, 106, 97–132. [Google Scholar] [CrossRef] [PubMed]
- Tucker, G.T.; Houston, J.B.; Huang, S.M. Optimizing drug development: Strategies to assess drug metabolism/transporter interaction potential-toward a consensus. Clin. Pharmacol. Ther. 2001, 70, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.G.; Dresser, G.K. Interactions between grapefruit juice and cardiovascular drugs. Am. J. Cardiovasc. Drugs 2004, 4, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.L.; Yu, H.L.; Li, D.; Meng, X.L.; Zhou, Z.Y.; Yu, Q.; Zhang, X.Y.; Wang, F.J.; Guo, C. In vitro inhibition of huanglian [Rhizoma coptidis (L.)] and its six active alkaloids on six cytochrome P450 isoforms in human liver microsomes. Phytother. Res. 2011. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, P.; Franklin, M.R. Human cytochrome p450 inhibition and metabolic-intermediate complex formation by goldenseal extract and its methylenedioxyphenyl components. Drug Metab. Dispos. 2003, 31, 1391–1397. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Tracy, T.S.; Remmel, R.P. Correlation between bilirubin glucuronidation and estradiol-3-gluronidation in the presence of model UDP-glucuronosyltransferase 1A1 substrates/inhibitors. Drug Metab. Dispos. 2011, 39, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Miners, J.O.; Bowalgaha, K.; Elliot, D.J.; Baranczewski, P.; Knights, K.M. Characterization of niflumic acid as a selective inhibitor of human liver microsomal UDP-glucuronosyltransferase 1A9: Application to the reaction phenotyping of acetaminophen glucuronidation. Drug Metab. Dispos. 2011, 39, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Sand, P.G.; Dreiseitel, A.; Stang, M.; Schreier, P.; Oehme, A.; Locher, S.; Hajak, G. Cytochrome P450 2C19 inhibitory activity of common berry constituents. Phytother. Res. 2010, 24, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Zhang, L.; Dai, R. An inhibition study of beauvericin on human and rat cytochrome P450 enzymes and its pharmacokinetics in rats. J. Enzyme Inhib. Med. Chem. 2009, 24, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.Y.; Kim, S.Y.; Kim, D.K.; Jeong, J.H.; Lee, H.S. Effects of eupatilin and jaceosidin on cytochrome p450 enzyme activities in human liver microsomes. Molecules 2010, 15, 6466–6475. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.A.; Lee, J.S.; Park, H.J.; Kim, J.W.; Kim, C.J.; Shim, I.S.; Kim, N.J.; Han, S.M.; Lim, S. Inhibition of cytochrome P450 activities by oleanolic acid and ursolic acid in human liver microsomes. Life Sci. 2004, 74, 2769–2779. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.F.; Zhou, Z.W.; Yang, L.P.; Cai, J.P. Substrates, inducers, inhibitors and structure-activity relationships of human Cytochrome P450 2C9 and implications in drug development. Curr. Med. Chem. 2009, 16, 3480–3675. [Google Scholar] [CrossRef] [PubMed]
- Newton, D.J.; Wang, R.W.; Lu, A.Y. Cytochrome P450 inhibitors. Evaluation of specificities in the in vitro metabolism of therapeutic agents by human liver microsomes. Drug Metab. Dispos. 1995, 23, 154–158. [Google Scholar] [PubMed]
- Zhang, Y.; Xu, H.; Chen, X.; Chen, C.; Wang, H.; Meng, F.; Yang, H.; Huang, L. Simultaneous quantification of 17 constituents from Yuanhu Zhitong tablet using rapid resolution liquid chromatography coupled with a triple quadrupole electrospray tandem mass spectrometry. J. Pharmaceut. Biomed. Anal. 2011. [Google Scholar] [CrossRef] [PubMed]
- Greenblatt, D.J.; Zhao, Y.; Venkatakrishnan, K.; Duan, S.X.; Harmatz, J.S.; Parent, S.J.; Court, M.H.; von Moltke, L.L. Mechanism of cytochrome P450-3A inhibition by ketoconazole. J. Pharm. Pharmacol. 2011, 63, 214–221. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |

| Enzymes | Marker reactions | IC50 (μM) | Ki (μM) (inhibition mode) | |
|---|---|---|---|---|
| no preincubation | with preincubation * | |||
| 1A2 | Phenacetin O-deethylation | No inhibition ** | No inhibition ** | - |
| 2A6 | Coumarin 7-hydroxylation | No inhibition ** | No inhibition ** | - |
| 2C8 | Amodiaquine N-deethylation | No inhibition ** | No inhibition ** | - |
| 2C9 | Diclofenac 4-hydroxylation | 26.2 ± 4.6 | 30.6 ± 4.1 | 7.1 ± 0.4 (noncompetitive) |
| 2C19 | S-Mephenytoin 4’-hydroxylation | 11.7 ± 1.4 | 19.2 ± 1.8 | 1.7 ± 0.5 (competitive) |
| 2D6 | Bufuralol 1’-hydroxylation | 64.5 ± 9.8 | 115.9 ± 7.6 | 27.3 ± 0.7 (competitive) |
| 3A | Midazolam 1’-hydroxylation | >200 | 67.4 ± 9.8 | - |
| Enzymes | Marker reactions | IC50 (μM) | Ki (μM) (inhibition mode) |
|---|---|---|---|
| UGT1A1 | 17β-Estradiol 3-glucuronidation | 137.1 ± 16.2 | 57.6 ± 13.0 (α = 0.555) |
| UGT1A4 | Trifluoperazine N-glucuronidation | No inhibition * | - |
| UGT1A9 | Propofol glucuronidation | 39.4 ± 6.0 | 37.3 ± 14.4 (competitive) |
| UGT2B7 | Azidothymidine glucuronidation | No inhibition * | - |
| Enzymes | Compound | SRM Transitions | Tube lens (V) | Collision energy (V) | |
|---|---|---|---|---|---|
| CYP1A2 | Metabolite | Acetaminophen | 152.19 > 110.19 | 59 | 23 |
| Internal standard | 13C2,15N-Acetaminophen | 155.05 > 111.29 | 58 | 21 | |
| CYP2A6 | Metabolite | 7-Hydroxycoumarin | 163.04 > 107.38 | 70 | 22 |
| Internal standard | d5-7-Hydroxycoumarin | 168.00 > 112.53 | 73 | 22 | |
| CYP2C8 | Metabolite | N-Desethylamodiaquine | 328.01 > 282.64 | 45 | 19 |
| Internal standard | 13C2,15N-Acetaminophen | 155.05 > 111.29 | 58 | 21 | |
| CYP2C9 | Metabolite | 4-Hydroxydiclofenac | 312.12 > 231.05 | 54 | 23 |
| Internal standard | 13C6-4-Hydroxydiclofenac | 318.49 > 237.28 | 54 | 20 | |
| CYP2C19 | Metabolite | 4-Hydroxymephenytoin | 235.03 > 150.19 | 50 | 27 |
| Internal standard | d3-4-Hydroxymephenytoin | 238.18 > 150.40 | 50 | 25 | |
| CYP2D6 | Metabolite | 1-Hydroxybufuralol | 278.08 > 186.31 | 54 | 19 |
| Internal standard | d9-1-Hydroxybufuralol | 287.12 > 187.09 | 54 | 20 | |
| CYP3A | Metabolite | 1-Hydroxymidazolam | 342.08 > 324.09 | 73 | 25 |
| Internal standard | d3-4-Hydroxymephenytoin | 238.18 > 150.4 | 60 | 25 | |
| UGT1A1 | Metabolite | 17β-Estradiol 3-glucuronide | 446.95 > 270.91 | 94 | 34 |
| Internal standard | Ezetimibe | 408.07 > 271.43 | 45 | 21 | |
| UGT1A4 | Product | Trifluoperazine N-glucuronide | 584.20 > 408.13 | 94 | 27 |
| Internal standard | Meloxicam | 352.05 > 115.38 | 63 | 20 | |
| UGT1A9 | Metabolite | Propofol glucuronide | 353.18 > 177.19 | 63 | 20 |
| Internal standard | Ezetimibe | 408.07 > 271.43 | 45 | 21 | |
| UGT2B7 | Metabolite | Azidothymidine glucuronide | 444.26 > 127.02 | 70 | 22 |
| Internal standard | Meloxicam | 352.05 > 115.38 | 63 | 20 | |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ji, H.Y.; Liu, K.H.; Lee, H.; Im, S.R.; Shim, H.J.; Son, M.; Lee, H.S. Corydaline Inhibits Multiple Cytochrome P450 and UDP-Glucuronosyltransferase Enzyme Activities in Human Liver Microsomes. Molecules 2011, 16, 6591-6602. https://doi.org/10.3390/molecules16086591
Ji HY, Liu KH, Lee H, Im SR, Shim HJ, Son M, Lee HS. Corydaline Inhibits Multiple Cytochrome P450 and UDP-Glucuronosyltransferase Enzyme Activities in Human Liver Microsomes. Molecules. 2011; 16(8):6591-6602. https://doi.org/10.3390/molecules16086591
Chicago/Turabian StyleJi, Hye Young, Kwang Hyeon Liu, Hyeri Lee, Sae Rom Im, Hyun Joo Shim, Miwon Son, and Hye Suk Lee. 2011. "Corydaline Inhibits Multiple Cytochrome P450 and UDP-Glucuronosyltransferase Enzyme Activities in Human Liver Microsomes" Molecules 16, no. 8: 6591-6602. https://doi.org/10.3390/molecules16086591







