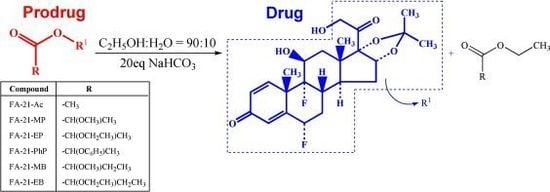
Investigation of Solvolysis Kinetics of New Synthesized Fluocinolone Acetonide C-21 Esters—An In Vitro Model for Prodrug Activation
Abstract
:1. Introduction
2. Results and Discussion
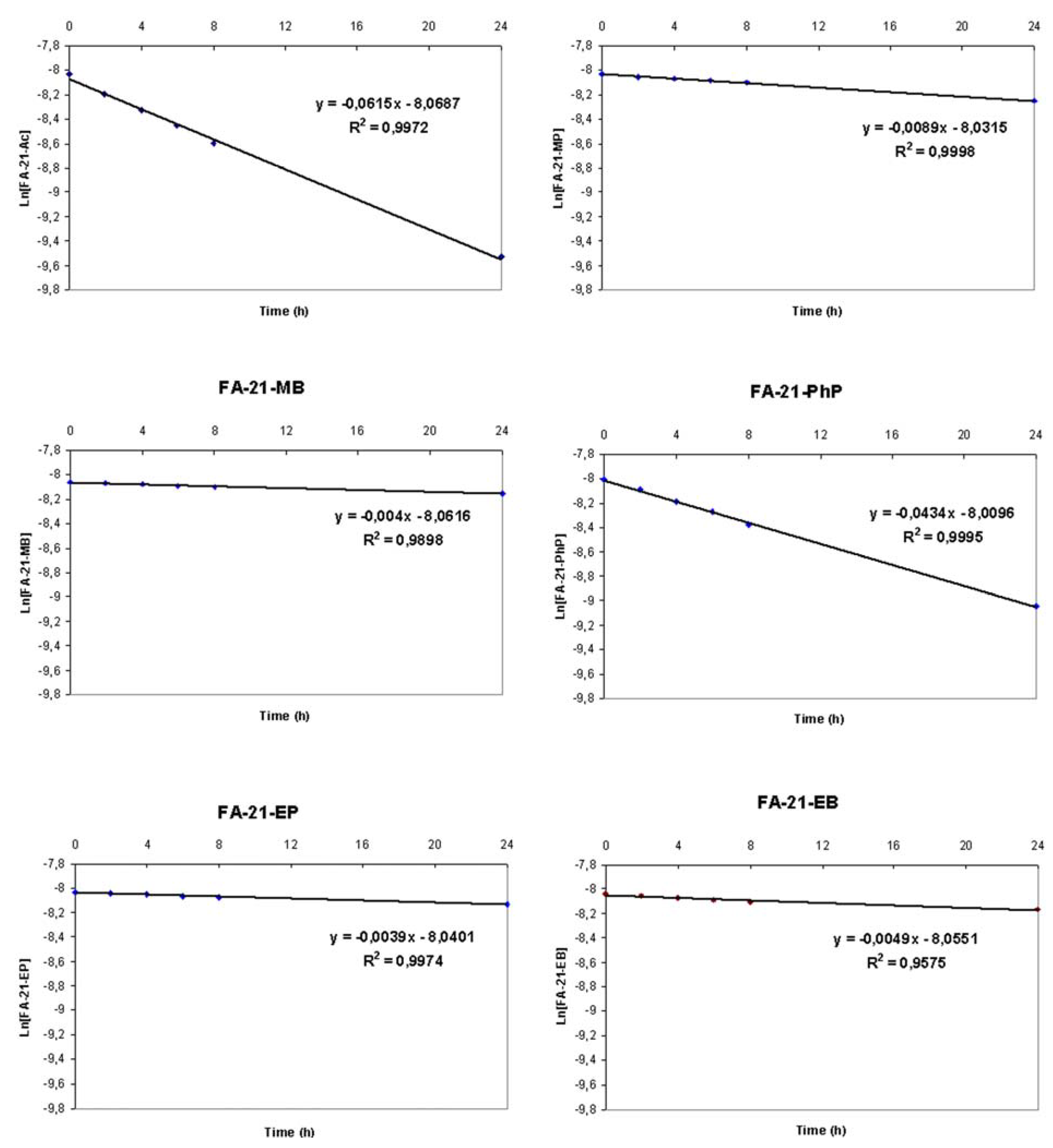
2.1. Solvolytic rate constants determination
- where: c(t) – concentration of corresponding ester after 2, 4, 6, 8 or 24 hours.
- c(0) – concentration of corresponding ester in freshly prepared mixtures (t = 0)
- c – concentration of FA (equal to the concentration of solvolyzed ester fraction)
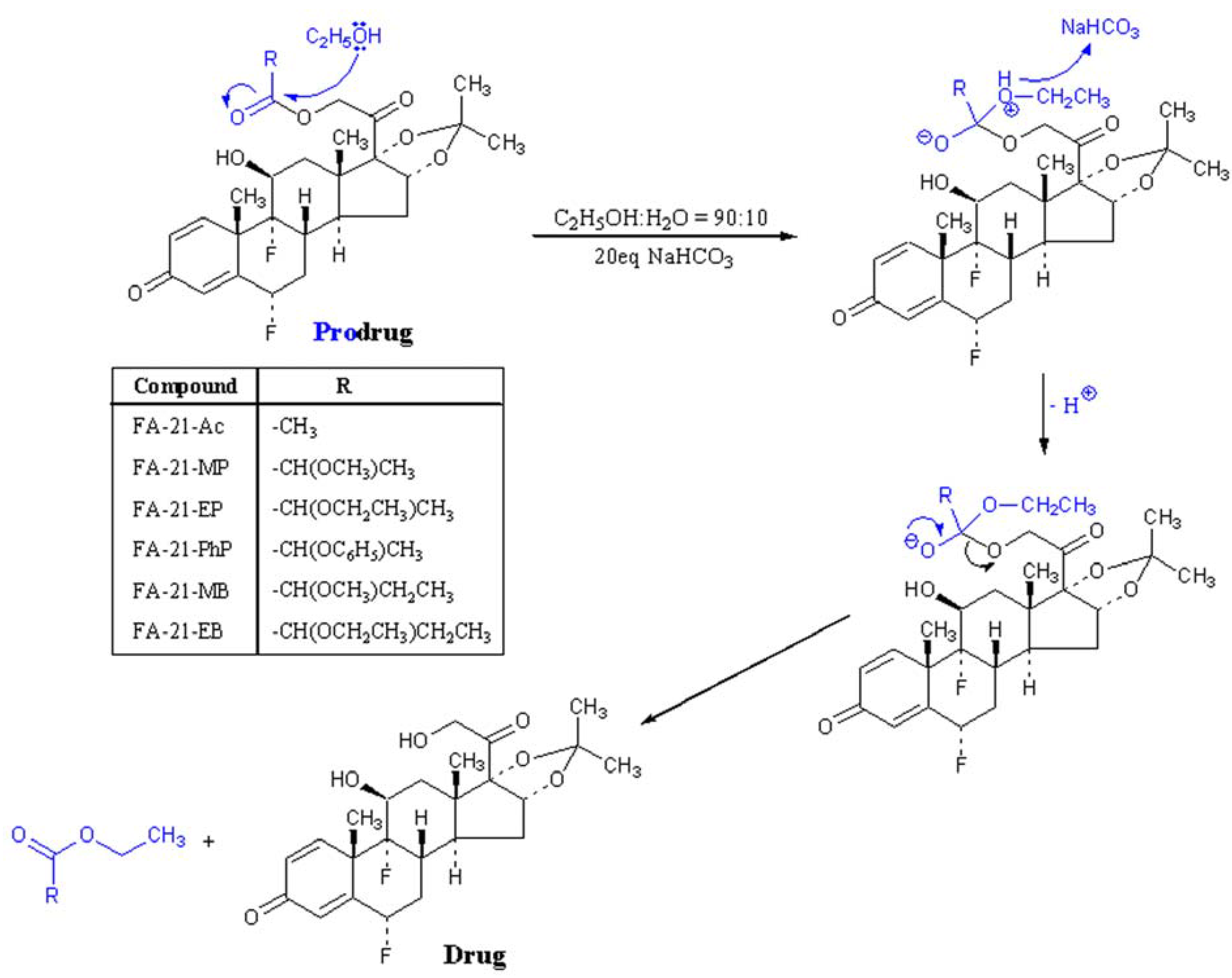
2.2. Mechanism of solvolysis of fluocinolone acetonide esters
2.3. Kinetics of esters solvolysis
2.4. Descriptor selection
| FA-21-Ac | FA-21-EB | FA-21-EP | FA-21-MB | FA-21-MP | FA-21-PhP | R | ||
|---|---|---|---|---|---|---|---|---|
| C-O2 bond length | 1.381 | 1.369 | 1.370 | 1.369 | 1.370 | 1.374 | 0.9627 | |
| C=O1 bond length | 1.229 | 1.232 | 1.232 | 1.232 | 1.232 | 1.228 | -0.9168 | |
| AM1 | C | 0.2991 | 0.2990 | 0.3062 | 0.2991 | 0.3069 | 0.2777 | -0.5215 |
| O2 | -0.2580 | -0.2237 | -0.2284 | -0.2234 | -0.2282 | -0.2851 | -0.8342 | |
| O1 | -0.2830 | -0.2950 | -0.2949 | -0.2943 | -0.2940 | -0.3227 | -0.0956 | |
| C-O2 | 0.5571 | 0.5226 | 0.5346 | 0.5225 | 0.5351 | 0.5628 | 0.9023 | |
| C-O1 | 0.5821 | 0.5940 | 0.6011 | 0.5934 | 0.6009 | 0.6004 | -0.5807 | |
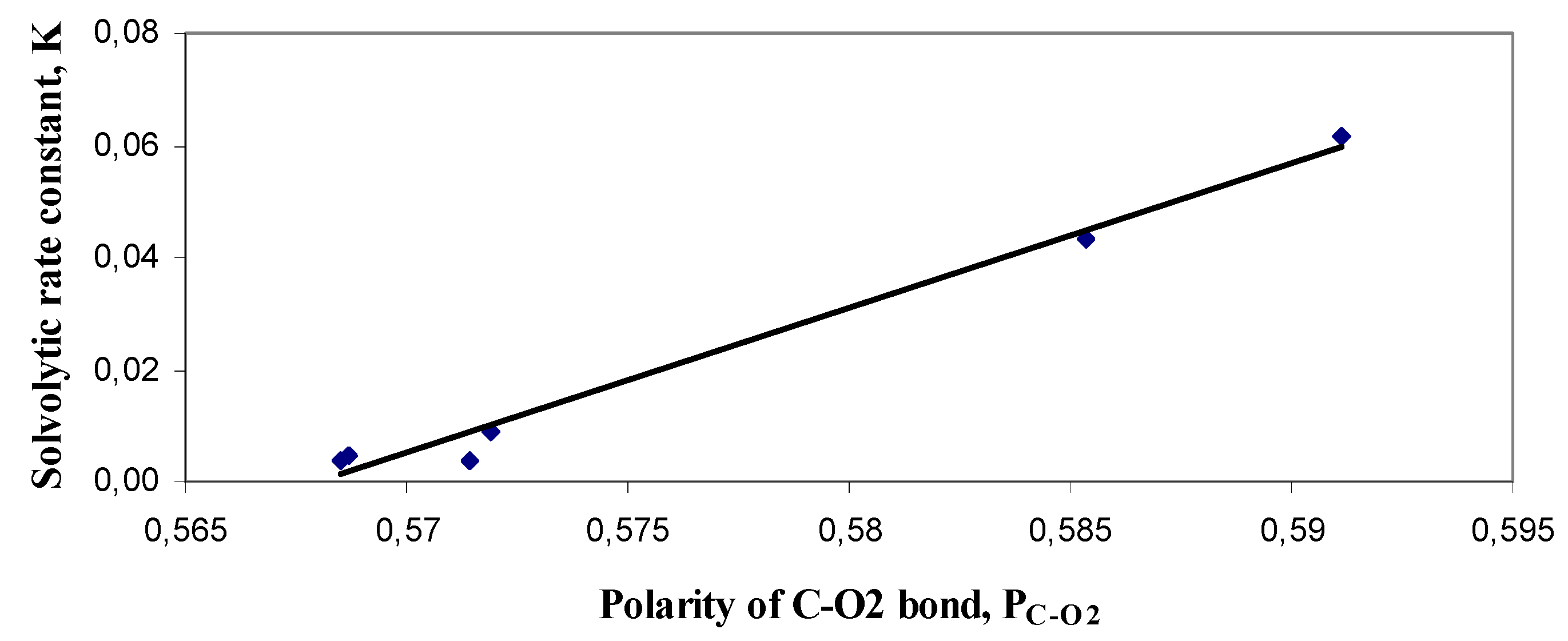
| PC-O2 | 0.7694 | 0.7155 | 0.7324 | 0.7153 | 0.7331 | 0.7732 | 0.9247 | |
| PC=O1 | 0.7154 | 0.7318 | 0.7405 | 0.7311 | 0.7403 | 0.7373 | -0.6644 | |
| HF/STO-3G gas phase | C | 0.3080 | 0.2971 | 0.3027 | 0.2975 | 0.3033 | 0.3025 | 0.7510 |
| O2 | -0.2595 | -0.2475 | -0.2515 | -0.2471 | -0.2511 | -0.2767 | -0.7309 | |
| O1 | -0.2407 | -0.2489 | -0.2478 | -0.2481 | -0.2468 | -0.2575 | 0.1229 | |
| C-O2 | 0.5676 | 0.5446 | 0.5542 | 0.5446 | 0.5544 | 0.5792 | 0.8315 | |
| C-O1 | 0.5488 | 0.5460 | 0.5505 | 0.5457 | 0.5502 | 0.5599 | 0.4620 | |
| PC-O2 | 0.7838 | 0.7455 | 0.7592 | 0.7456 | 0.7595 | 0.7958 | 0.8771 | |
| PC=O1 | 0.6745 | 0.6727 | 0.6782 | 0.6723 | 0.6778 | 0.6876 | 0.3561 | |
| HF/6-31G gas phase | C | 0.7654 | 0.7898 | 0.8195 | 0.7917 | 0.8212 | 0.8161 | -0.4782 |
| O2 | -0.6927 | -0.6792 | -0.6837 | -0.6784 | -0.6833 | -0.7509 | -0.5802 | |
| O1 | -0.5207 | -0.5390 | -0.5423 | -0.5383 | -0.5413 | -0.5598 | 0.2239 | |
| C-O2 | 1.4582 | 1.4689 | 1.5033 | 1.4701 | 1.5044 | 1.5670 | 0.1414 | |
| C-O1 | 1.2862 | 1.3288 | 1.3618 | 1.3300 | 1.3625 | 1.3759 | -0.4076 | |
| PC-O2 | 2.0137 | 2.0110 | 2.0595 | 2.0126 | 2.0611 | 2.1531 | 0.2631 | |
| PC=O1 | 1.5807 | 1.6371 | 1.6778 | 1.6386 | 1.6786 | 1.6896 | -0.4605 | |
| B3LYP/STO-3G gas phase | C | 0.2314 | 0.2233 | 0.2277 | 0.2237 | 0.2283 | 0.2232 | 0.4277 |
| O2 | -0.1879 | -0.1783 | -0.1807 | -0.1778 | -0.1802 | -0.2010 | -0.7492 | |
| O1 | -0.2095 | -0.2172 | -0.2167 | -0.2165 | -0.2157 | -0.2207 | 0.4194 | |
| C-O2 | 0.4193 | 0.4016 | 0.4084 | 0.4015 | 0.4085 | 0.4242 | 0.8752 | |
| C-O1 | 0.4409 | 0.4405 | 0.4443 | 0.4402 | 0.4440 | 0.4439 | -0.0451 | |
| PC-O2 | 0.5790 | 0.5498 | 0.5595 | 0.5496 | 0.5596 | 0.5828 | 0.9118 | |
| PC=O1 | 0.5418 | 0.5427 | 0.5474 | 0.5423 | 0.5470 | 0.5452 | -0.3450 | |
| B3LYP/6-31G gas phase | C | 0.5089 | 0.5138 | 0.5387 | 0.5167 | 0.5410 | 0.5518 | -0.0727 |
| O2 | -0.4682 | -0.4582 | -0.4610 | -0.4574 | -0.4605 | -0.5214 | -0.5609 | |
| O1 | -0.3784 | -0.3959 | -0.3997 | -0.5167 | -0.3988 | -0.4059 | 0.4213 | |
| C-O2 | 0.9771 | 0.9720 | 0.9997 | 0.9742 | 1.0016 | 1.0731 | 0.3315 | |
| C-O1 | 0.8874 | 0.9097 | 0.9383 | 1.0334 | 0.9398 | 0.9577 | -0.4469 | |
| PC-O2 | 1.3494 | 1.3306 | 1.3696 | 1.3336 | 1.3722 | 1.4745 | 0.4106 | |
| PC=O1 | 1.0906 | 1.1207 | 1.1560 | 1.2732 | 1.1578 | 1.1760 | -0.4686 | |
| B3LYP/6-31G gas phase | C | 0.5089 | 0.5138 | 0.5387 | 0.5167 | 0.5410 | 0.5518 | -0.0727 |
| O2 | -0.4682 | -0.4582 | -0.4610 | -0.4574 | -0.4605 | -0.5214 | -0.5609 | |
| O1 | -0.3784 | -0.3959 | -0.3997 | -0.5167 | -0.3988 | -0.4059 | 0.4213 | |
| C-O2 | 0.9771 | 0.9720 | 0.9997 | 0.9742 | 1.0016 | 1.0731 | 0.3315 | |
| C-O1 | 0.8874 | 0.9097 | 0.9383 | 1.0334 | 0.9398 | 0.9577 | -0.4469 | |
| PC-O2 | 1.3494 | 1.3306 | 1.3696 | 1.3336 | 1.3722 | 1.4745 | 0.4106 | |
| PC=O1 | 1.0906 | 1.1207 | 1.1560 | 1.2732 | 1.1578 | 1.1760 | -0.4686 | |
| HF/6-31G CPCM ethanol | C | 0.3188 | 0.3057 | 0.3086 | 0.3062 | 0.3089 | 0.3107 | 0.9101 |
| O2 | -0.2633 | -0.2597 | -0.2616 | -0.2591 | -0.2612 | -0.2736 | -0.6169 | |
| O1 | -0.2719 | -0.2765 | -0.2765 | -0.2752 | -0.2748 | -0.2745 | 0.8871 | |
| C-O2 | 0.5821 | 0.5655 | 0.5702 | 0.5654 | 0.5701 | 0.5843 | 0.9243 | |
| C-O1 | 0.5907 | 0.5822 | 0.5850 | 0.5815 | 0.5837 | 0.5852 | 0.8641 | |
| PC-O2 | 0.8038 | 0.7741 | 0.7811 | 0.7740 | 0.7810 | 0.8028 | 0.9578 | |
| PC=O1 | 0.7260 | 0.7173 | 0.7208 | 0.7164 | 0.7192 | 0.7186 | 0.7311 | |
| B3LYP/STO-3G COSMO ethanol | C | 0.2370 | 0.2277 | 0.2287 | 0.2280 | 0.2293 | 0.2281 | 0.7636 |
| O2 | -0.1910 | -0.1877 | -0.1883 | -0.1872 | -0.1881 | -0.1979 | -0.6912 | |
| O1 | -0.2395 | -0.2424 | -0.2426 | -0.2943 | -0.2940 | -0.3227 | -0.0345 | |
| C-O2 | 0.4280 | 0.4154 | 0.4171 | 0.4153 | 0.4174 | 0.4260 | 0.9853 | |
| C-O1 | 0.4766 | 0.4701 | 0.4713 | 0.5223 | 0.5233 | 0.5508 | 0.1160 | |
| PC-O2 | 0.5911 | 0.5687 | 0.5714 | 0.5685 | 0.5719 | 0.5853 | 0.9924 | |
| PC=O1 | 0.5857 | 0.5791 | 0.5807 | 0.6435 | 0.6447 | 0.6764 | 0.0966 | |
| Steric hindrance | 1.24 | 1.36 | 1.34 | 1.37 | 1.35 | 1.40 | -0.5356 | |
| cLogP | 2.79 | 3.79 | 3.26 | 3.40 | 2.87 | 4.59 | 0.0533 | |
| K | 0.0615 | 0.0049 | 0.0039 | 0.0040 | 0.0089 | 0.0434 | 1.0000 | |
2.6. Relationships between solvolytic rate constant and selected descriptors
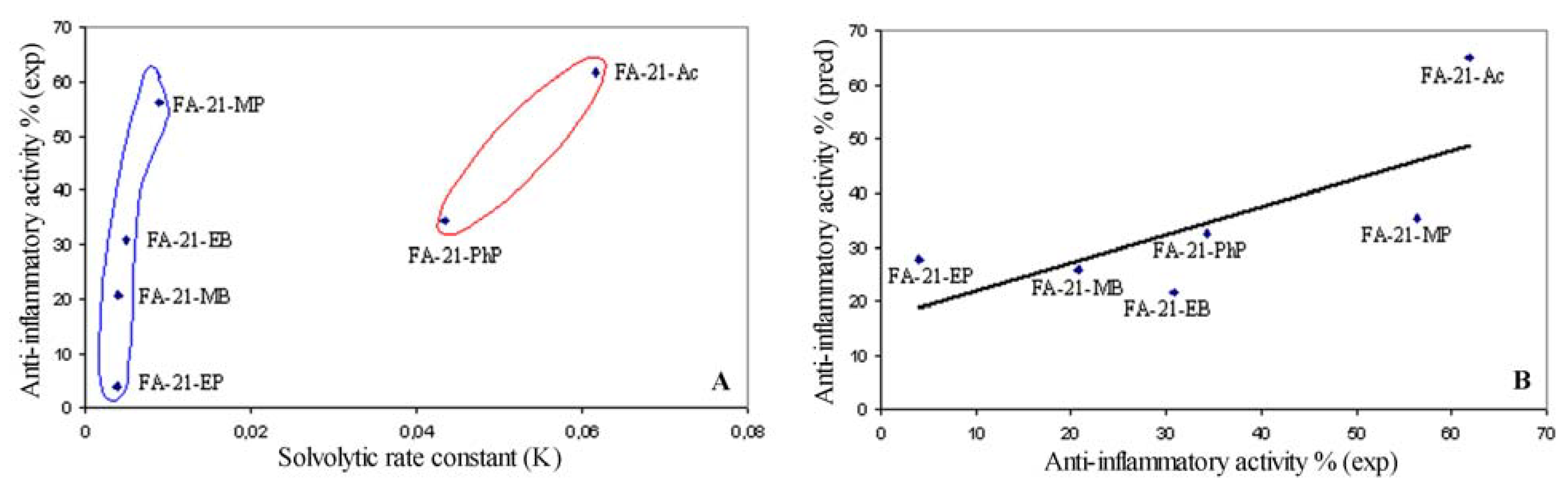
2.7. Relationship between solvolitic rate constant and anti-inflammatory activity
3. Experimental
3.1. General
3.2. General procedure for synthesis of fluocinolone acetonide α-alkoxyalkanoyl and α-aryloxy-alkanoyl C-21 monoesters
3.3. Solvolytic process evaluation method
3.3. Computational detail
3.4. Determination of anti-inflammatory activity in the test of inhibition of croton oil induced mice ear edema
4. Conclusions
Acknowledgements
References and Notes
- Sweetman, S.C. Martindale-The Complete Drug Reference, 34th ed; Pharmaceutical Press: London, UK, 2005. [Google Scholar]
- Katz, M.; Gans, E.H. Topical Corticosteroids, Structure-activity and the glucocorticoid receptor: Discovery and development – a process of “planned serendipity”. J. Pharm. Sci. 2008, 97, 2936–2947. [Google Scholar] [CrossRef]
- Markovic, B.; Vladimirov, S.; Pitic, D.; Savic, V.; Jacevic, V.; Dobric, S. Synthesis ad anti-inflammatory activity of new α-oxyalcanoyl esters of fluocinolone acetonide. In Proceedings ofHungarian-Austrian-Czech-German-Greek-Italian-Polish-Slovak Joint Meeting on Medicinal Chemistry, Budapest, Hungary, 24-27 June 2009; Medimond S.r.l. - Monduzzi Editore International Proceedings Division: Bologna, Italy, 2009; pp. 41–44. [Google Scholar]
- Weidersberg, S.; Leopold, C.S.; Guy, R.H. Bioavailability and bioequivalence of topical glucocorticoids. Eur. J. Pharm. Biopharm. 2008, 68, 453–466. [Google Scholar] [CrossRef]
- Avery, M.A.; Woolfrey, J.R. Anti-inflammatory steroids. In Burger’s Medicinal Chemistry & Drug Discovery, 6th; Abraham, D.J., Ed.; John Wiley & Sons: New York, NY, USA, 2003; Volume 3, pp. 747–853. [Google Scholar]
- Pörtner, M.; Möllmann, H.; Rohdewald, P. Glucocorticoid receptors in human synovial tissue and relative receptor affinities of glucocorticoid-21-esters. Pharm. Res. 1988, 5, 623–627. [Google Scholar] [CrossRef]
- Zhang, H.; Qu, X.; Ando, H. A simple method for reaction rate prediction of ester hydrolysis. J. Mol. Struct. Theochem. 2005, 725, 31–37. [Google Scholar] [CrossRef]
- Massova, I.; Kollman, P.A. Quantum mechanical study of β-Lactam reactivity: The effect of solvatation on barriers of reaction and stability of transition states and reaction intermediates. J. Phys. Chem. B 1999, 103, 8628–8638. [Google Scholar] [CrossRef]
- Markovic, B.; Vladimirov, S.; Cudina, O.; Savic, V.; Karljikovic-Rajic, K. An application of second-order UV-derivative spectrophotometry for study of solvolysis of a novel fluocinolone acetonide ester. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2010, 75, 930–935. [Google Scholar] [CrossRef]
- Grasa, G.A.; Singh, R.; Nolan, S.P. Transesterification/acylation reactions catalyzed by molecular catalysts. Synthesis 2004, 7, 971–985. [Google Scholar]
- Otera, J. Reaction with esters: Transesterification. In Esterification – Methods, Reactions, Applications; Wiley-VCH: Weinheim, Germany, 2003; pp. 52–100. [Google Scholar]
- Williams, D.B.G.; Sibiya, M.S.; Van Heerden, P.S.; Kirk, M.; Harris, R. Verkade super base-catalyzed transesterification of propylene carbonate with methanol to co-produce dimethyl carbonate and propylene glycol. J. Mol. Catal. A 2009, 304, 147–152. [Google Scholar] [CrossRef]
- Jursic, B.S.; Zdravkovski, Z. Rates of hydrolysis of N-acetylazoles: Semiempirical calculations compared to experimental values. J. Mol. Struct. Theochem 1994, 303, 177–183. [Google Scholar] [CrossRef]
- Fife, T.H.; Chauffe, L. General base and general acid catalyzed intramolecular aminolysis of esters. Cyclization of esters of 2-aminomethylbenzoic acid to phthalimidine. J. Org Chem. 2000, 65, 3579–3586. [Google Scholar] [CrossRef]
- Zhan, C.G.; Landry, D.W.; Ornstein, R.L. Energy barriers for alkaline hydrolysis of carboxylic acid esters in aqueous solution by reaction field calculations. J. Phys. Chem. A 2000, 104, 7672–7678. [Google Scholar] [CrossRef]
- James, K.C.; Nicholls, P.J.; Richards, G.T. Correlation of androgenic activities of the lower testosterone esters in rat, with Rm values and hydrolysis rates. Eur. J. Med. Chem. 1975, 10, 55–58. [Google Scholar]
- Brennan, C.; Dixon, J.A.; Scott, W.J.; Redman, A.; Jones, B.D.; Phillips, B.; Wickens, P.; Enyedy, I.; Kumarasinghe, E.; Kreiman, C.; Dumas, J.; Khire, U.; Chuang, C.; Kluender, H.; Hong, Z.; Wang, L.; Bierer, D. 2-Aminoarylcarboxamides useful as cancer chemotherapeutic agents. WO Patent 2006002383, 2006. [Google Scholar]
- Dewar, M.J.S.; Zoebisch, E.G.; Healy, E.F.; Stewart, J.J.P. Development and use of quantum mechanical molecular models. 76. AM1: A new general purpose quantum mechanical molecular model. J. Am.Chem. Soc. 1985, 107, 3902–3909. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A.;. Vreven, T.; Kudin, K.N.; Burant, J.C.; Millam, J.M.; Iyengar, S.S.; Tomasi, J.; Barone, V.; Mennucci, B.; Cossi, M.; Scalmani, G.; Rega, N.; Petersson, G.A.; Nakatsuji, H.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Klene, M.X.Li.; Knox, J.E.; Hratchian, H.P.; Cross, J.B.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R.E.; Yazyev, O.; Austin, A.J.; Cammi, R.; Pomelli, C.; Ochterski, J.W.; Ayala, P.Y.; Morokuma, K.; Voth, G.A.; Salvador, P.; Dannenberg, J.J.; Zakrzewski, V.G.; Dapprich, S.; Daniels, A.D.; Strain, M.C.; Farkas, O.; Malick, D.K.; Rabuck, A.D.; Raghavachari, K.; Foresman, J.B.; Ortiz, J.V.; Cui, Q.; Baboul, A.G.; Clifford, S.; Cioslowski, J.; Stefanov, B.B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Martin, R.L.; Fox, D.J.; Keith, T.; Al-Laham, M.A.; Peng, C.Y.; Nanayakkara, A.; Challacombe, M.; Gill, P.M.W.; Johnson, B.; Chen, W.; Wong, M.W.; Gonzalez, C.; Pople, J.A. Gaussian 03, Revision C.01, 2004.
- Dykstra, E.C.; Jasien, P.G. Derivative Hartree-Fock theory to all orders. Chem. Phys. Lett. 1984, 109, 388–393. [Google Scholar] [CrossRef]
- Becke, A.D. Density functional exchange-energy approximation with correct asymptotic behevior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Becke, A.D. Density functional thermochemistry III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Colle-Savetti correlation energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Samples Availability: Samples of the compounds are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Markovic, B.D.; Dobricic, V.D.; Vladimirov, S.M.; Cudina, O.A.; Savic, V.M.; Karljikovic-Rajic, K.D. Investigation of Solvolysis Kinetics of New Synthesized Fluocinolone Acetonide C-21 Esters—An In Vitro Model for Prodrug Activation. Molecules 2011, 16, 2658-2671. https://doi.org/10.3390/molecules16032658
Markovic BD, Dobricic VD, Vladimirov SM, Cudina OA, Savic VM, Karljikovic-Rajic KD. Investigation of Solvolysis Kinetics of New Synthesized Fluocinolone Acetonide C-21 Esters—An In Vitro Model for Prodrug Activation. Molecules. 2011; 16(3):2658-2671. https://doi.org/10.3390/molecules16032658
Chicago/Turabian StyleMarkovic, Bojan D., Vladimir D. Dobricic, Sote M. Vladimirov, Olivera A. Cudina, Vladimir M. Savic, and Katarina D. Karljikovic-Rajic. 2011. "Investigation of Solvolysis Kinetics of New Synthesized Fluocinolone Acetonide C-21 Esters—An In Vitro Model for Prodrug Activation" Molecules 16, no. 3: 2658-2671. https://doi.org/10.3390/molecules16032658
APA StyleMarkovic, B. D., Dobricic, V. D., Vladimirov, S. M., Cudina, O. A., Savic, V. M., & Karljikovic-Rajic, K. D. (2011). Investigation of Solvolysis Kinetics of New Synthesized Fluocinolone Acetonide C-21 Esters—An In Vitro Model for Prodrug Activation. Molecules, 16(3), 2658-2671. https://doi.org/10.3390/molecules16032658