Drug Resistance Modulation in Staphylococcus Aureus, a New Biological Activity for Mesoionic Hydrochloride Compounds
Abstract
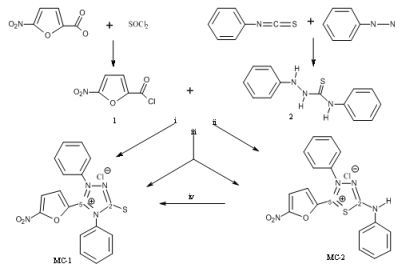
:1. Introduction
2. Results and Discussion

| Strain (efflux protein) | Tetracycline | Erythromycin | Norfloxacin | MC-1 | MC-2 |
|---|---|---|---|---|---|
| IS-58 (TetK) | 32 | - | - | 64 | 64 |
| RN-4220 (MrsA) | - | 256 | - | 32 | 64 |
| SA-1199B (NorA) | - | - | 64 | 64 | 64 |
| Strain /(efflux protein)/Antibiotic (MIC µg/mL) | Antibiotic MIC µg/mL with (*)MIC reduction factor in the presence of MC-1/MC-2(16 µg/mL) | |
|---|---|---|
| Antibiotic + MC-1 | Antibiotic + MC-2 | |
| IS-58/(TetK)/Tetracycline (32) | 2 (16 *) | 1 (32 *) |
| RN-4220/(MrsA)/ Erythromycin (256) | 64 (4 *) | 64 (4 *) |
| SA-1199B/(NorA)/Norfloxacin (64) | 64 (0 *) | 64 (0 *) |
3. Experimental
3.1. General
3.2. Biological Activity
4. Conclusions
Acknowledgements
References
- Athayde-Filho, P.F.; Miller, J.; Simas, A.M. Mesoionic compounds: Amphiphilic heterocyclic betaines. Synthesis 2000, 11, 1565–1568. [Google Scholar]
- Lira, B.F.; Athayde-Filho, P.F.; Miller, J.; Simas, A.M., Dias; Vieira, M.J. Synthesis and characterization of some new mesoionic 1,3- thiazolium-5-thiolates via cyclodehydration and in situ 1,3-dipolar cycloaddition/cycloreversion. Molecules 2002, 7, 791–800. [Google Scholar] [CrossRef]
- Rakov, N.; Araújo, C.B.; Athayde-Filho, P.F.; Rocha, G.B.; Miller, J.; Simas, A.M. Two-photon absorption in mesoionic compounds pumped at the visible and at the infrared. Chem. Phys. Lett. 2000, 332, 13–18. [Google Scholar] [CrossRef]
- Pilla, V.; Araújo, C.B.; Athayde-Filho, P.F.; Miller, J.; Simas, AM. Nonlinear absorption of new mesoionic compounds. Opt. Commun. 2006, 264, 225–228. [Google Scholar] [CrossRef]
- Bosco, C.A.C.; Maciel, G.S.; Rakov, N.; Araújo, C.B.; Acioli, L.H.; Simas, AM; Athayde-Filho, P.F.; Miller, J. Probing the nuclear susceptibility of mesoionic compounds using two-beam coupling with chirp-controlled pulses. Chem. Phys. Lett. 2007, 449, 101–106. [Google Scholar] [CrossRef]
- Nascimento, R.S.T.R.; Morais, C.R.S., Lira; Morais, S.A.; Athayde-Filho, P.F.; Lucena, L.F.L.; Souza, A.G.; Campos, G.B. Synthesis and characterization of nanocomplexes of Eu(III) and Er(III) coordinate with 2(4-clorophenil)-3-phenyl-1,3,4-thiadiazoleo-5-tiolate mesoionic. J. Alloys Comp. 2010, 495, 603–605. [Google Scholar] [CrossRef]
- Morais, S.A.; Morais, C.R.S.; Athayde-Filho, P.F.; Lira, B.F.; Nascimento, R.S.T.R. A kinetic study of the thermaldecomposition of mesoionic compounds within scope of its application in nonlinear optical devices. J. Therm. Anal. Calorim. 2009, 97, 437–441. [Google Scholar] [CrossRef]
- Lira, B.F.; Morais, S.A.; Rocha, G.B.; Miller, J.; Moura, G.L.C.; Simas, AM; Peppe, C.; Athayde-Filho, P.F. 1,3-Thiazolium-5-thiolates mesoionic compounds: Semiempirical evaluation of their first static hyperpolarizabilities and synthesis of new examples. J. Braz. Chem. Soc. 2010, 21, 934–940. [Google Scholar] [CrossRef]
- Kier, L.B.; Roche, E.B. Medicinal chemistry of the mesoionic compounds. J. Pharm. Sci. 1966, 56, 149–168. [Google Scholar]
- Ollis, W.D.; Ramsden, C.A. Meso-ionic compounds. Adv. Heterocycl. Chem. 1976, 19, 1–122. [Google Scholar] [CrossRef]
- Newton, C.G.; Ramsden, C.A. Meso-ionic heterocycles. Tetrahedron 1982, 38, 2965–3011. [Google Scholar] [CrossRef]
- Cavalcante, K.V.M.; Correia, N.A.; Dias, K.L.G.; Silva, D.F.; Silva-Filho, J.C.; Araújo, I.G.A.; Lira, B.F.; Athayde-Filho, P.; Medeiros, I.A. dothelium-derived nitric oxide contributes to the vasorelaxant response induced by mesoionic 2-(4-chlorophenyl)-3-methyl-4-(4-methoxyphenyl)-1,3-thiazolium-5-thyolate (CMMTT) in rats. J. Pharmacol. Sci. 2009, 110, 29–35. [Google Scholar] [CrossRef]
- Athayde-Filho, P.F.; Miller, J.; Simas, A.M.; Sena, K.X.F.R.; Chiappeta, A.A. Synthesis, characterization and evaluation of the activity of ten mesoionic compounds against microorganisms. Acta Farm. Bonaerense 1999, 18, 17–22. [Google Scholar]
- Athayde, P.F.; Miller, J.; Thomas, G.; Araújo, C.C. Synthesis and spasmolytic activity of mesoionic 1,4-diphenyl-5-(5-nitro-2-furanyl)-1,3,4-triazolium-2-thiolate hydrochloride. Heterocycl. Commun. 1996, 2, 573–580. [Google Scholar]
- Chopra, I.; Hesse, L.; O’neil, A.J. Exploiting current understanding of antibiotic action for discovery of new drugs. J. Appl. Microbiol. Symp. Suppl. 2002, 92, 4S–15S. [Google Scholar] [CrossRef]
- Lynch, A.S. Efflux systems in bacterial pathogens: an opportunity for therapeutic intervention? An industry view. Biochem. Pharmacol. 2006, 71, 949–956. [Google Scholar] [CrossRef]
- Pidoock, L.J.V. Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacterial. Clin. Microbiol. Rev. 2006, 19, 382–402. [Google Scholar] [CrossRef]
- Gibbons, S. Anti-staphylococcal plant natural products. Nat. Prod. Rep. 2004, 21, 263–277. [Google Scholar] [CrossRef]
- Maciel, M.A.M.; Echevarria, A.; Rumjanek, V.M. Isolation and characterization ofacyl-thiosemicarbazides as intermediates in mesoionic compounds synthesis. Quim. Nova 1998, 21, 569–572. [Google Scholar] [CrossRef]
- Echevarria, A.; Galembeck, S.E.; Maciel, M.A.M.; Miller, J. Reaction of aroyl chlorides with 1,4-diphenylthiosemicarbazide: formation of both 1,3,4-thiadiazolium-2-aminides and 1,3,4-triazolium-2-thiolate. Heterocycl. Commun. 1995, 1, 129–136. [Google Scholar]
- Lira, B.F.; Miller, J.; Simas, A.M.; Athayde-Filho, P.F.; Dias, A.F.; Silva, R.O.; Oliveira, V.C. Synthesis and complete assignments of 1H and 13C-NMR spectra of mesoionic 2-(p-trifluoromethylphenyl)-3-methyl-4-(p-tolyl)-1,3- thiazolium-5-thiolate and 2-(p-chlorophenyl)-3-methyl–4-(p-isopropylphenyl)-1,3-thiazolium-5-thiolate. ARKIVOC 2004, vi, 12–21. [Google Scholar]
- Montanari, C.A.; Sandall, J.P.B.; Miyata, Y.; Miller, J. Structural studies on some 1,3,4-thiadiazolium-2-aminides and their rearrangement isomers using 15N and 13C-NMR spectroscopy. J. Chem. Soc. Perkin Trans. 2 1994, 2571–2575. [Google Scholar]
- Oliveira, C.S. Síntese, caracterização e avaliação farmacológica de novos compostos mesoiônicos. Ph.D. Thesis, Universidade Federal da Paraíba, João Pessoa, Paraíba, Brazil, 2009. [Google Scholar]
- Kawase, M.; Sakagami, H.; Motohashi, N. The chemistry of bioactive mesoionic heterocycles. Top. Heterocycl. Chem. 2009, 16, 135–152. [Google Scholar]
- Rozenski, J.; Raunter, C.J.; Verplanken, H. Quantitative structure-activity relationships for antimicrobial nitroheterocyclic drugs. Quant. Struct.-Act. Relat. 1995, 14, 134–141. [Google Scholar] [CrossRef]
- Markham, P.N.; Neyfakh, A.A. Efflux-mediated drug resistance in Gram-positive bacteria. Curr. Opin. Microbiol. 2001, 4, 509–514. [Google Scholar] [CrossRef]
- Robeya, R.W.; Shuklab, S; Finleya, E.M.; Oldhamc, R.K.; Barnettc, D.; Ambudkarb, S.V.; Fojoa, T.; Batesa, S.E. Inhibition of P-glycoprotein (ABCB1)- and multidrug resistance associated protein 1 (ABCC1)-mediated transport by the orally administered inhibitor, CBT-1®. Biochem. Pharmacol. 2008, 75, 1302–1312. [Google Scholar] [CrossRef]
- Kaatz, G.W.; Seo, S.M.; Ruble, C.A. Efflux-mediated fluoroquinolone resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 1993, 37, 1086–1094. [Google Scholar] [CrossRef]
- Kaatz, G.W.; Seo, S.M. Inducible NorA-mediated multidrug resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 1995, 39, 2650–2655. [Google Scholar] [CrossRef]
- Ross, J.I.; Farrell, A.M.; Eady, E.A.; Cove, J.H.; Cunliffe, W.J. Characterisation and molecular cloning of the novel macrolide-streptogramin B resistance determinant from Staphylococcus epidermidis. J. Antimicrob. Chemother. 1989, 24, 851–862. [Google Scholar] [CrossRef]
- Gibbons, S.; Udo, E.E. The effect of reserpine, a modulator of multidrug efflux pumps, on the in vitro activity of tetracycline against clinical isolates of methicillin resistant Staphylococcus aureus (MRSA) possessing the tet(K) determinant. Phytother. Res. 2000, 14, 139–140. [Google Scholar] [CrossRef]
- CLSI—Clinical and Laboratory Standards Institute/NCCLS. Performance Standards for Antimicrobial Susceptibility Testing; Fifteenth Informational Supplement. CLSI/NCCLS document M100-S15; Wayne, P.A., Ed.; Clinical and Laboratory Standards Institute, 2005.
- Stavri, M.; Piddock, L.J.V.; Gibbons, S. Bacterial efflux pump inhibitors from natural sources. J. Antimicrob. Chemother. 2007, 59, 1247–1260. [Google Scholar] [CrossRef]
- Falcão-Silva, V.; Silva, D.A.; Souza, M.F.V.; Siquiera-Junior, J.P. Modulation of drug resistance in Staphylococcus aureus by a kaempferol glycoside from Herissantia tiubae (Malvaceae). Phytoter. Res. 2009, 23, 1367–1370. [Google Scholar] [CrossRef]
- Silva, D.A.; Falcão-Silva, V.S.; Gomes, A.Y.S.; Costa, D.A.; Lemos, V.S.; Agra, M.F.; Braz-Filho, R.; Siqueira-Junior, J.P.; Souza, M.F.V. Triterpenes and phenolic compounds isolated from the aerial parts of Herissantia tiubae and evaluation of 5,4’,-dihydroxy-3,6,7,8,3’-pentamethoxyflavone as a modulator of bacterial drug resistance. Pharm. Biol. 2009, 47, 279–284. [Google Scholar] [CrossRef]
- Sample Availability: Samples are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Soares de Oliveira, C.; Dos Santos Falcão-Silva, V.; Siqueira-Júnior, J.P.; Harding, D.P.; Lira, B.F.; Lorenzo, J.G.F.; Barbosa-Filho, J.M.; Filgueiras de Athayde-Filho, P. Drug Resistance Modulation in Staphylococcus Aureus, a New Biological Activity for Mesoionic Hydrochloride Compounds. Molecules 2011, 16, 2023-2031. https://doi.org/10.3390/molecules16032023
Soares de Oliveira C, Dos Santos Falcão-Silva V, Siqueira-Júnior JP, Harding DP, Lira BF, Lorenzo JGF, Barbosa-Filho JM, Filgueiras de Athayde-Filho P. Drug Resistance Modulation in Staphylococcus Aureus, a New Biological Activity for Mesoionic Hydrochloride Compounds. Molecules. 2011; 16(3):2023-2031. https://doi.org/10.3390/molecules16032023
Chicago/Turabian StyleSoares de Oliveira, Cledualdo, Vivyanne Dos Santos Falcão-Silva, José Pinto Siqueira-Júnior, David Peter Harding, Bruno Freitas Lira, Jorge Gonçalo Fernandes Lorenzo, José Maria Barbosa-Filho, and Petrônio Filgueiras de Athayde-Filho. 2011. "Drug Resistance Modulation in Staphylococcus Aureus, a New Biological Activity for Mesoionic Hydrochloride Compounds" Molecules 16, no. 3: 2023-2031. https://doi.org/10.3390/molecules16032023