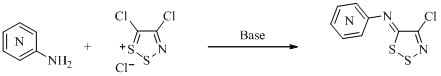Synthesis of [(4-Chloro-5H-1,2,3-dithiazol-5-ylidene)amino]azines
Abstract
:1. Introduction

2. Results and Discussion
2.1. Studies on N-(4-Chloro-5H-1,2,3-dithiazol-5-ylidene)pyridinamines

| Aminopyridine | Base (pKb) | Yields of 1a, 1g or 1k (%) |
|---|---|---|
| Pyridin-2-amine | Pyridine (8.8) | 1a (69) |
| 2,6-Lutidine (7.4) | 1a (72) | |
| Dabco (5.2) | 1a (55) | |
| Et3N (3.4) | 1a (69) | |
| i-Pr2NEt (2.6) | 1a (73) | |
| DBU (1.1) | 1a (58) | |
| DBN (0.5) | 1a (47) | |
| Pyridin-3-amine | Pyridine (8.8) | 1g (24) |
| 2,6-Lutidine (7.4) | 1g (45) | |
| Dabco (5.2) | 1g (8) | |
| Et3N (3.4) | 1g (43) | |
| i-Pr2NEt (2.6) | 1g (57) | |
| DBU (1.1) | 1g (16) | |
| DBN (0.5) | 1g (13) | |
| Pyridin-4-amine | Pyridine (8.8) | 1k (traces) |
| 2,6-Lutidine (7.4) | 1k (traces) | |
| Dabco (5.2) | 1k (3) | |
| Et3N (3.4) | 1k (23) | |
| i-Pr2NEt (2.6) | 1k (13) | |
| DBU (1.1) | 1k (13) | |
| DBN (0.5) | 1k (traces) |


2.2. Synthesis of a [(4-Chloro-5H-1,2,3-dithiazol-5-ylidene)amino]azine Library
| Azine | Product Yields (%) | ||||||
|---|---|---|---|---|---|---|---|
| Pyridine | Lutidine | DABCO | Et3N | i-Pr2NEt | DBU | DBN | |
| 1a (pyrid-2-yl) | 69 | 72 | 55 | 69 | 73 | 58 | 47 |
| 1b (3-MeO-pyrid-2-yl) | 71 | 67 | 55 | 60 | 66 | 42 | 40 |
| 1c (3,5-Cl2-pyrid-2-yl) | 69 | 70 | 52 | 44 | 43 | 44 | 39 |
| 1d (3,5-Br2-pyrid-2-yl) | 65 | 70 | 56 | 48 | 40 | 27 | 15 |
| 1e (3-O2N-pyrid-2-yl) | 45 | 62 | 48 | 32 | 14 | 23 | 12 |
| 1f (pyrazin-2-yl) | 65 | 63 | 51 | 63 | 61 | 40 | 36 |
| 1g (pyrid-3-yl) | 23 | 45 | 8 | 42 | 57 | 16 | 10 |
| 1h (2-HO-pyrid-3-yl) | 53 | 19 | 11 | 9 | 18 | 12 | 5 |
| 1i (2-Cl-pyrid-3-yl) | 75 | 82 | 85 | 71 | 72 | 50 | 45 |
| 1j (4-Cl-pyrid-3-yl) | 65 | 76 | 60 | 70 | 69 | 21 | 19 |
| 1k (pyrid-4-yl) | trace | trace | 3 | 21 | 13 | 13 | 10 |
| 1l (2,6-Me2-pyrid-4-yl) | 5 | trace | 19 | 22 | 10 | 9 | 5 |
| 1m (5-NC-pyrimid-4-yl) | 61 | 55 | 35 | 45 | 40 | 39 | 15 |
3. Experimental
3.1. General
3.2. General Procedure for the Synthesis of [(4-Chloro-5H-1,2,3-dithiazol-5-ylidene)amino]azines
4. Conclusions
Acknowledgments
References
- Konstantinova, L.S.; Bol’shakov, O.I.; Obruchnikova, N.V.; Laborie, H.; Tanga, A.; Sopéna, V.; Lanneluc, I.; Picot, L.; Sablé, S.; Thiéry, V.; Rakitin, O.A. One-pot synthesis of 5-phenylimino, 5-thieno or 5-oxo-1,2,3-dithiazoles and evaluation of their antimicrobial and antitumor activity. Bioorg. Med. Chem. Lett. 2009, 19, 136–141. [Google Scholar]
- Cottenceau, G.; Besson, T.; Gautier, V.; Rees, C.W.; Pons, A.M. Antibacterial evaluation of novel N-arylimino-1,2,3-dithiazoles and N-arylcyanothioformamides. Bioorg. Med. Chem. Lett. 1996, 6, 529–532. [Google Scholar] [CrossRef]
- Thiery, V.; Rees, C.W.; Besson, T.; Cottenceau, G.; Pons, A.M. Antimicrobial activity of novel N-quinolinyl and N-naphthylimino-1,2,3-dithiazoles. Eur. J. Med. Chem. 1998, 33, 149–153. [Google Scholar]
- Joseph, R.W.; Antes, D.L.; Osei-Gyimah, P. Antimicrobial Compounds with Quick Speed of Kill. US Patent 5688744, 1997. [Google Scholar]
- Moore, J.E. Certain 4-Halo-5-aryl-1,2,3-dithiazole Compounds and their Preparation. US Patent 4059590, 1977. [Google Scholar]
- Appel, R.; Janssen, H.; Haller, I.; Plempel, M. 1,2,3-Dithiazolderivate, Verfahren zu ihrer Herstellung Sowie ihre Verwendung als Arzneimittel. DE Patent 2848221, 1980. [Google Scholar]
- Besson, T.; Rees, C.W.; Cottenceau, G.; Pons, A.M. Antimicrobial evaluation of 3,1-benzoxazin-4-ones, 3,1-benzothiazin-4-ones, 4-alkoxyquinazolin-2-carbonitriles and N-arylimino-1,2,3-dithiazoles. Bioorg. Med. Chem. Lett. 1996, 6, 2343–2348. [Google Scholar] [CrossRef]
- Mayer, R.; Foerster, E.; Matauschek, B. Verfahren zur Herstellung von Aromatisch oder Heteroaromatisch Substituierten Cyanthioformamiden. DD Patent 212387 1984. [Google Scholar]
- Konstantinova, L.S.; Rakitin, O.A. Synthesis and properties of 1,2,3-dithiazoles. Russ. Chem. Rev. 2008, 77, 521–546. [Google Scholar] [CrossRef]
- Rees, C.W. Polysulfur-nitrogen heterocyclic chemistry. J. Heterocycl. Chem. 1992, 29, 639–651. [Google Scholar] [CrossRef]
- Besson, T.; Dozias, M.J.; Guillard, J.; Rees, C.W. New route to 2-Cyano-benzothiazoles via N-Arylimino-1,2,3-dithiazoles. J. Chem. Soc., Perkin Trans. 1 1998, 3925–3926. [Google Scholar]
- Rakitin, O.A.; Rees, C.W.; Vlasova, O.G. Direct synthesis of 2-Cyano-benzimidazoles and the generation of S2. Tetrahedron Lett. 1996, 37, 4589–4592. [Google Scholar] [CrossRef]
- Christoforou, I.C.; Koutentis, P.A.; Michaelidou, S.S. 1,2,3-Dithiazole chemistry in heterocyclic synthesis. ARKIVOC 2006, 7, 207–223. [Google Scholar]
- Besson, T.; Guillaumet, G.; Lamazzi, C.; Rees, C.W. Synthesis of 3,1-benzoxazines, 3,1-benzothiazines and 3,1-benzoxazepines via N-arylimino-1,2,3-dithiazoles. Synlett 1997, 704–706. [Google Scholar]
- Lee, H.; Kim, K. A new procedure to N-arylcyanothioformamides from 5-arylimino-4-chloro-5H-1,2,3-dithiazoles. Bull. Korean Chem. Soc. 1992, 13, 107–108. [Google Scholar]
- Michaelidou, S.S.; Koutentis, P.A. The synthesis of 2-cyano cyanothioformanilides from 2-(4-chloro-5H-1,2,3-dithiazol-5-ylideneamino)benzonitriles using DBU. Synthesis 2009, 4167–4174. [Google Scholar]
- Besson, T.; Emayan, K.; Rees, C.W. 1,2,3-Dithiazoles and new routes to 3,1-benzoxazin-4-ones, 3,1-benzothiazin-4-ones and N-arylcyanothioformamides. J. Chem. Soc. Perkin Trans. 1 1995, 2097–2102. [Google Scholar]
- Lee, H.; Kim, K. Reactions of 5-(arylimino)-4-chloro-5H-1,2,3-dithiazoles with primary and secondary alkylamines: Novel synthesis of (arylimino)cyanomethyl alkylamino disulfides and their mechanisms of formation. J. Org. Chem. 1993, 58, 7001–7008. [Google Scholar] [CrossRef]
- Besson, T.; Guillard, J.; Rees, C.W. Rapid synthesis of 2-cyanobenzothiazole, isothiocyanates and cyanoformanilide derivatives of dapsone. J. Chem. Soc. Perkin Trans. 1 2000, 563–566. [Google Scholar]
- Besson, T.; Guillard, J.; Rees, C.W.; Thiéry, V. New syntheses of aryl isothiocyanates. J. Chem. Soc. Perkin Trans. 1 1998, 889–892. [Google Scholar]
- Baraldi, P.G.; Pavani, M.G.; Nuñez, M.C.; Brigidi, P.; Vitali, B.; Gambari, R.; Romagnoli, R. Antimicrobial and antitumor activity of n-heteroimmine-1,2,3-dithiazoles and their transformation in triazolo-, imidazo-, and pyrazolopirimidines. Bioorg. Med. Chem. 2002, 10, 449–456. [Google Scholar] [CrossRef]
- Lee, H.-S.; Chang, Y.-G.; Kim, K. A facile synthesis of 3-substituted 2-cyanoquinazolin-4(3H)-ones and 3-alkyl-2-cyanothieno[3,2-d]pyrimidin-4(3H)-ones via 1,2,3-dithiazoles. J. Heterocycl. Chem. 1998, 35, 659–668. [Google Scholar] [CrossRef]
- Cuadro, A.M.; Alvarez-Builla, J. 4,5-Dichloro-1,2,3-dithiazolium chloride (Appel’s Salt): reactions with N-nucleophiles. Tetrahedron 1994, 50, 10037–10046. [Google Scholar] [CrossRef]
- English, R.F.; Rakitin, O.A.; Rees, C.W.; Vlasova, O.G. Conversion of imino-1,2,3-dithiazoles into 2-cyanobenzothiazoles, cyanoimidoyl chlorides and diatomic sulfur. J. Chem. Soc. Perkin Trans. 1 1997, 201–205. [Google Scholar]
- Konstandinova, L.S.; Rakitin, O.A.; Rees, C.W.; Sivadasan, S.; Torrobas, T. New route to 2-cyanobenzimidazoles. Tetrahedron 1998, 54, 9639–9650. [Google Scholar] [CrossRef]
- Kalogirou, A.S.; Koutentis, P.A. The degradation of 4,5-dichloro-1,2,3-dithiazolium chloride in wet solvents. Tetrahedron 2009, 65, 6859–6862. [Google Scholar] [CrossRef]
- Bordwell pKa Table (Acidity in DMSO). Available online: http://www.chem.wisc.edu/areas/reich/pkatable/ accessed on 1 June 2011.
- Stewart, R.; Harris, M.G. Comparison of the acidities and basicities of amino-substituted nitrogen heterocycles. J. Org. Chem. 1978, 43, 3123–3126. [Google Scholar] [CrossRef]
- L’abbe, G.; D’hooge, B.; Dehaen, W. Unusual behavior of 4,5-dichloro-1,2,3-dithiazolium chloride (Appel’s salt) with 5-aminopyrazoles: A synthetic method of 1H-pyrazolo[3,4-d]-thiazoles. J. Chem. Soc., Perkin Trans. 1 1995, 2379–2380. [Google Scholar]
- L’abbe, G.; Bastin, L.; Dehaen, W.; Toppet, S.; Delbeke, P.; Vlieghe, D.; van Meerveek, L. Reactions of 5-chloro-1,2,3-dithiazolium salts with activated methylene compounds. J. Chem. Soc., Perkin Trans. 1 1994, 2545–2551. [Google Scholar]
- Samples Availability: Samples of the compounds are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Koutentis, P.A.; Koyioni, M.; Michaelidou, S.S. Synthesis of [(4-Chloro-5H-1,2,3-dithiazol-5-ylidene)amino]azines. Molecules 2011, 16, 8992-9002. https://doi.org/10.3390/molecules16118992
Koutentis PA, Koyioni M, Michaelidou SS. Synthesis of [(4-Chloro-5H-1,2,3-dithiazol-5-ylidene)amino]azines. Molecules. 2011; 16(11):8992-9002. https://doi.org/10.3390/molecules16118992
Chicago/Turabian StyleKoutentis, Panayiotis A., Maria Koyioni, and Sophia S. Michaelidou. 2011. "Synthesis of [(4-Chloro-5H-1,2,3-dithiazol-5-ylidene)amino]azines" Molecules 16, no. 11: 8992-9002. https://doi.org/10.3390/molecules16118992
APA StyleKoutentis, P. A., Koyioni, M., & Michaelidou, S. S. (2011). Synthesis of [(4-Chloro-5H-1,2,3-dithiazol-5-ylidene)amino]azines. Molecules, 16(11), 8992-9002. https://doi.org/10.3390/molecules16118992