Bovine and Human Serum Albumin Interactions with 3-Carboxyphenoxathiin Studied by Fluorescence and Circular Dichroism Spectroscopy
Abstract
:1. Introduction
2. Results and Discussion
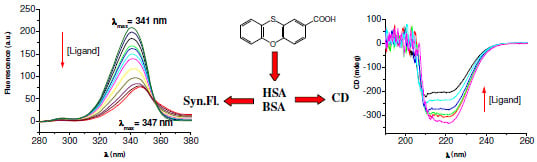
2.1. Fluorescence Spectra of the I–Albumin Systems
2.2. Stern-Volmer Analysis
2.3. Determination of the Binding Parameters
2.4. Synchronous Fluorescence Spectroscopy
2.5. Energy Transfer between 3-carboxyphenoxathiin and BSA/ HSA
2.6. Circular Dichroism Spectra
3. Experimental
3.1. Materials
3.2. Apparatus and Methods
4. Conclusions
Acknowledgments
References
- Moreno, F.; Cortijo, M.; Gonzalez-Jimenez, J. Interaction of Acrylodan with Human Serum Albumin. A Fluorescence Spectroscopic Study. J. Photochem. Photobiol. 1999, 70, 695–700. [Google Scholar] [CrossRef]
- Sato, T.; Saito, Y.; Chikuma, M.; Saito, Y.; Nagai, S. Determination of albumin in bronchoalveolar lavage fluid by flow-injection fluorometry using Chromazurol S. Biol. Pharm. Bull. 2008, 31(3), 336–339. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.J.; Haugland, R.P.; Singer, V.L. Development and characterization of the NanoOrange protein quantitation assay: A fluorescence-based assay of proteins in solution. Biotechniques 2003, 34, 850–854. [Google Scholar] [PubMed]
- Li, Y.; Yao, X.; Jin, J.; Chen, X.; Hu, Z. Interaction of rhein with human serum albumin investigation by optical spectroscopic technique and modeling studies. Biochim. Biophys. Acta 2007, 1774, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, S.; Gavriliu, D.; Maior, O.; Hillebrand, M. Excited states properties of some phenoxathiin derivatives. J. Photochem. Photobiol. 1999, 124, 67–73. [Google Scholar] [CrossRef]
- Oana, V; Tintaru, V; Gavriliu, V; Maior, V; Hillebrand, M. Experimental and Theoretical Study of the Inclusion Complexes of Some Phenoxathiin Derivatives with β-Cyclodextrin. J. Phys. Chem. 2002, 106, 257–263. [Google Scholar]
- Cristian, A. M.; Birla, L.; Gavriliu, D.; Maior, O.; Hillebrand, M. Steady state fluorescence study of some phenoxathiin derivatives-protein interaction. Rev. Roum. Chim. 2002, 47, 769–775. [Google Scholar]
- Varlan, A.; Hillebrand, M. Study on the interaction of 2-carboxyphenoxathiin with bovine serum albumin and human serum albumin by fluorescence spectroscopy and circular dichroism. Rev. Roum. Chim. 2010, 55(1), 69–77. [Google Scholar]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Plenum Press: New York, NY, USA, 1983; p. 44. [Google Scholar]
- Eftink, M.R.; Ghiron, C.A. Fluorescence quenching studies with proteins. Anal. Biochem. 1981, 114, 199–227. [Google Scholar] [CrossRef]
- Thipperudrappa, J.; Biradar, D.S.; Lagare, M.T.; Hanagodimath, S.M.; Inamdar, S.R.; Kadadevaramath, J.S. Fluorescence quenching of BPBD by aniline in benzene–acetonitrile mixtures. J. Photochem. Photobiol. 2006, 177, 89–93. [Google Scholar] [CrossRef]
- Wang, N.; Ye, L.; Yan, F.; Xu, R. Spectroscopic studies on the interaction of azelnidipine with bovine serum albumin. Int. J. Pharm. 2008, 351, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Kamat, B.P.; Seetharamappa, J. In vitro study on the interaction of mechanism of tricyclic compounds with bovine serum albumin. J. Pharm. Biomed. Anal. 2004, 35, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.M.T.; Seetharamappa, J.; Kandagal, P.B.; Manjunatha, D.H.; Ashoka, S. Spectroscopic investigations on the mechanism of interaction of bioactive dye with bovine serum albumin. Dyes Pigm. 2007, 74, 665–671. [Google Scholar] [CrossRef]
- Melavanki, R.M.; Kusanur, R.A.; Kadadevaramath, J.S.; Kulakarni, M.V. Quenching mechanisms of 5BAMC by aniline in different solvents using Stern–Volmer plots. J. Lumin. 2009, 129, 1298–1303. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Zhang, H.-M.; Zhang, G.-C.; Tao, W.-H.; Tang, S.-H. Binding of brucine to human serum albumin. J. Mol. Struct. 2007, 830, 40–45. [Google Scholar] [CrossRef]
- Chakraborty, B.; Basu, S. Interaction of BSA with proflavin: A spectroscopic approach. J. Lumin. 2009, 129, 34–39. [Google Scholar] [CrossRef]
- Kang, J.; Liu, V.; Xie, V.; Li, V.; Jiang, V.; Wang, V. Interactions of human serum albumin with chlorogenic acid and ferulic acid. Biochim. Biophys. Acta 2004, 1674, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Jiang, V.; Xie, V.; Zheng, D.; Liu, Y.; Li, X.-Y.; Chen, X. Spectroscopic studies on the interaction of cinnamic acid and its hydroxyl derivatives with human serum albumin. J. Mol. Struct. 2004, 692, 71–80. [Google Scholar]
- Zhou, B.; Qi, Z.-D.; Xiao, Q.; Dong, J.-X.; Zhang, Y-Z.; Liu, Y. Interaction of loratadine with serum albumins studied by fluorescence quenching method. J. Biochem. Bioph. Methods 2007, 70, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Sułkowska, A.; Maciazek-Jurczyk, M.; Bojko, B.; Rownicka, J.; Zubik-Skupien, I.; Temba, E.; Pentak, D.; Sułkowski, W.W. Competitive binding of phenylbutazone and colchicine to serum albumin in multidrug therapy: A spectroscopic study. J. Mol. Struct. 2008, 881, 97–106. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, Q.; Fan, Y.; Shen, H. Study on the binding of luteolin to bovine serum albumin. Spectrochim. Acta 2008, 69, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Cheema, M. A; Taboada, P.; Barbosa, S.; Juárez, J.; Gutiérrez-Pichel, M.; Siddiq, M.; Mosquera, V. Human serum albumin unfolding pathway upon drug binding: A thermodynamic and spectroscopic description. J. Chem. Thermodynamics 2009, 41, 439–447. [Google Scholar] [CrossRef]
- Divsalar, A.; Saboury, A.; Mansoori-Torshizi, H.; Hemmatinejad, B. Comparative and Structural Analysis of the Interaction between β-Lactoglobulin type A and B with a New Anticancer Component (2,2′-Bipyridin n-Hexyl Dithiocarbamato Pd(II) Nitrate). Bull. Korean Chem. Soc. 2006, 27, 1801–1808. [Google Scholar]
- Wang, Y.Q.; Zhang, H.-M.; Zhang, G.-C.; Tao, W.-H.; Fei, Z.-H.; Liu, Z.-T. Spectroscopic studies on the interaction between silicotungstic acid and bovine serum albumin. J. Pharm. Biomed. Anal. 2007, 43, 1869–1875. [Google Scholar] [CrossRef] [PubMed]
- Scatchard, G. The attraction of proteins for small molecules and ions. Ann. NY Acad. Sci. 1949, 51, 660–672. [Google Scholar] [CrossRef]
- Bi, S.; Ding, L.; Tian, Y.; Song, D.; Zhou, X.; Liu, X.; Zhang, H. Investigation of the interaction between flavonoids and human serum albumin. J. Mol. Struct. 2004, 703, 37–45. [Google Scholar] [CrossRef]
- Bi, S.; Song, D.; Tian, Y.; Zhou, X.; Liu, Z.; Zhang, H. Molecular spectroscopic study on the interaction of tetracyclines with serum albumins. Spectrochim. Acta 2005, 61, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Zou, J.-W.; Yi, P.-G.; Shang, Z.-C.; Hu, G.-X.; Yu, Q.-S. Binding interaction of gatifloxacin with bovine serum albumin. Anal. Sci. 2004, 20, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Q.; Zhang, H.-M.; Zhang, G.-C.; Tao, W.-H.; Tang, S.-H. Interaction of the flavonoid hesperidin with bovine serum albumin: A fluorescence quenching study. J. Lumin. 2007, 126, 211–218. [Google Scholar] [CrossRef]
- Bogdan, M.; Pirnau, A.; Floare, C.; Bugeac, C. Binding interaction of indomethacin with human serum albumin. J. Pharm. Biomed Anal. 2008, 47, 981–984. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Liang, Y.-Z.; Wang, P. Characterization of the interaction between furosemide and bovine serum albumin. J. Mol. Struct. 2008, 872, 190–196. [Google Scholar] [CrossRef]
- Chen, X.; Fan, J.-C.; Wang, Y.; Fan, C.-P.; Shang, Z.-C. Fluorometric Study on the Interaction between Lomefloxacin and Bovine Lactoferrin. Anal. Sci. 2006, 22, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.N. Recent advances in molecular luminescence analysis. Proc. Anal. Div. Chem. Soc. 1979, 16, 203–208. [Google Scholar]
- Chen, G. Z.; Huang, X. Z.; Xu, J. G.; Zheng, Z. Z.; Wang, Z. B. Method of Fluorescence Analysis, 2nd ed.; Science Press: Beijing, China, 1990; p. 112. [Google Scholar]
- Hu, Y.J.; Liu, Y.; Wang, J.B.; Xiao, X.H.; Qu, S.S. Study of the interaction between monoammonium glycyrrhizinate and bovine serum albumin. J. Pharm. Biomed. Anal. 2004, 36, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wang, Y.; Zhang, H.; Tang, S.; Tao, W. Human serum albumin interaction with paraquat studied using spectroscopic methods. Pestic. Biochem. Physiol. 2007, 87, 23–29. [Google Scholar] [CrossRef]
- Fan, J.-C.; Chen, X.; Wang, Y.; Fan, C.-P.; Shang, Z.-C. Binding interactions of pefloxacin mesylate with bovine lactoferrin and human serum albumin. J. Zhejiang Univ. Sci. 2006, 7, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Förster, T.; Sinanoglu, O. Modern Quantum Chemistry; Academic Press: New York, NY, USA, 1966; p. 93. [Google Scholar]
- Cui, F.L.; Fan, J.; Ma, D.L.; Liu, M.C.; Chen, X.G.; Hu, Z.D. A study of the interaction between a new reagent and serum albumin by fluorescence spectroscopy. Anal. Lett. 2003, 36, 2151–2166. [Google Scholar] [CrossRef]
- Yuan, J.-L.; Zhong, I.; Liu, Z.-G.; Hu, Z.; Zou, G.-L. Study on interaction between apigenin and human serum albumin by spectroscopy and molecular modeling. J. Photochem. Photobiol. 2007, 191, 104–113. [Google Scholar] [CrossRef]
- He, Y.; Wang, Y.; Tang, L.; Liu, H.; Chen, W.; Zheng, Z.; Zou, G. Binding of Puerarin to human serum albumin: A spectroscopic analysis and molecular docking. J. Fluoresc. 2008, 18, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.N.; Islam, B.; Yennamalli, R.; Sultan, A.; Subbarao, N.; Khan, A.U. Interaction of mitoxantrone with human serum albumin: Spectroscopic and molecular modeling studies. Eur. J. Pharm. Sci. 2008, 35, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Valeur, B. Molecular Fluorescence: Principles and Applications; WILEY-VCH: Weinheim, Germany, 2002. [Google Scholar]
- Kragh-Hansen, U. Molecular aspects of ligand binding to serum albumin. Pharmacol. Rev. 1981, 33, 17–53. [Google Scholar] [PubMed]
- Peters, T. Serum albumin. Adv. Protein Chem. 1985, 37, 161–245. [Google Scholar] [PubMed]
- Feng, X.-Z.; Lin, Z.; Yang, L.-J.; Wang, C.; Bai, C.-l. Investigation of the interaction between acridine orange and bovine serum albumin. Talanta 1998, 47, 1223–1229. [Google Scholar] [CrossRef]
- Kandagal, P.B.; Ashoka, S.; Seetharamappa, J.; Shaikh, S.M.T.; Jadegoud, Y.; Ijare, O.B. Study of the interaction of an anticancer drug with human and bovine serum albumin: Spectroscopic approach. J. Pharm. Biomed. Anal. 2006, 41, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-P.; Wei, Y.-l.; Dong, C. Study on the interaction of 3,3-bis(4-hydroxy-1-naphthyl)-phthalide with bovine serum albumin by fluorescence spectroscopy. J. Photochem. Photobiol. 2006, 177, 6–11. [Google Scholar] [CrossRef]
- Zsila, F.; Bikadi, Z.; Simonyi, M. Probing the binding of the flavonoid, quercetin to human serum albumin by circular dichroism, electronic absorption spectroscopy and molecular modelling methods. Biochem. Pharmacol. 2003, 65, 447–456. [Google Scholar] [CrossRef]
- Li, D.; Zhu, J.; Jin, J.; Yao, X. Studies on the binding of nevadensin to human serum albumin by molecular spectroscopy and modeling. J. Mol. Struct. 2007, 846, 34–41. [Google Scholar] [CrossRef]
- Gao, H.; Lei, L.D.; Liu, J.Q.; Kong, Q.; Chen, X.G.; Hu, Z.D. The Study on the Interaction Between Human Serum Albumin and a New Reagent with Antitumour Activity by Spectrophotometric Methods. J. Photochem. Photobiol. 2004, 167, 213–221. [Google Scholar] [CrossRef]









| Equation (1), d/p < 1 | Equation (1), d/p > 1 | Equation (2) | |||||
|---|---|---|---|---|---|---|---|
| Protein | KSV×105 (M-1) domain 1 | r2 | KSV×105 (M-1) domain 2 | r2 | KKSV×105 (M-1) | V×105 (M-1) | r2 |
| HSA | 3.30 ± 0.03 | 0.999 | 5.60 ± 0.10 | 0.999 | 2.10 ± 0.05 | 0.76 ± 0.02 | 0.999 |
| BSA | 3.10 ± 0.74 | 0.991 | 7.40 ± 0.32 | 0.991 | 0.40 ± 0.10 | 1.90 ± 0.09 | 0.999 |
| Protein | Equation (6) | Equation (7) | ||
|---|---|---|---|---|
| n | K×105 (M-1) | n | K×105 (M-1) | |
| HSA | 0.80 ± 0.12 | 8.10 ± 0.02 | 0.70 ± 0.04 | 5.60 ± 0.20 |
| BSA | 1.00 ± 0.02 | 10.00 ± 0.30 | 1.30 ± 0.03 | 9.20 ± 0.36 |
| Ligand | Protein | J (cm3 l mol-1) | E | R0 (nm) | r (nm) |
|---|---|---|---|---|---|
| (I) | BSA | 1.634×10-15 | 0.500 | 1.862 | 1.862 |
| HSA | 1.748×10-15 | 0.516 | 1.883 | 1.863 |
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Varlan, A.; Hillebrand, M. Bovine and Human Serum Albumin Interactions with 3-Carboxyphenoxathiin Studied by Fluorescence and Circular Dichroism Spectroscopy. Molecules 2010, 15, 3905-3919. https://doi.org/10.3390/molecules15063905
Varlan A, Hillebrand M. Bovine and Human Serum Albumin Interactions with 3-Carboxyphenoxathiin Studied by Fluorescence and Circular Dichroism Spectroscopy. Molecules. 2010; 15(6):3905-3919. https://doi.org/10.3390/molecules15063905
Chicago/Turabian StyleVarlan, Aurica, and Mihaela Hillebrand. 2010. "Bovine and Human Serum Albumin Interactions with 3-Carboxyphenoxathiin Studied by Fluorescence and Circular Dichroism Spectroscopy" Molecules 15, no. 6: 3905-3919. https://doi.org/10.3390/molecules15063905