Abstract
Brominated anthraquinones can be synthesized directly from bromothiophenes when these are reacted with 1,4-naphthoquinones in the presence of meta-chloroperoxy-benzoic acid. The bromoanthraquinones are versatile building blocks in the preparation of arylated anthraquinones and of extended π-systems with interspersed anthraquinone units.
1. Introduction
Arylated anthraquinones 1 (Figure 1) have elicited interest in Physical Organic Chemistry [,] due to the interaction of the attached aryl groups with the π-system of the anthraquinone core, as evidenced in the corresponding UV and luminescence spectra [,], in the redox behavior of the molecules [,], and their NMR shift values. Specifically, the interaction of the substituents on the anthraquinone C=O function has been subjected to investigation []. In practical applications, arylated anthraquinones have also been used as stabilizers of light-modulating fluids such as of fluids comprised of liquid polybenzyltoluenes []. Our interest in these molecules is in the study of their electrochemical behavior. In the following, a new direct preparation of arylated anthraquinones from bromothiophenes is presented.
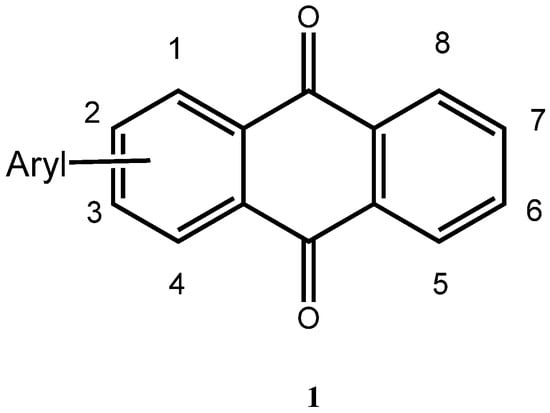
Figure 1.
General Structure of arylated Anthraquinones.
A number of synthetic routes to arylated anthraquinones are known. It has been shown by Bergmann et al. [,] that [4+2]-cycloaddition reactions of phenylbutadienes 2 with either 1,4-naphthoquinone (3a) or with p-benzoquinone give 1-phenylanthraquinone (4a) and 1,4-diphenylanthraquinone (4b) (from 1,4-naphthoquinone) and 1,5-diphenylanthraquinone and 1,4,5,8-tetraphenylanthraquinone (from p-benzoquinone), respectively (Scheme 1). The Diels-Alder approach has also been used for the synthesis of arenoanthraquinones such as of benz[a]anthracene-7,12-diones []. For the preparation of 1,4-diarylanthraquinones, Gautrot et al. [] started from 1,4-dihydroxy-9,10-anthraquinone, which was transformed into its bistriflate 5 [] and subsequently subjected to coupling reaction with arylboronic acids (Scheme 2) [].
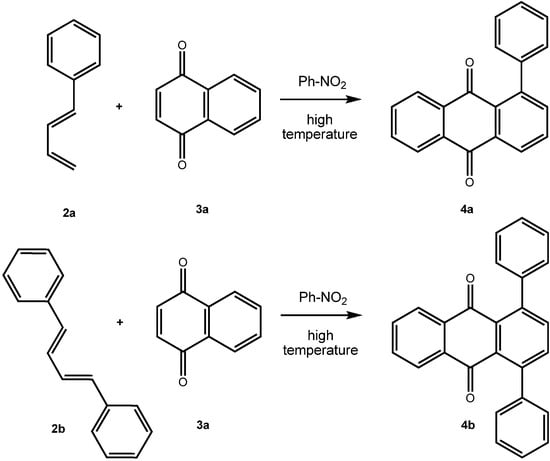
Scheme 1.
Aryl substituted anthraquinones by [4+2]-cycloaddition reaction [,].
Coupling reactions have also been carried out with 1-diazoanthraquinone, which was prepared from the corresponding 1-aminoanthraquinone []. In order to have a versatile strategy to prepare aryl substituted anthraquinones in hand, we wanted to use haloanthraquinones as key intermediates, which we could subsequently transform into the target compounds by Suzuki cross coupling reactions. Again, preparative routes to haloanthraquinones are known. Thus, Battegay and Claudin prepared a number of dibromoanthraquinones from the corresponding diaminoanthraquinones by Sandmeyer reactions [] and sulfonic acid functionalities could also be transformed to bromo substituents at elevated temperatures [].
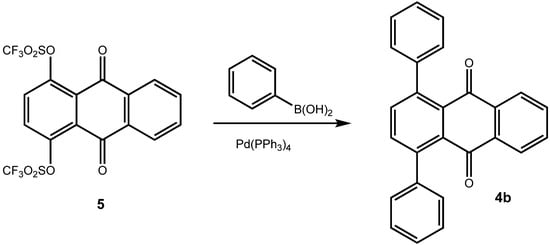
Scheme 2.
1,4-Diphenylanthraquinone by Suzuki-type coupling with aryl triflates [].
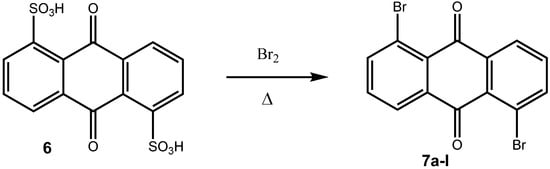
Scheme 3.
Bromination of an anthraquinone-disulfonic acid to a dibromoanthraquinone [].
Based on our good experience in using thiophene S-oxides, either in situ [,,] or in purified form [,,], as dienes in the preparation of multi-functionalised arenes [,], we decided to utilize halogenated thiophene S-oxides as transient intermediates to prepare bromoanthraquinones. While most thiophenes themselves are unreactive or react sluggishly [,,] and thiophene S,S-dioxides [] often necessitate high temperatures to participate in [4+2]-cycloaddition reactions, thiophene S-oxides have been found to be reactive dienes in Diels-Alder type reactions. While a number of thiophene S-oxides [,,,,,,,], especially those with electron donating substituents have been isolated, thiophene S-oxides can be reacted in situ [,,]. Thiophene S-oxides undergo cycloaddition reactions, when thiophenes are oxidized in the presence of a dienophile.
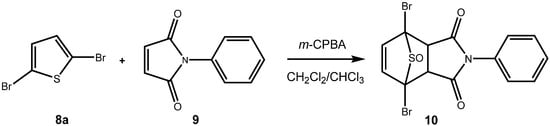
Scheme 4.
In situ preparation and cycloaddition of a dibrominated thiophene S-oxide [].
From our understanding, in halogenated thiophenes, the sulfur is more difficult to oxidize with peracids or with hydrogen peroxide than in the corresponding donor substituted thiophenes. On the other hand, oxidized halothiophenes – halothiophene S-oxides and halothiophene S,S-dioxides – should be more reactive dienes than their electron-donor substituted counterparts. Therefore, in all likelihood, halothiophene S-oxides would have to be used in situ. In fact, Torssell has reported on one example of a successful oxidative cycloaddition of a monobrominated thiophene with 1,4-naphthoquinone (3a), where the cycloadduct was produced in poor yield []. Our own work [] on the oxidative cycloaddition of brominated and chlorinated thiophenes (eg, 8a) to maleimides (eg, to 9) indicated that halothiophene S-oxides can be produced in situ and can be reacted with electron poor dienophiles (Scheme 4).
2. Results and Discussion
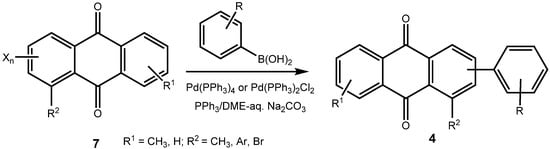
In the present case, a variety of brominated thiophenes 8 were submitted to oxidative cycloaddition reactions with 1,4-naphthoquinones 3. Heated solutions of thiophene 8 and 1,4-naphthoquinone (3a) were treated with meta-chloroperbenzoic acid in small portions over 48 h. Under these conditions, cycloaddition between intermediately formed thiophene S-oxides and 1,4-naphthoquinone 3 takes place, where the formulated, primary sulfoxy-bridged cycloadduct 11 loses the SO-bridge with concomitant aromatization (Scheme 5). The bromoanthraquinones 7 can be obtained, albeit in very moderate yield (Table 1). A number of more polar side products formed, depending on the substrate. One important type of side product are hydroxyanthraquinones 12 (Figure 2). That bromothiophene S-oxides are involved here, has been shown in the reaction under analogous conditions of 2,5-dibromothiophene (8a), 2,3,4,5-tetrabromothiophene (8e) and 2,5-dichlorothiophene with N-phenylmaleimide (9), where halogenated 7-thiabicyclo[2.2.1]heptene S-oxides 10 could be isolated (Scheme 4) []. Nevertheless, even in cases where halothiophene S-oxides are oxidized further to halothiophene S,S-dioxides, cycloaddition reactions may be expected to proceed as electron poor thiophene S,S-dioxides have been found to undergo cycloaddition reactions readily [,,], so that under the present conditions, halothiophene S,S-dioxides can also contribute to the reaction.
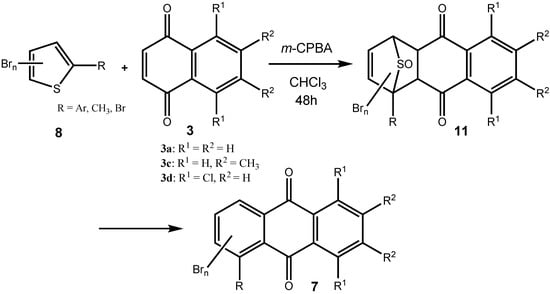
Scheme 5.
Preparation of bromoanthraquinones by oxidative cycloaddition of thiophenes to quinines.

Table 1.
Preparation of bromoanthraquinones by oxidative cycloaddition of thiophenes to quinines.
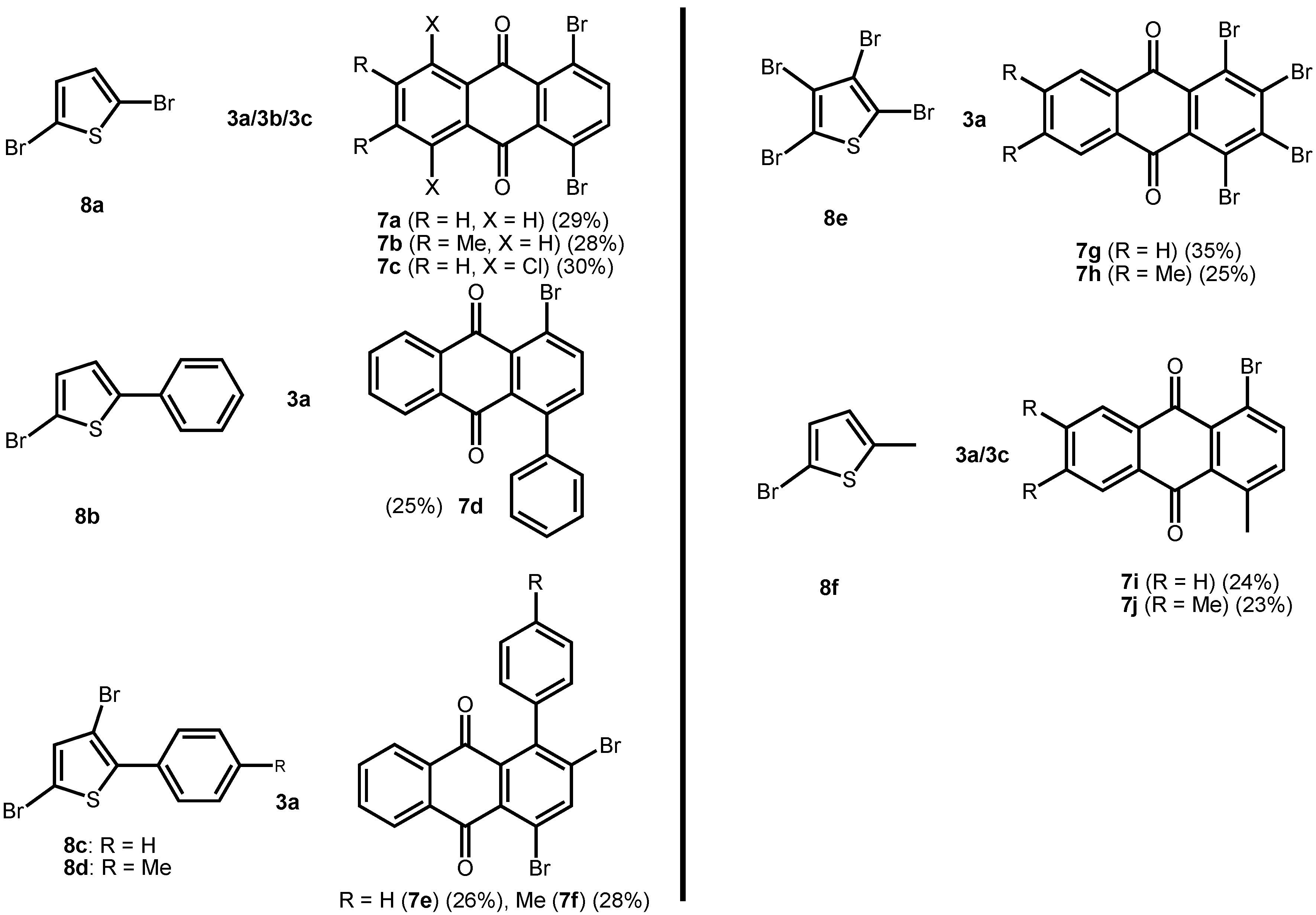 |
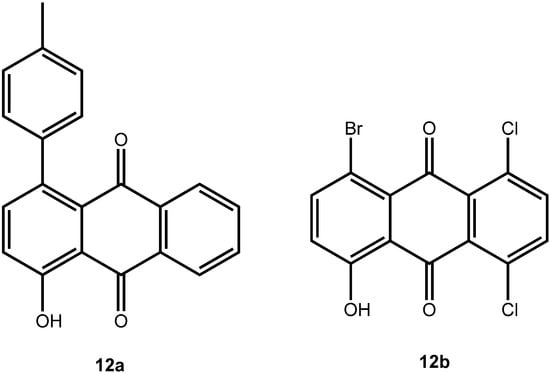
Figure 2.
Hydroxyanthraquinones as side products in the oxidative cycloaddition reactions of thiophenes to quinines.
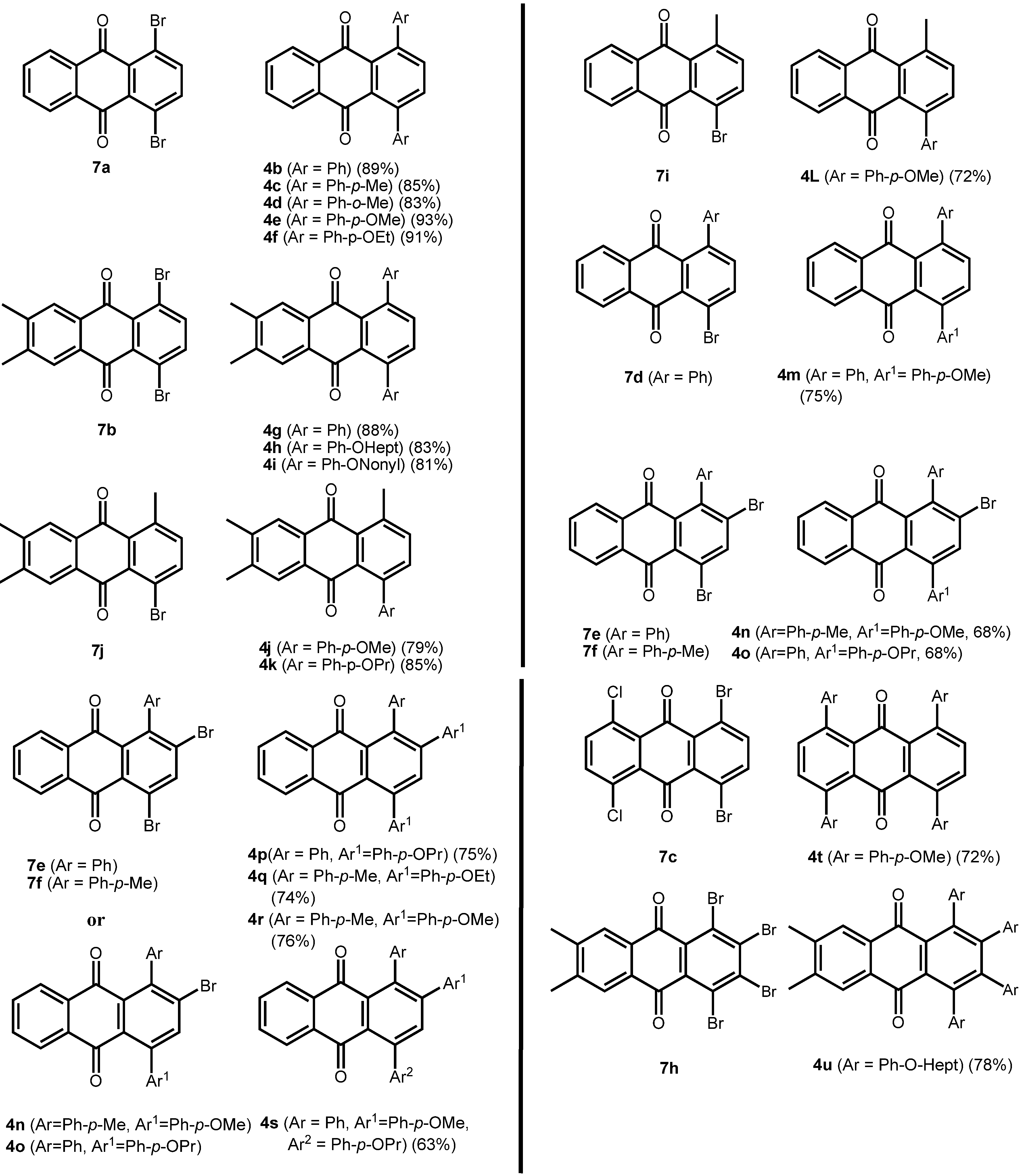
The brominated anthraquinones obtained were subjected to Suzuki-Miyaura cross coupling reactions with a variety of arylboronic acids. Either Pd(PPh3)4/PPh3 or Pd(PPh3)2Cl2/PPh3 was used as catalyst in a biphasic reaction medium of DME and aq. Na2CO3. The corresponding arylated anthraquinones were obtained in good yield. In the case of the 1-aryl-2,4-dibromoanthraquinones, the first aryl group enters selectively into the 4-position, ie., away from the aryl function already present in the anthraquinone system (Figure 3). Prolonged reaction times and an excess of arylboronic acid make the 2-position accessible, also. In this manner it is possible to provide anthraquinones with three different aryl substituents in positions 1, 2 and 4.
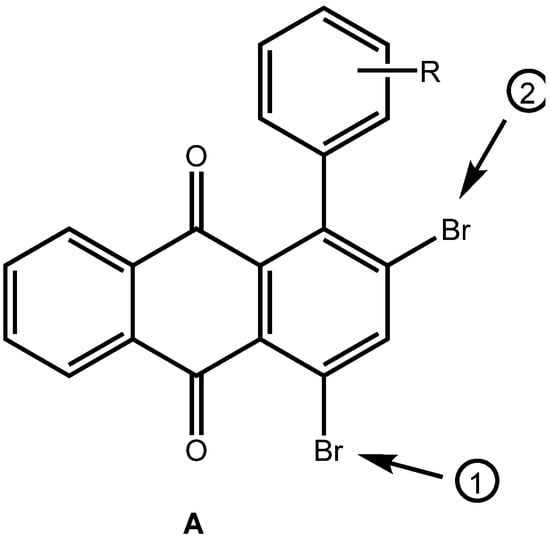
Figure 3.
Order of entry of further aryl substituents by Suzuki-Miyaura cross-coupling reaction.
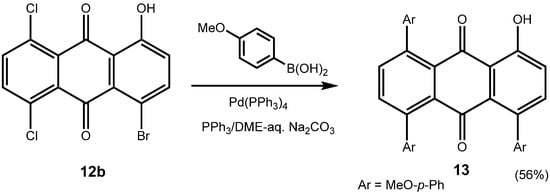
Equally interesting is the fact that chlorinated anthraquinones exchange the chloro-substituent readily, and thus they undergo Suzuki-Miyaura cross coupling reactions with ease, too, even when using a common catalyst such as Pd(PPh3)4. Thus, 1,4-dibromo-5,8-dichloroanthraquinone (7c) can be converted to the 1,4,5,8-tetra-arylanthraquinone 4t (see continued Table 2), using Pd(PPh3)4 as a catalyst, as can be 1-bromo-5,8-dichloro-4-hydroxyanthraquinone (12b) to 13 (Scheme 7).
Scheme 6.
Arylated anthraquinones by Suzuki-Miyaura coupling of dibromoanthraquinones.

Table 2.
Arylated anthraquinones by Suzuki-Miyaura coupling of dibromoanthraquinones.
 |
Scheme 7.
Suzuki-Miyaura coupling of chloroanthraquinones.
The anthraquinones obtained show spectral data typical for this species of compounds. Thus, in the mass spectra, many of the anthraquinones prepared above have [M+-CO] and [M+-2CO] fragmentation peaks that are typical for anthraquinones [,]. In their carbon NMR spectra, the carbonyl functions resonate at 184 – 185 ppm. In 1,2-aryl-substituted anthraquinones, the influence of the proximity of the π-system of one aryl group on the protons of the other can be noted by a high-field shift.
The UV-VIS spectra of most of the solutions of the arylated anthraquinones in acetonitrile show at least three distinct bands, usually associated with π-π* transitions [,]. The strongest band, normally called a ‘benzoid band’ [], is located at around λ = 250 nm for most of the compounds, which is in accordance to data gathered from other substituted anthraquinones. It could be shown that the substitution pattern of the aryl substituent in the anthraquinone has little influence on the wavelength of this absorption band. Methylation of the C6/C7 positions in the anthraquinone core leads to a shift of Δλ= 10 nm, where λmax = 263 nm. A longer-wave π-π*-transition (often called a ‘quinoid band’ []) can be found as a shoulder at λ = 265 – 270 nm for the 1,4-diarylated anthraquinones. Again, there is very little influence of the substitution pattern of the aryl groups at C1 and C4 on the wavelength of this band. Also, 1,2,4-triarylated anthraquinones show this band within the same wavelength region. Where identifiable, this transition is shifted to lower energy for 6,7-methylated anthraquinones (eg., for 4k, λ = 279 nm). A shift to higher wavelength is also found for the β-bromo substituted anthraquinone 4n (λ = 275 nm). Two further π-π* transitions can be noted, although they cannot be identified for all compounds measured. The first is found at around λ = 300 nm. The π-π* transition with the longest wavelength can be noted at λ = 350 – 380 nm for the compounds measured. Substituent dependence of this transition has been reported for mono-substituted anthraquinones [,], and also in our case a substituent-dependence can be noted.
3. Experimental Section
Warning: Working with meta-chloroperoxybenzoic acid at elevated temperatures is hazardous. The reactions should be carried out in a well-ventilated hood. Protections against an explosion should be set up. (The authors themselves have not experienced any difficulties with these reactions. The above measures may be seen as protective precautions).
General
Melting points were measured on a Yanaco microscopic hot stage and are uncorrected. IR spectra were measured with JASCO IR-700 and Nippon Denshi JIR-AQ2OM machines. 1H- and 13C-NMR spectra were recorded with a JEOL EX-270 (1H at 270 MHz and 13C at 67.8 MHz) and JEOL Lambda 400 spectrometer (1H at 395 MHz and 13C at 99.45 MHz). The chemical shifts are relative to TMS (solvent CDCl3, unless otherwise noted). Mass spectra were measured with a JMS-01-SG-2 spectrometer [electron impact mode (EI), 70 eV or fast atom bombardment (FAB)]. Column chromatography was carried out on Wakogel 300.
The oxidative cycloaddition reactions were carried out with commercially available meta-chloroperbenzoic acid (m-CPBA, 70-75 w%, Acros), which was used without further purification. Pd(PPh3)4 (TCI), Pd(PPh3)2Cl2 (TCI), 2,5-dibromothiophene (8a) (Aldrich), 2-methylthiophene (TCI), 2-bromothiophene (Aldrich), thiophene (Wako) and 2,5-dichlorothiophene (Aldrich) were acquired commercially. 2,3,4,5-Tetrabromothiophene (8e) (thiophene, Br2, CHCl3) [], 2-bromo-5-methyl-thiophene (8f) (2-methylthiophene, NBS, CHCl3, AcOH), 2-bromo-5-phenylthiophene (8b) and 2-bromo-5-(p-tolyl)thiophene (a. 2-bromothiophene, Aryl-B(OH)2, Pd(PPh3)4, DME, aq. Na2CO3; b. N-bromosuccinimide [NBS], CHCl3, AcOH) [] were prepared analogous to known procedures. 2,4-Dibromo-5-arylthiophenes, 8c and 8d, were synthesized by brominating 2-arylthiophenes using an excess [] of NBS. 5,8-Dichloro-1,4-naphthoquinone (3c) was prepared by oxidative cycloaddition of 2,5-dichlorothiophene to p-benzoquinone []. 2,3-Dimethyl-5,8-naphthoquinone (3b) was prepared by cycloaddition of 2,3-dimethylbuta-1,3-diene to p-benzoquinone under EuCl3 catalysis (96 h, ClCH2CH2Cl, rt) [] with subsequent base catalysed enolisation [,] of the 4a,5,8,8a-tetrahydro-6,7-dimethyl-1,4-naphthoquinone formed and oxidation of the 6,7-dimethyl-5,8-dihydronaphthalene-1,4-diol (Ag2O, Na2SO4, benzene) [] to 6,7-dimethyl-5,8-dihydro-1,4-naphthoquinone, which in a last step was dehydrogenated (DDQ, benzene, reflux). p-Methoxyphenylboronic acid (TCI), o-methoxyphenylboronic acid (TCI), phenylboronic acid (TCI), and p-tolylboronic acid (Aldrich) were acquired commercially. p-Ethoxy- and p-propoxyphenylboronic acids were prepared from the corresponding p-alkoxy-bromobenzenes (a. n-BuLi, B(OEt)3, THF; b. HCl) [].
1,4-Dibromoanthraquinone (7a) [,]. To a stirred solution of dibromothiophene (8a, 1.00 g, 4.16 mmol) and 1,4-naphthoquinone (3a, 517 mg, 3.47 mmol) in CHCl3 (20 mL) at 75 ºC was added m-CPBA (70w%, 4.76 g) in small portions. After 48 h, the mixture was cooled and poured into an aq. sat. Na2CO3 solution. After the mixture was stirred for 15 min. at rt, it was extracted with chloroform (3 X 25 mL). The organic phase was dried over anhydrous MgSO4 and concentrated in vacuo. The residue was subjected to column chromatography on silica gel (hexane/ether/CHCl3 8:1:1) to give 7a (370 mg, 29%); δH (270 MHz, CDCl3) 7.78 – 7.81 (2H, m), 7.81 (2H, s), 8.20 – 8.23 (2H, m); δC (67.8 MHz, CDCl3) 122.1 (2C, Cquat), 126.9 (2C, CH), 133.5 (2C, Cquat), 133.6 (2C, Cquat), 134.2 (2C, CH), 140.6 (2C, CH), 181.6 (2C, Cquat, CO); MS (EI, 70 eV) m/z (%) 368 ([81Br2]M+) (50), 366 ([81Br79Br]M+) (100), 364 ([79Br2]M+) (51), 340 ([81Br2]M+-CO) (15), 338 ([81Br79Br]M+-CO) (30), 336 ([79Br2]M+ - CO) (15), 312 ([81Br2]M+-2CO) (10), 310 ([81Br79Br]M+-2CO) (21), 308 ([79Br2]M+-2CO) (11), 287 (11), 285 (11), 231 (15), 229 (15), 150 (73). HRMS Found: 365.8716. Calcd. for C14H6O279Br81Br: 365.8715.
Selected data of other bromoanthraquinones
1,4-Dibromo-6,7-dimethylanthraquinone (7b). Yellow solid; δH (270 MHz, CDCl3) 2.42 (6H, s, 2 CH3), 7.77 (s, 2H), 7.94 (s, 2H); δC (67.8 MHz, CDCl3) 20.3 (2C, CH3), 122.1 (2C, Cquat), 127.8 (2C, CH), 131.5 (2C, Cquat), 133.7 (2C, Cquat), 140.4 (2C, CH), 144.5 (2C, Cquat), 181.8 (2C, Cquat, CO); MS (EI, 70 eV) m/z (%) 396 ([81Br2]M+, 50), 394 ([81Br79Br]M+, 100), 392 ([79Br2]M+, 50), 368 ([81Br2]M+-CO, 12), 366 ([81Br79Br]M+ - CO, 25), 364 ([79Br2]M+ - CO, 13). HRMS Found: 393.9033. Calcd. for C16H10O279Br81Br: 393.9028.
1,4-Dibromo-5,8-dichloroanthraquinone (7c). Colorless solid; δH (270 MHz, CDCl3) 7.60 (2H, s), 7.72 (2H, s); MS (EI, 70 eV) m/z 438 (3.3), 436 (9.2), 434 (9.6), 432 (3.9), 149 (34), 58 (100). HRMS Found: 433.7930. Calcd. for C14H4O235Cl37Cl79Br2: 433.7933.
2,4-Dibromo-1-(4-methylphenyl)anthraquinone (7f). Yellow solid, mp. 183 ºC; δH (270 MHz, CDCl3) 2.47 (3H, s, CH3), 7.01 (2H, d, 3J = 8.1 Hz), 7.31 (2H, d, 3J = 8.1 Hz), 7.50 – 7.80 (2H, m), 7.96 – 8.00 (1H, m), 8.20 – 8.23 (1H, m), 8.38 (1H, s); δC (67.8 MHz, CDCl3) 21.6, 122.1, 126.9, 127.6 (2C), 128.0, 129.0, 129.2 (2C), 131.3, 133.2, 133.4, 133.5, 134.0, 134.1, 137.3, 137.4, 143.6, 143.9, 182.1, 182.2; MS (EI, 70 eV) m/z (%) 456 ([81Br79Br]M+) (18), 299 (100). HRMS Found: 455.9188. Calcd. for C21H12O281Br79Br: 455.9185.
1,2,3,4-Tetrabromoanthraquinone (7g) []. Orange solid; mp. 200 ºC; δH (270 MHz, CDCl3) 7.76 – 7.79 (2H, m), 8.11 – 8.14 (2H, m); δC (67.8 MHz, CDCl3) 125.0 (2C, Cquat), 126.8 (2C, CH), 133.6 (2C, Cquat), 134.3 (2C, CH), 139.0 (2C, Cquat), 181.8 (2C, Cquat, CO); MS (FAB, 3-nitrobenzyl alcohol) m/z (%) 527 ([81Br379Br]MH+) (0.2), 526 ([81Br379Br]M+) (0.1), 525 ([81Br279Br2]MH+) (0.3), 524 ([81Br279Br2]M+) (0.2), 523 ([81Br79Br3]MH+) (0.2). HRMS Found: 524.6993. Calcd. for C14H5O279Br281Br2: 524.6983 (MH+, FAB).
1,2,3,4-Tetrabromo-6,7-dimethylanthraquinone (7h). Slowly solidifying yellow oil; δH (270 MHz, CDCl3) 2.42 (6H, s, 2 CH3), 7.86 (2H, s); δC (67.8 MHz, CDCl3) 20.3 (2C, CH3), 127.7 (2C), 131.5 (2C), 134.5 (2C), 135.4 (2C), 138.7 (2C), 144.5 (2C), 181.8 (2C, CO); MS (FAB, 3-nitrobenzyl alcohol) m/z (%) 552 ([81Br279Br2]M+) (0.2)
1-Bromo-4-methylanthraquinone (7i) []. Beige colored solid; δH (270 MHz, CDCl3) 2.79 (3H, s, CH3), 7.36 (1H, d, 3J = 8.4 Hz), 7.74 – 7.78 (2H, m), 7.87 (1H, d, 3J = 8.4 Hz), 8.15 – 8.24 (2H, m); δC (67.8 MHz, CDCl3) 23.6 (CH3), 120.2 (Cquat), 126.6 (CH), 126.9 (CH), 127.8 (Cquat), 132.9 (Cquat), 133.8 (2C, CH), 134.4 (Cquat), 137.9 (CH), 140.2 (CH), 140.4 (Cquat), 141.9 (Cquat), 182.9 (Cquat, CO), 184.5 (Cquat, CO); MS (EI, 70 eV) m/z (%) 302 ([81Br]M+, 97), 300 ([79Br]M+, 100), 274 ([81Br]M+-CO, 15), 272 ([79Br]M+-CO, 15), 193 (59), 165 (90). HRMS Found: 301.9764. Calcd. for C15H9O281Br: 301.9767. Found: 299.9789. Calcd. for C15H9O279Br: 299.9786.
1-Bromo-4,6,7-trimethylanthraquinone (7j). Yellow solid, mp. 194 ºC; δH (270 MHz, CDCl3) 2.42 (6H, s, 2 CH3), 2.73 (3H, s, CH3), 7.33 (1H, d, 3J = 8.4 Hz), 7.82 (1H, d, 3J = 8.4 Hz), 7.91 (1H, s), 7.95 (1H, s); δC (67.8 MHz, CDCl3) 20.2 (2C, CH3), 20.7 (CH3), 120.0 (Cquat), 127.4 (CH), 127.8 (CH), 131.7 (Cquat), 131.8 (Cquat), 132.6 (Cquat), 134.1 (Cquat), 137.7 (CH), 140.0 (CH), 141.8 (Cquat), 143.8 (Cquat, 2C), 183.2 (Cquat, CO), 184.8 (Cquat, CO); MS (EI, 70 eV) m/z (%) 330 ([81Br]M+) (100), 328 ([79Br]M+) (100), 315 ([81Br]M+-CH3) (38), 313 ([79Br]M+-CH3) (39), 302 ([81Br]M+-CO) (26), 300 ([81Br]M+-CO) (28), 287 ([81Br]M+-CH3-CO) (26), 285 ([79Br]M+-CO-CH3) (26), 221 (55), 178 (83). HRMS Found: 328.0097. Calcd. for C17H13O279Br: 328.0099.
1,4-Bis(4-methylphenyl)anthraquinone (4c) []. Under an inert atmosphere, a solution of 7a (324 mg, 0.89 mmol), 4-methylphenylboronic acid (385 mg, 2.83 mmol), Pd(PPh3)2Cl2 (30 mg, 4.0.10-5 mol) and triphenylphosphine (30 mg, 0.11 mmol) in a solvent mixture of DME (10 mL) and aq. Na2CO3 (2.32 g Na2CO3 in 15 mL H2O, 6 mL) was kept at 65 ºC for 18h. Thereafter the cooled solution was poured into water (25 mL) and extracted with chloroform (3 x 15 mL). The combined organic phase was dried over anhydrous MgSO4 and was concentrated in vacuo. Column chromatography of the residue on silica gel (hexane/CHCl3/ether 3:1:1) gave 4c (293 mg, 85%) as an orange solid; mp. 265 ºC; δH (270 MHz, CDCl3) 2.45 (6H, s, 2 CH3), 7.18 (4H, d, 3J = 7.6 Hz), 7.27 (4H, d, 3J = 7.6 Hz), 7.53 (2H, s), 7.65 – 7.70 (2H, m), 8.05 - 8.09 (2H, m); δC (67.8 MHz, CDCl3) 21.3 (2C, CH3), 126.7 (2C, CH), 127.9 (4C, CH), 128.9 (4C, CH), 132.8 (2C, Cquat), 133.7 (2C, CH), 134.1 (2C, Cquat), 136.5 (2C, CH), 136.8 (2C, Cquat), 139.4 (2C, Cquat), 143.9 (2C, Cquat), 184.2 (2C, CO); MS (EI, 70 eV) m/z (%) = 388 (M+) (83), 373 (M+-CH3) (100), 179 (40). HRMS Found: 388.1469. Calcd. for C28H20O2: 388.1463. Found: C, 84.36; H, 5.12%. Calcd. for C28H20O2.H2O: C, 84.61; H, 5.33%. UV-Vis spectrum (CH3CN, nm) λmax 253 (44700), 268 (sh, 21310), 298 (9350), 358 (2470).
Selected data for other arylated anthraquinones
1,4-Diphenylanthraquinone (4b) [,] Yellow solid; δH (270 MHz, CDCl3) 7.29 – 7.35 (4H, m), 7.43 – 7.48 (6H, m), 7.56 (2H, s), 7.66 – 7.71 (2H, m), 8.05 – 8.08 (2H, m); δC (67.8 MHz, CDCl3) 126.8 (2C, CH), 127.2 (2C, CH), 127.9 (4C, CH), 128.2 (4C, CH), 132.7 (2C, Cquat), 133.7 (2C, CH), 134.0 (2C, Cquat), 136.4 (2C, CH), 142.3 (2C, Cquat), 144.1 (2C, Cquat), 184.0 (2C, Cquat, CO); MS (FAB, 3-nitrobenzyl alcohol) m/z (%) 361 (MH+) (5.6). HRMS Found: 361.1232. Calcd. for C26H17O2: 361.1229 (MH+, FAB); UV-Vis spectrum (CH3CN, nm) λmax 253 (36370), 269 (sh, 19190), 288 (sh, 7320).
1,4-Bis(4-methoxyphenyl)anthraquinone (4e). Orange needles; mp. 231 ºC; δH (270 MHz, CDCl3) 3.89 (6H, s, 2 OCH3), 7.00 (4H, d, 3J = 8.6 Hz), 7.26 (4H, d, 3J = 8.6 Hz), 7.53 (2H, s), 7.68 – 7.72 (2H, m), 8.06 – 8.09 (2H, m); δC (67.8 MHz, CDCl3) 55.2 (2C, OCH3), 113.7 (4C, CH), 126.7 (2C, CH), 129.3 (4C, CH), 133.7 (2C, CH), 132.9 (2C, Cquat), 134.1 (2C, Cquat), 134.5 (2C, Cquat), 136.6 (2C, CH), 143.6 (2C, Cquat), 158.9 (2C, Cquat), 184.3 (2C, Cquat, CO); MS (EI, 70 eV) m/z (%) 420 (M+) (100), 389 (32), 333 (18), 313 (13), 276 (17). HRMS Found: 420.1367. Calcd. for C28H20O4: 420.1362; UV-Vis spectrum (CH3CN, nm) λmax 253 (59610), 271 (sh, 23890), 313 (13280).
1,4-Bis(4-ethoxyphenyl)anthraquinone (4f). Orange needles; mp. 239 ºC; δH (270 MHz, CDCl3) 1.47 (3H, t, CH3, 3J = 7.0 Hz), 4.12 (2H, q, OCH2, 3J = 7.0 Hz), 6.98 (4H, d, 3J = 8.4 Hz), 7.24 (4H, d, 3J = 8.4 Hz), 7.53 (2H, s), 7.67 – 7.70 (2H, m), 8.05 – 8.09 (2H, m); δC (67.8 MHz, CDCl3) 14.9 (2C, CH3), 63.4 (2C, OCH2), 114.2 (4C, CH), 126.7 (2C, CH), 129.3 (4C, CH), 132.9 (2C, Cquat), 133.6 (2C, CH), 134.2 (2C, Cquat), 134.3 (2C, Cquat), 136.6 (2C, CH), 143.6 (2C, Cquat), 158.3 (2C, Cquat), 184.3 (2C, Cquat, CO); MS (FAB, 3-nitrobenzyl alcohol) m/z (%) 449 (MH+) (7.5). HRMS Found: 449.1749. Calcd. for C30H25O4: 449.1753. Found: C, 79.57; H, 5.47%. Calcd. for C30H24O4.0.2H2O: C, 79.70; H, 5.44%; UV-Vis spectrum (CH3CN, nm) λmax 253 (49430), 269 (sh, 21220), 314 (10910).
1,4-Diphenyl-6,7-dimethylanthraquinone (4g). Yellow needles, mp. 232 ºC; δH (270 MHz, CDCl3) 2.34 (6H, s, 2 CH3), 7.30 – 7.34 (4H, m), 7.43 – 7.50 (6H, m), 7.53 (2H, s), 7.82 (2H, s); δC (67.8 MHz, CDCl3) 20.1 (2C, CH3), 127.0 (2C, CH), 127.7 (2C, CH), 127.9 (4C, CH), 128.1 (4C, CH), 132.0 (2C, Cquat), 132.9 (2C, Cquat), 136.1 (2C, CH), 142.5 (2C, Cquat), 143.7 (2C, Cquat), 143.9 (2C, Cquat), 184.1 (2C, Cquat, CO); MS (FAB, 3-nitrobenzyl alcohol) m/z (%) 389 (MH+) (5.3). HRMS Found: 389.1539. Calcd. for C28H21O2: 389.1542 (FAB). Found: C, 85.80; H, 5.18%. Calcd. for C28H20O2.0.2H2O: C, 85.78; H, 5.24%; UV-Vis spectrum (CH3CN, nm) λmax 263 (41490), 338 (4480).
1,4-Bis(4-heptoxyphenyl)-6,7-dimethylanthraquinone (4h). Beige solid; mp. 181 ºC; δH (270 MHz, CDCl3) 0.92 (6H, t, 3J = 6.2 Hz, 2 CH3), 1.34 – 1.60 (16H, m), 1.78 – 1.86 (4H, m), 2.35 (6H, s, 2 CH3), 4.03 (4H, t, 3J = 6.5 Hz), 6.98 (4H, d, 3J = 8.6 Hz), 7.23 (4H, d, 3J = 8.6 Hz), 7.50 (2H, s), 7.83 (2H, s); δC (67.8 MHz, CDCl3) 14.1 (2C, CH3), 20.2 (2C, CH2), 22.7 (2C, CH2), 26.1 (2C, CH2), 29.1 (2C, CH2), 29.4 (2C, CH2), 31.8 (2C, CH2), 67.9 (2C, OCH2), 114.1 (4C, CH), 127.7 (2C, CH), 129.2 (4C, CH), 132.1 (2C, Cquat), 133.0 (2C, Cquat), 134.4 (2C, Cquat), 136.4 (2C, Cquat), 143.5 (2C, Cquat), 158.4 (2C, Cquat), 184.5 (2C, Cquat, CO); MS (FAB, 3-nitrobenzyl alcohol) m/z (%) 617 (MH+) (0.5). HRMS Found: 617.3629. Calcd. for C42H49O4: 617.3631 (MH+, FAB)
1,4-Bis(4-nonyloxyphenyl)-6,7-dimethylanthraquinone (4i). Yellow-orange solid; mp. 176 ºC; δH (270 MHz, CDCl3) 0.88 (6H, t, 3J = 4.3 Hz, 2 CH3), 1.30 (20H, m), 1.44 – 1.49 (4H, m), 1.78 – 1.85 (4H, m), 2.35 (6H, s, 2 CH3), 4.03 (4H, t, 3J = 6.5 Hz), 6.98 (4H, d, 3J = 8.6 Hz), 7.23 (4H, d, 3J = 8.6 Hz), 7.50 (2H, s), 7.83 (2H, s); δC (67.8 MHz, CDCl3) 14.1 (2C, CH3), 20.2 (2C, CH2), 22.7 (2C, CH2), 26.1 (2C, CH2), 29.3 (2C, CH2), 29.4 (2C, CH2), 29.5 (2C, CH2), 29.6 (2C, CH2), 31.9 (2C, CH2), 67.9 (2C, OCH2), 114.1 (4C, CH), 127.6 (2C, CH), 129.2 (4C, CH), 132.1 (2C, Cquat), 133.0 (2C, Cquat), 134.4 (2C, Cquat), 136.4 (2C, Cquat), 143.5 (2C, Cquat), 158.4 (2C, Cquat), 184.5 (2C, Cquat, CO); MS (FAB, 3-nitrobenzyl alcohol) m/z (%) 673 (MH+) (100). HRMS Found: 673.4254. Calcd. for C46H57O4: 673.4257 (MH+, FAB).
1-(4-Methoxyphenyl)-4,6,7-trimethylanthraquinone (4j). Solid; δH (270 MHz, CDCl3) 2.30 (3H, s, CH3), 2.40 (3H, s, CH3), 2.86 (3H, s, CH3), 3.87 (3H, s, OCH3), 6.96 (2H, d, 3J = 8.6 Hz), 7.19 (2H, d, 3J = 8.6 Hz), 7.40 (1H, d, 3J = 7.8 Hz), 7.50 (1H, d, 3J = 7.8 Hz), 7.80 (1H, s), 7.94 (1H, s); δC (67.8 MHz, CDCl3) 20.1 (CH3), 20.2 (CH3), 23.8 (CH3), 55.2 (OCH3), 113.5 (2C, CH), 127.4 (CH), 127.5 (CH), 129.1 (2C, CH), 132.1 (Cquat), 132.2 (Cquat), 132.9 (Cquat), 133.0 (Cquat), 135.1 (Cquat), 136.7 (CH), 136.8 (CH), 141.1 (Cquat), 142.5 (Cquat), 143.3 (Cquat), 143.4 (Cquat), 158.6 (Cquat), 184.7 (Cquat, CO), 185.9 (Cquat, CO); MS (EI, 70 eV) m/z (%) 356 (M+) (84), 355 (100), 325 (32), 312 (14). HRMS Found: 356.1413. Calcd. for C24H20O3: 356.1412; UV-Vis spectrum (CH3CN, nm) λmax 263 (49630), 278 (sh, 19470), 339 (4880).
1-(4-Propoxyphenyl)-4,6,7-trimethylanthraquinone (4k). Yellow solid; mp. 215 ºC; δH (270 MHz, CDCl3) 1.07 (3H, t, 3J = 7.6 Hz, CH3), 1.56 (3H, s, CH3), 1.85 (2H, dt, 3J = 7.6 Hz, 3J = 6.5 Hz), 2.35 (3H, s, CH3), 2.41 (3H, s, CH3), 3.99 (2H, t, 3J = 6.5 Hz, OCH2), 6.95 (2H, d, 3J = 8.6 Hz), 7.17 (2H, d, 3J = 8.6 Hz), 7.41 (1H, d, 3J = 7.8 Hz), 7.50 (1H, d, 3J = 7.8 Hz), 7.81 (1H, s), 7.94 (1H, s); δC (67.8 MHz, CDCl3) 10.6 (CH3), 20.1 (CH3), 20.2 (CH3), 22.7 (CH2), 23.8 (CH3), 69.4 (OCH2), 114.1 (2C, CH), 127.5 (CH), 127.6 (CH), 129.1 (2C, CH), 132.1 (Cquat), 132.2 (Cquat), 132.8 (Cquat), 133.0 (Cquat), 134.8 (Cquat), 136.7 (CH), 136.8 (CH), 141.1 (Cquat), 142.6 (Cquat), 143.3 (Cquat), 143.4 (Cquat), 158.2 (Cquat), 184.7 (Cquat, CO), 185.9 (Cquat, CO); MS (EI, 70 eV) m/z (%) 384 (M+) (68), 341 (M+-(CH2)2CH3) (100). HRMs Found: 384.1718. Calcd. for C26H24O3: 384.1725 UV-Vis spectrum (CH3CN, nm) λmax 263 (57850), 279 (sh, 20490), 330 (5580, ill-defined).
1-(4-Methoxyphenyl)-4-methylanthraquinone (4l) []. Yellow-orange needles; mp. 221 ºC; δH (270 MHz, CDCl3) 2.88 (3H, s, CH3), 3.88 (3H, s, OCH3), 6.97 (2H, d, 3J = 8.6 Hz), 7.20 (2H, d, 3J = 8.6 Hz), 7.44 (1H, d, 3J = 8.1 Hz), 7.53 (1H, d, 3J = 8.1 Hz), 7.67 – 7.75 (2H, m), 8.04 – 8.07 (1H, m), 8.19 – 8.23 (1H, m); δC (67.8 MHz, CDCl3) 23.8 (CH3), 55.2 (OCH3), 113.6 (2C, CH), 126.6 (CH), 129.2 (2C, CH), 132.8 (Cquat), 132.9 (Cquat), 133.5 (CH), 133.6 (CH), 134.1 (Cquat), 134.2 (Cquat), 134.8 (Cquat), 137.0 (CH, 3C), 141.3 (Cquat), 142.6 (Cquat), 158.7 (Cquat), 184.6 (Cquat, CO), 184.7 (Cquat, CO); MS (FAB, 3-nitrobenzyl alcohol) m/z (%) 329 (MH+) (14). HRMS Found: 329.1183. Calcd. for C22H17O3: 329.1178 (MH+, FAB). Found: C, 79.89; H, 4.73%. Calcd. for C22H16O3.0.1H2O: C, 80.03; H, 4.91%; UV-Vis spectrum (CH3CN, nm) λmax 253 (38343), 269 (sh, 15440), 302 (5400), 354 (2505).
1-(4-Methoxyphenyl)-4-phenylanthraquinone (4m). Beige solid; MS (FAB, 3-nitrobenzyl alcohol) m/z (%) 391 (MH+) (7.6). HRMS Found: 391.1340. Calcd. for C27H19O3: 391.1334 (MH+, FAB); UV-Vis spectrum (CH3CN, nm) λmax 253 (38520), 271 (sh, 18390), 306 (7980).
2-Bromo-1-(4-methylphenyl)-4-(4-methoxyphenyl)anthraquinone (4n). Orange needles; mp. 208 ºC; δH (270 MHz, CDCl3) 2.49 (3H, s, CH3), 3.89 (3H, s, OCH3), 7.01 (2H, d, 3J = 8.9 Hz), 7.08 (2H, d, 3J = 8.1 Hz), 7.27 (2H, d, 3J = 8.9 Hz), 7.33 (2H, d, 3J = 8.1 Hz), 7.66 – 7.70 (2H, m), 7.92 (1H, s), 8.00 – 8.07 (2H, m); MS (FAB, 3-nitrobenzyl alcohol) m/z (%) 485 ([81BrM]H+) (7.2), 484 (81BrM+) (8.0), 483 ([79BrM]H+, 8.9), 482 (79BrM+) (6.0). HRMS Found: 483.0595. Calcd. for C28H20O379Br (MH+, FAB); UV-Vis spectrum (CH3CN, nm) λmax 258 (37150), 275 (sh, 16870), 309 (8100).
2-Bromo-1-phenyl-4-(4-propoxyphenyl)anthraquinone (4o). Yellow solid; mp. 183 ºC; δH (270 MHz, CDCl3) 1.08 (3H, t, 3J = 7.3 Hz, CH3), 1.86 (2H, dt, 3J = 7.3 Hz, 3J = 6.5 Hz), 4.01 (2H, t, 3J = 6.5 Hz, OCH2), 7.00 (2H, d, 3J = 8.4 Hz), 7.17 – 7.21 (2H, m), 7.25 (2H, d, 3J = 8.4 Hz), 7.48 – 7.54 (3H, m), 7.66 – 7.70 (2H, m), 7.93 (1H, s), 7.98 – 8.02 (1H, m), 8.04 – 8.07 (1H, m); δC (67.8 MHz, CDCl3) 10.6 (CH3), 22.7 (CH2), 69.5 (OCH2), 114.3 (2C, CH), 126.7 (CH), 126.9 (CH), 127.4 (CH), 127.9 (2C, CH), 128.3 (2C, CH), 129.2 (2C, CH), 131.7 (Cquat), 132.3 (Cquat), 132.9 (Cquat), 133.6 (Cquat), 133.8 (CH), 133.9 (CH), 134.3 (Cquat), 141.0 (CH), 141.2 (Cquat), 142.9 (Cquat), 145.1 (Cquat), 155.8 (Cquat), 183.1 (Cquat, CO), 183.7 (Cquat, CO); MS (EI, 70 eV) m/z (%) 498 ([81Br]M+) (100), 496 ([79Br]M+) (98), 455 ([81Br]M+-(CH2)2CH3) (84), 453 ([79Br]M+-(CH2)2CH3) (81). HRMS Found: 496.0677. Calcd. for C29H21O379Br: 496.0674.
1-(4-Methylphenyl)-2,4-bis(4-ethoxyphenyl)anthraquinone (4q). Yellow needles, mp. 230 ºC; δH (270 MHz, CDCl3) 1.37 (3H, t, 3J = 7.0 Hz, CH3), 1.46 (3H, t, 3J = 7.0 Hz, CH3), 2.36 (3H, s, CH3), 3.97 (2H, d, 3J = 7.0 Hz, OCH2), 4.11 (2H, d, 3J = 7.0 Hz, OCH2), 6.67 (2H, d, 3J = 8.9 Hz), 6.91 – 6.99 (6H, m), 7.08 (2H, d, 3J = 7.8 Hz), 7.29 (2H, d, 3J = 8.9 Hz), 7.57 (1H, s), 8.00 – 8.04 (2H, m), 8.06 – 8.09 (2H, m); δC (67.8 MHz, CDCl3) 14.7 (CH3), 14.9 (CH3), 21.4 (CH3), 63.3 (OCH2), 63.4 (OCH2), 113.7 (2C, CH), 114.1 (2C, CH), 126.5 (CH), 126.7 (CH), 128.6 (2C, CH), 129.2 (2C, CH), 129.4 (2C, CH), 130.7 (2C, CH), 131.3 (Cquat), 132.0 (Cquat), 133.4 (CH), 133.5 (CH), 134.1 (Cquat), 134.2 (Cquat), 134.3 (Cquat), 134.5 (Cquat), 135.9 (Cquat), 137.0 (Cquat), 138.9 (CH), 141.6 (Cquat), 143.4 (Cquat), 147.4 (Cquat), 158.0 (Cquat), 158.3 (Cquat), 184.1 (Cquat, CO), 184.9 (Cquat, CO); MS (FAB, 3-nitrobenzyl alcohol) m/z (%) 539 (MH+) (31). HRMS Found: 539.2219. Calcd. for C37H31O4: 539.2222. Found: C, 82.26; H, 5.62%. Calcd. for C37H30O4: C, 82.50; H, 5.61%; UV-Vis spectrum (CH3CN, nm) λmax 250 (57030), 268 (sh, 31850), 298 (sh, 19440), 359 (6340).
1-(4-Methylphenyl)-2,4-bis(4-methoxyphenyl)anthraquinone (4r). Orange solid; mp. 230 ºC; δH (270 MHz, CDCl3) 2.36 (3H, s, CH3), 3.75 (3H, s, OCH3), 3.88 (3H, s, OCH3), 6.69 (2H, d, 3J = 8.6 Hz), 6.93 – 6.96 (4H, m), 6.99 (2H, d, 3J = 8.6 Hz), 7.08 (2H, d, 3J = 8.6 Hz), 7.31 (2H, d, 3J = 8.6 Hz), 7.57 (1H, s), 7.65 – 7.69 (2H, m), 8.00 – 8.11 (2H, m); δC (67.8 MHz, CDCl3) 21.4 (CH3), 55.1 (OCH3), 55.2 (OCH3), 113.1 (2C, CH), 113.6 (2C, CH), 126.5 (CH), 126.7 (CH), 128.6 (2C, CH), 129.2 (2C, CH), 129.4 (2C, CH), 130.7 (2C, CH), 131.4 (Cquat), 132.2 (Cquat), 133.5 (CH), 133.6 (CH), 134.1 (Cquat), 134.3 (Cquat), 134.5 (Cquat), 134.6 (Cquat), 135.9 (Cquat), 137.0 (Cquat), 138.9 (CH), 141.6 (Cquat), 143.4 (Cquat), 147.4(Cquat), 158.6 (Cquat), 158.9 (Cquat), 184.1 (Cquat, CO), 184.9 (Cquat, CO). MS (FAB, 3-nitrobenzyl alcohol) m/z (%) 511 (MH+) (13). HRMS Found: 511.1905. Calcd. for C35H27O4: 511.1909 (MH+, FAB); UV-Vis spectrum (CH3CN, nm) λmax 250 (50 085), 269 (sh, 26790), 300 (sh, 15980), 365 (4960).
2-(4-Methoxyphenyl)-1-phenyl-4-(4-propoxyphenyl)anthraquinone (4s). Light yellow needles; mp. 233 ºC; δH (270 MHz, CDCl3) 1.08 (3H, t, 3J = 7.6 Hz, CH3), 1.86 (2H, tt, 3J = 7.6 Hz, 3J = 6.2 Hz), 3.74 (3H, s, OCH3), 4.00 (t, 2H, 3J = 6.2 Hz, OCH2), 6.68 (2H, d, 3J = 8.6 Hz), 6.94 (2H, d, 3J = 8.6 Hz), 6.99 (2H, d, 3J = 8.6 Hz), 7.27 – 7.30 (3H, m), 7.04 – 7.08 (2H, m), 7.30 (2H, d, 3J = 8.6 Hz), 7.59 (1H, s), 7.65 – 7.71 (2H, m), 8.00 – 8.03 (1H, m), 8.07 – 8.10 (1H, m); δC (67.8 MHz, CDCl3) 10.6 (CH3), 22.7 (CH2), 55.1 (OCH3), 69.4 (OCH2), 113.2 (CH, 2C), 114.2 (CH, 2C), 126.5 (CH), 126.6 (CH), 126.7 (CH), 127.7 (CH, 2C), 129.4 (CH, 4C), 130.7 (CH, 2C), 131.4 (Cquat), 132.6 (Cquat), 133.5 (CH), 133.6 (CH), 134.1 (Cquat), 134.2 (Cquat), 134.4 (Cquat), 138.9 (Cquat), 140.2 (Cquat), 141.5 (Cquat), 143.7 (Cquat), 147.3 (Cquat), 158.6 (Cquat), 158.7 (Cquat), 184.1 (Cquat, CO), 184.8 (Cquat, CO). MS (FAB, 3-nitrobenzyl alcohol) m/z (%) 525 (MH+) (9), 524 (M+) (11). HRMS Found: 524.1990. Calcd. For C36H28O4: 524.1988. UV-Vis spectrum (CH3CN, nm) λmax 249 (22370), 268 (sh, 12560), 298 (sh, 7490), 382 (2320).
1,4,5,8-Tetrakis(4-methoxyphenyl)anthraquinone (4t). Pale orange solid; mp. 251 ˚C; δH (270 MHz, CDCl3) 3.84 (12H, s, 4 OCH3), 6.85 (8H, d, 3J = 8.4 Hz), 7.21 (8H, d, 3J = 8.4 Hz), 7.48 (4H, s); δC (67.8 MHz, CDCl3) 55.2 (4C, OCH3), 113.4 (8C, CH), 130.3 (8C, CH), 131.9 (4C, Cquat), 134.5 (4C, CH), 135.5 (4C, Cquat), 140.3 (4C, Cquat), 159.0 (4C, Cquat), 188.4 (2C, Cquat, CO); MS (FAB, 3-nitrobenzyl alcohol) m/z (%) 633 (MH+) (1.0). HRMS Found: 633.2286. Calcd. for C42H33O6: 633.2277 (MH+, FAB).
1,2,3,4-Tetrakis(4-heptoxyphenyl)anthraquinone (4u). Slowly crystallizing orange oil; δH (270 MHz, CDCl3) 0.88 (12H, t, 3J = 7.0 Hz, 4 CH3), 1.28 – 1.31 (32H, m), 1.66 (4H, m), 1.72 (4H, m), 2.32 (6H, s, 2 CH3), 3.74 (4H, t, 3J = 6.5 Hz), 3.90 (4H, t, 3J = 6.5 Hz), 6.39 (4H, d, 3J = 8.6 Hz), 6.52 (4H, d, 3J = 8.6 Hz), 6.70 (4H, d, 3J = 8.6 Hz), 6.86 (4H, d, 3J = 8.6 Hz), 7.78 (2H, s); δC (67.8 MHz, CDCl3) 14.0 (2C, CH3), 14.1 (2C, CH3), 20.1 (2C), 22.5 (2C), 22.6 (2C), 25.9 (2C), 26.1 (2C), 29.0 (2C), 29.1 (2C), 29.2 (2C), 29.4 (2C), 31.7 (2C), 31.8 (2C), 67.7 (4C, OCH2), 112.9 (4C), 113.5 (4C), 128.5 (2C), 130.2 (4C), 131.2 (2C), 131.6 (4C), 132.4 (2C), 132.8 (2C), 133.2 (2C), 142.6 (2C), 143.2 (2C), 148.3 (2C), 157.2 (2C), 156.7 (2C), 184.8 (2C, CO); MS (FAB, 3-nitrobenzyl alcohol) m/z (%) 997 (MH+) (100). HRMS Found: 997.6355. Calcd. for C68H85O5: 997.6346.
1-Hydroxy-4,5,8-tris(4-methoxyphenyl)anthraquinone (13). Reddish solid; mp. 238 ˚C; δH (270 MHz, CDCl3) 3.82 (3H, s, OCH3), 3.83 (3H, s, OCH3), 3.90 (3H, s, OCH3), 6.84 (2H, d, 3J = 8.6 Hz), 6.87 (2H, d, 3J = 8.6 Hz), 7.00 (2H, d, 3J = 8.6 Hz), 7.14 – 7.29 (7H, m), 7.46 (1H, d, 3J = 7.8 Hz), 7.47 (1H, d, 3J = 8.4 Hz), 7.56 (1H, d, 3J = 7.8 Hz), 12.21 (s, 1H, OH); δC (67.8 MHz, CDCl3) 55.2 (2C, OCH3), 55.3 (OCH3), 113.6 (6C, CH), 117.1 (Cquat), 122.1 (CH), 129.4 (2C, CH), 129.9 (2C, CH), 130.2 (2C, CH), 131.0 (Cquat), 132.2 (Cquat), 132.5 (Cquat), 134.0 (Cquat), 134.1 (Cquat), 134.2 (Cquat), 136.0 (CH), 136.4 (CH), 136.8 (Cquat), 139.5 (CH), 142.0 (Cquat), 142.9 (Cquat), 158.8 (Cquat), 159.0 (2C, Cquat), 161.0 (Cquat), 188.0 (Cquat, CO), 189.5 (Cquat, CO); MS (FAB, 3-nitrobenzyl alcohol) m/z (%) 543 (MH+) (1.4). HRMS Found: 543.1805. Calcd. for C35H27O6: 543.1808 (MH+, FAB).
4. Conclusions
Bromoanthraquinones can be synthesized by an oxidative cycloaddition to suitably substituted naphthoquinones. Bromoanthraquinones can be reacted further to arylated anthraquinones via Suzuki-Miyaura coupling. Initial results show that also chloro substituted anthraquinones undergo Suzuki reactions in presence of the commercially available Pd(PPh3)4. The UV-VIS spectra of most of the solutions of the arylated anthraquinones in acetonitrile show at least three distinct bands associated with π-π* transitions. Substituent dependence of the longest wavelength transition of the three bands can be noted.
Acknowledgements
This work has been supported in part by the Global Centre of Excellence of Kyushu University on New Carbon Resources.
References and Notes
- Josien, J.M.; Fuson, N. The infrared study of the valence bond C=O in polycyclic compounds. Bull. Soc. Chim. Fr. 1952, 389–397. [Google Scholar]
- Gautrot, J.E.; Hodge, P.; Cupertino, D.; Helliwell, M. Experimental evidence for carbonyl-π electron cloud interactions. New J. Chem. 2006, 30, 1801–1807. [Google Scholar] [CrossRef]
- Showalter, H.D.H.; Berman, E.M.; Johnson, J.L.; Hunter, W.E. A facile synthesis of functionalized 9,10-anthracenediones via tosylate and triflatephenolic activation. Tetrahedron Lett. 1985, 26, 157–160. [Google Scholar] [CrossRef]
- Shcheglova, N.A.; Shigorin, D.N.; Dokunikhin, N.S. Characteristics of luminescence and absorption spectra of 1- and 2-alkyl- and arylanthraquinones in solutions. II. Luminescence spectra at 77 K. Zh. Fiz. Khim. 1966, 40, 1048–1055, [Chem. Abstr. 1966, 65, 9958b]. [Google Scholar]
- Shcheglova, N.A.; Shigorin, D.N.; Dokunikhin, N.S. Spectra of luminescence and absorption of α - and β -alkyl and aryl derivatives of anthraquinone in solutions. I. Absorption spectra of α - and β -alkyl and aryl derivatives of anthraquinone. Zh. Fiz. Khim. 1965, 39, 3039–3043, [Chem. Abstr. 1965, 64, 12058f]. [Google Scholar]
- Orser, D.A. (General Electric Company, Schenectady, N.Y., USA) Light-modulating medium for image projection apparatus. US Pat. 3764549 9 Oct. 1973. [Chem. Abstr. 1973, 79, 141431j]. [Google Scholar]
- Weizmann, C.; Bergmann, E.; Haskelberg, L. Phenylated phthalic acids and anthracene derivatives. J. Chem. Soc. 1939, 391–397. [Google Scholar] [CrossRef]
- Bergmann, E.; Haskelberg, L.; Bergmann, F. Successive diene addition and dehydrogenation in nitrobenzene solution without isolation of the hydroaromatic intermediate. J. Org. Chem. 1942, 7, 303–306. [Google Scholar] [CrossRef]
- Muschik, G.M.; Tomaszewski, J.E.; Sato, R.I. Synthesis of the 1-,2-,3-,4-hydroxy isomers of benz[a]anthracene-7,12-dione, benz[a]anthracene, and 7,12-dimethylbenz[a]anthracene. J. Org. Chem. 1979, 44, 2150–2153. [Google Scholar] [CrossRef]
- Puchkov, V.A. Synthesis of 1-phenylanthraquinone. Zh. Vsesoy. Khim. Obshch. im. D. I. Mendeleeva 1961, 6, 238–239, [Chem. Abstr. 1961, 55, 19877g]. [Google Scholar]
- Battegay, M.; Claudin, J. Dibromoanthraquinones. Bull. Soc. Chim. Fr. 1921, 29, 1017–1027. [Google Scholar]
- Li, Y.Q.; Thiemann, T.; Sawada, T.; Tashiro, M. Novel crown ethers by oxidative cycloaddition of thiopheno crown ethers. J. Chem. Soc., Perkin Trans. 1 1994, 2323–2329. [Google Scholar]
- Thiemann, T.; Li, Y.Q.; Mataka, S.; Tashiro, M. Intramolecular oxidative cycloaddition of thiophenes. J. Chem. Res. (S) 1995, 384–384, (M) 1995, 2364-2379. [Google Scholar]
- Thiemann, T.; Li, Y.Q.; Thiemann, C.; Sawada, T.; Ohira, D.; Tashiro, M.; Mataka, S. Oxidative cycloaddition of structures with multi core thiophenes. Heterocycles 2000, 1215–1230. [Google Scholar]
- Li, Y.Q.; Matsuda, M.; Thiemann, T.; Sawada, T.; Mataka, S. Lewis acid catalysed oxidative cycloaddition of thiophenes. Synlett. 1996, 461–464. [Google Scholar]
- Li, Y.Q.; Thiemann, T.; Sawada, T.; Mataka, S.; Tashiro, M. Lewis acid catalysis in the oxidative cycloaddition of thiophenes. J. Org. Chem. 1997, 62, 7926–7936. [Google Scholar] [CrossRef]
- Li, Y.Q.; Thiemann, T.; Mimura, K.; Sawada, T.; Mataka, S.; Tashiro, M. Oxidative cycloaddition of thiophenophanes – [n](2,5)-parathiophenophane (n=8,10-12.14). [8](2,4)metathiophenophane and [2.2](2,5)parametathiophenophane. Eur. J. Org. Chem. 1998, 1841–1850. [Google Scholar]
- for reviews, see: Thiemann, T.; Dongol, K.G. Thiophene-S-oxides. J. Chem. Res. (S) 2002, 303–308, (M) 2002, 701-708 and ref.18. [Google Scholar] [CrossRef]
- Thiemann, T.; Walton, D.J.; Brett, A.O.; Iniesta, J.; Marken, F.; Li, Y.Q. The chemistry of thiophene-S-oxides and related compounds. ARKIVOC 2009, Vol. ix, 96–113. [Google Scholar]
- Exceptions are strained thiophenes: Nakayama, J.; Kuroda, K. Synthesis and reactivities of a highly strained thiophene with two fused four-membered rings, 1,2,4,5-tetahydrodicyclobuta[b,d]thiophene. J. Am. Chem. Soc. 1993, 115, 4612–4617, and ref. 21, 22. [Google Scholar] [CrossRef]
- Thiophenes with very effective electron-donor substituents: Barker, J.M.; Huddleston, P.R.; Shutler, S.W. Preparation and reactions of 2,5-dimethoxythiophene. J. Chem. Soc., Perkin Trans. 1 1975, 2483–2484. [Google Scholar]
- Wynberg, H.; Helder, R. One-step ring enlargement of a thiophene to a thiepin. Tetrahedron Lett. 1972, 3647–3650. [Google Scholar] [CrossRef]
- This is underscored by theoretical calculations, see, cf.: Jursic, B.S.; Coupe, D. AM1 semiempirical searching for suitable thiophene derivatives that will enable thiophenes to act as a diene in the Diels-Alder reactions. J. Heterocycl. Chem. 1995, 32, 483–489. [Google Scholar]
- Furukawa, N.; Zhang, S.; Sato, S.; Higaki, M. Simple procedure for the synthesis of 2,5-bis(silylated) thiophene S-oxides with m-chloroperbenzoic acid in the presence of BF3(Et2O). Heterocycles 1997, 44, 61–66. [Google Scholar] [CrossRef]
- Nakayama, J.; Nagasawa, H.; Sugihara, Y.; Ishii, A. Synthesis, isolation, and full characterization of the parent thiophene 1,1-dioxide. J. Am. Chem. Soc. 1997, 119, 9077–9078. [Google Scholar] [CrossRef]
- Furukawa, N.; Zhang, S.-Z.; Horn, E.; Takahashi, S.; Sato, S.; Yokoyama, M.; Yamaguchi, K. X-ray crystallographic analysis of 2,5-bis(diphenylmethylsilyl)thiophenemonooxide and the Diels-Alder reaction of thiophenemonooxide with dienophiles. Heterocycles 1998, 47, 793–809. [Google Scholar] [CrossRef]
- Pouzet, P.; Erdelmeier, I.; Ginderow, D.; Mornon, J.-P.; Dansette, P.M.; Mansuy, D. Thiophene 1-oxides. V. comparison of the crystal structures and thiophene ring aromaticity of 2,5-diphenylthiophene, its sulfoxide and sulfone. J. Heterocycl. Chem. 1997, 34, 1567–1574. [Google Scholar] [CrossRef]
- Pouzet, P.; Erdelmeier, I.; Ginderow, D.; Mornon, J.-P.; Dansette, P.M.; Mansuy, D. Thiophene S-oxides: convenient preparation, first complete structural characterization and unexpected dimerization of one of them, 2,5-diphenylthiophene 1-oxide. J. Chem. Soc., Chem. Commun. 1995, 473–474. [Google Scholar]
- Torssell, K. Diels-Alder reactions of thiophene oxides generated in situ. Acta Chem. Scand., Ser. B 1976, 30, 353–357. [Google Scholar] [CrossRef]
- Thiemann, T.; Sa e Melo, M.L.; Campos Neves, A.S.; Li, Y.Q.; Mataka, S.; Tashiro, M.; Geiβler, U.; Walton, D. Preparation and electrooxidative SO-extrusion of halogenated 7-thiabicyclo[2.2.1]heptene 7-oxides. J. Chem. Res. (S) 1998, 346–347. [Google Scholar]
- Raasch, M.S. Annulations with tetrachlorothiophene 1,1-dioxide. J. Org. Chem. 1980, 45, 856–867. [Google Scholar] [CrossRef]
- Raasch, M.S. Addition-rearrangement reactions of halogenated thiophene dioxides with furans. J. Org. Chem. 1980, 45, 867–870. [Google Scholar] [CrossRef]
- Hamon, D.P.G.; Spurr, P.R. A simple construction of the iceane skeleton via an intramolecular Diels-Alder reaction. J. Chem. Soc., Chem. Commun. 1982, 372–373. [Google Scholar] [CrossRef]
- Hesse, M.; Meier, H.; Zeeh, B. Spectroscopic Methods in Organic Chemistry; Georg Thieme Verlag: Stuttgart, Germany, 1997; p. 246. [Google Scholar]
- Wraight, E.S. Some Newer Phys. Methods Struct. Chem., Proc. Symp. 1967, 67–67, [Chem. Abstr. 1969, 70, 62337u].
- Yoshida, Z.; Takabayashi, F. Electronic spectra of monosubstituted anthraquinones and solvent effects. Tetrahedron 1968, 24, 933–943. [Google Scholar] [CrossRef]
- Peters, R.H.; Sumners, H. Spectra of anthraquinone derivatives. J. Chem. Soc. 1953, 2101–2110. [Google Scholar] [CrossRef]
- cf., Ertas, E.; Ozturk, T. A new reaction of P4S10 and Lawesson's reagent; a new method for the synthesis of dithieno[3,2-b;2',3'-d]thiophenes. Tetrahedron Lett. 2004, 45, 3405–3407. [Google Scholar] [CrossRef]
- see also: Goldberg, Y.; Alper, H. Electrophilic halogenation of aromatics and heteroaromatics with N-halosuccinimides in a solid/liquid system using an H+ ion exchanger or ultrasonic irradiation. J. Mol. Cat. 1994, 88, 377–383. [Google Scholar] [CrossRef]
- Gjoes, N.; Gronowitz, S. Substitution reactions of 2-phenylthiophene. Acta Chem. Scand. 1972, 26, 1851–1859. [Google Scholar] [CrossRef]
- This reaction has been reported to proceed under YbCl3 catalysis: Fang, X.; Warner, B.P.; Watkin, J.G. Ytterbium trichloride-catalyzed Diels-Alder reactions of un-activated dienes. Synth. Commun. 2000, 30, 2669–2676. [Google Scholar] We have found that it proceeds also in the presence of EuCl3.
- Here, we have used a biphasic system of 4N aq. NaOH and ether under ultrasonication. The enolisation has been reported to also go very well in the presence of triethylamine (Et3N) or in the presence of acids such as HCl: Fernandez, M.P.; Gonzalez, B.; Pardo, M.; Soto, J.L. 1,3-Dipolar cycloaddition of nitrile oxides with Diels-Alder adducts of p-benzoquinone and 1,4-naphthoquinone. Anal. Quim. 1994, 90, 477–482. [Google Scholar] (for Et3N), also see ref. []
- Kotha, S.; Manivannan, E. Synthesis of functionalized cis-syn-cis triquinanes. Ind. J. Chem., Sect. B 2002, 41B, 808–811, (for HCl). [Google Scholar]
- We used Ag2O in benzene as the oxidation agent as described for the oxidation of other hydroquinones to quinones. The reaction gives quantitative yields of 6,7-dimethyl-5,8-dihydro-1,4-naphthoquinone, when carried out at rt. Specifically for the transformation of 6,7-dimethyl-5,8-dihydronaphthalene-1,4-diol to 6,7-dimethyl-5,8-dihydro-1,4-naphthoquinone, the use of MnO2 in acetone has been described, also: ref. [].
- cf., Fuchibe, K.; Akiyama, T. Low-valent niobium-mediated double activation of C-F/C-H bonds: fluorene synthesis from o-arylated alpha,alpha,alpha-trifluorotoluene derivatives. J. Am. Chem. Soc. 2006, 128, 1434–1435. [Google Scholar] [CrossRef]
- Kondo, M.; Murata, M.; Nishihara, H.; Nishibori, E.; Aoyagi, S.; Yoshida, M.; Kinoshita, Y.; Sakata, M. Guest-induced instant and reversible crystal-to-crystal transformation of 1,4-bis(ferrocenylethynyl)anthraquinone. Angew. Chem. Int. Ed. Engl. 2006, 45, 5461–5464. [Google Scholar] [CrossRef]
- Hofmann, A. Substituted benzoylbenzoic acids. Monatsh. Chem. 1915, 36, 805–824. [Google Scholar] [CrossRef]
- Castle, R.N.; Kudo, H.; Lee, M.L. The synthesis of some monomethylanthracenamines. Coll. Czech Chem. Commun. 1991, 56, 2269–2277. [Google Scholar] [CrossRef]
- Mizuno, H.; Kubota, S. (Seiko Epson Corp.), Method of distinguishing right from left contact lenses by coloring. JP Pat. 04011214 1992. [Chem. Abstr. 1992, 116, 221636]. [Google Scholar]
- Ganushchak, N.I.; Dombrovskii, A.V.; Vislobitskaya, O.A. Syntheses based on Diels-Alder condensations. I. 1-Methyl-4-arylanthraquinones. Zh. Obshch. Khim. 1963, 33, 2532–2534. [Google Scholar]
- Sample Availability: Samples of the compounds 4b-4f, 4m are available from the authors.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).