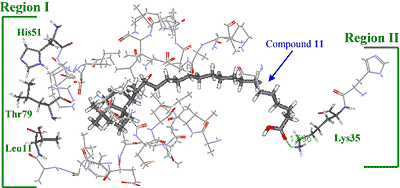
The Inhibitory Action of Kohamaic Acid A Derivatives on Mammalian DNA Polymerase β
Abstract
:Introduction
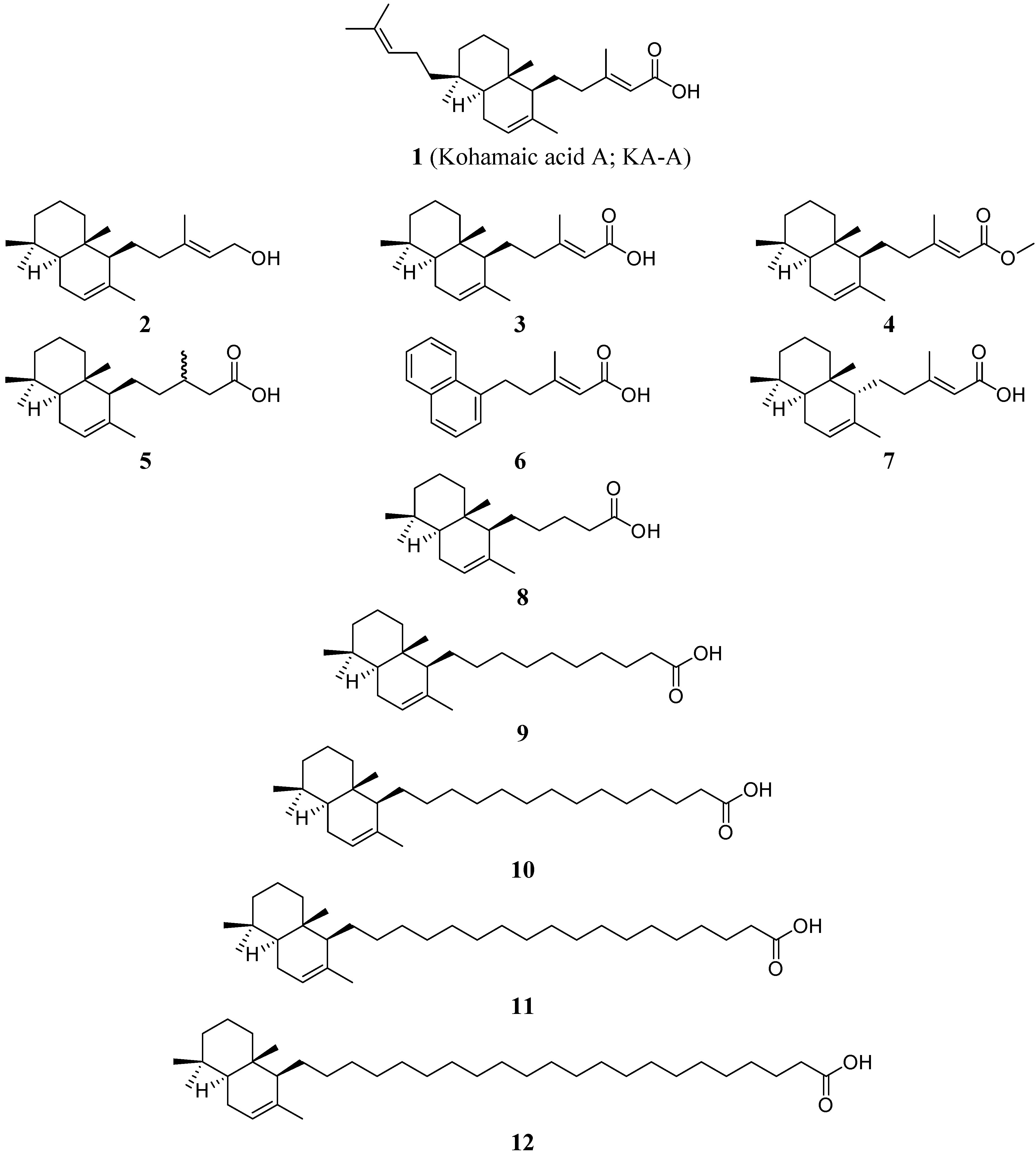
Results and Discussion
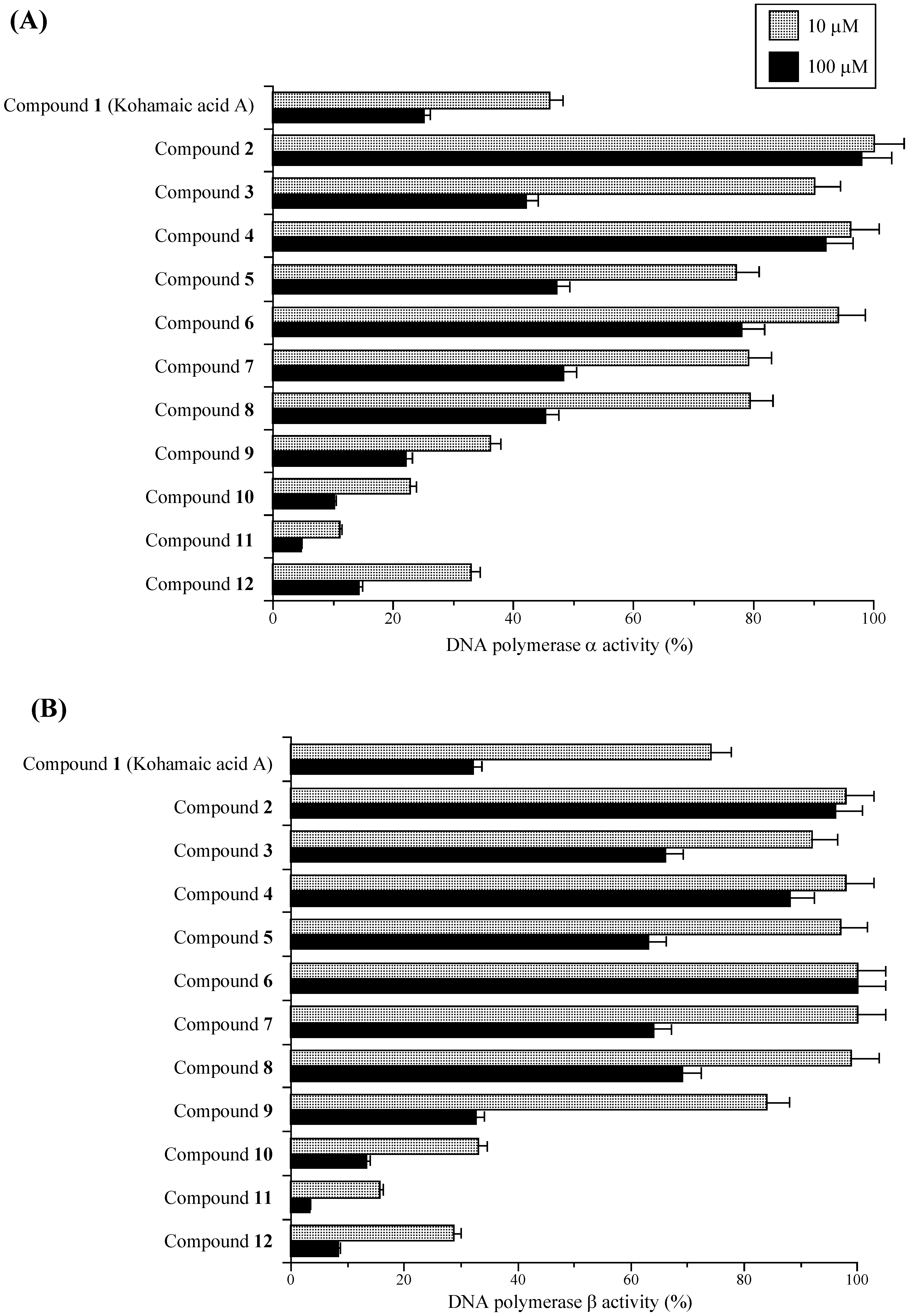
Effects of KA-A derivatives on the activities of mammalian DNA polymerases α and β
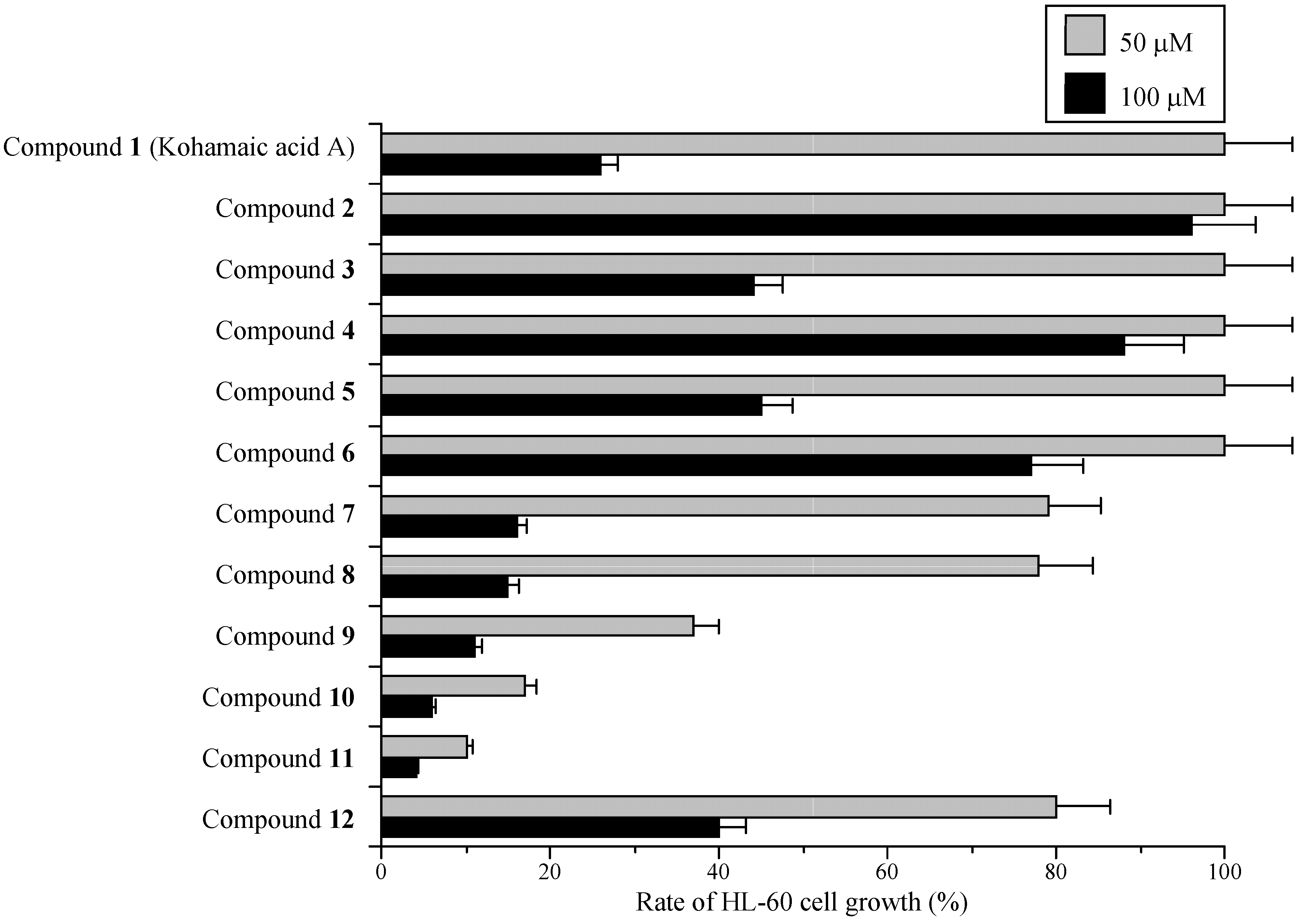
Effects of KA-A derivatives on cultured human cancer cells
Inhibitory effect of compound 11 on the activities of DNA polymerases and other DNA metabolic enzymes
| Enzyme | IC50 values (μM) |
|---|---|
| Mammalian DNA polymerases | |
| Calf DNA polymerase α | 4.21 ± 0.21 |
| Rat DNA polymerase β | 5.50 ± 0.28 |
| Human DNA polymerase γ | 8.76 ± 0.44 |
| Human DNA polymerase δ | 4.89 ± 0.24 |
| Human DNA polymerase ε | 3.22 ± 0.16 |
| Human DNA polymerase η | 7.45 ± 0.37 |
| Human DNA polymerase ι | 7.84 ± 0.39 |
| Human DNA polymerase κ | 7.20 ± 0.36 |
| Human DNA polymerase λ | 4.67 ± 0.23 |
| Fish DNA polymerase | |
| Cherry salmon DNA polymerase δ | 4.30 ± 0.22 |
| Plant DNA polymerase | |
| Cauliflower DNA polymerase I (α-like) | >200 |
| Prokayotic DNA polymerases | |
| E. coli DNA polymerase I (Klenow fragment) | >200 |
| Taq DNA polymerase | >200 |
| T4 DNA polymerase | >200 |
| Other DNA metabolic enzymes | |
| Calf Primase of DNA polymerase α | >200 |
| T4 Polynucleotide kinase | >200 |
| Bovine Deoxyribonuclease I | >200 |
Effect of interaction of nucleic acid, protein and compound 11
| Compounds added to the reaction mixture | Relative activity of pol β (%) |
|---|---|
| Without compound 11 | |
| None (control) | 100 ± 5.0 |
| + 50 μM poly (rC) | 100 ± 4.6 |
| + 200 μg/ml BSA | 100 ± 8.9 |
| + 0.05 % NP-40 | 100 ± 5.9 |
| + 0.1 % NP-40 | 100 ± 8.5 |
| 10 μM compound 11 | |
| 10 μM compound 11 | 15.5 ± 0.78 |
| 10 μM compound 11 + 50 μM poly (rC) | 15.2 ± 0.74 |
| 10 μM compound 11 + 200 μg/ml BSA | 15.9 ± 1.1 |
| 10 μM compound 11 + 0.05 % NP-40 | 96.1 ± 7.6 |
| 10 μM compound 11 + 0.1 % NP-40 | 100 ± 8.8 |
| 100 μM compound 11 | |
| 100 μM compound 11 | 3.2 ± 0.16 |
| 100 μM compound 11 + 50 μM poly (rC) | 3.1 ± 0.15 |
| 100 μM compound 11 + 200 μg/ml BSA | 3.3 ± 0.19 |
| 100 μM compound 11 + 0.05 % NP-40 | 62.5 ± 4.1 |
| 100 μM compound 11 + 0.1 % NP-40 | 95.0 ± 7.6 |
Mode of inhibition of DNA polymerase β by compound 11
| Enzyme | DNA Substrate | Compound 11 (μM) | Km a) (μM) | Vmax a) (pmol / h) | Ki b) (μM) | Inhibitory mode a) |
|---|---|---|---|---|---|---|
| Pol β | Template | 0 | 6.74 | 111 | 1.96 | Competitive |
| -primer c) | 3 | 8.77 | ||||
| 6 | 12.7 | |||||
| 9 | 22.0 | |||||
| Nucleotide d) | 0 | 3.05 | 62.5 | 2.36 | Competitive | |
| substrate | 3 | 4.03 | ||||
| 6 | 5.81 | |||||
| 9 | 10.8 |
Three-dimensional modeling of the interaction of KA-A derivatives with DNA polymerase β

| Compound | Energy (kcal/mol) | ||
|---|---|---|---|
| Coulomb | van der Waals | Total | |
| 7 | -12.81 | -6.89 | -19.70 |
| 8 | -4.48 | -7.79 | -12.27 |
| 9 | -56.61 | -9.44 | -66.05 |
| 10 | -65.42 | -10.20 | -75.62 |
| 11 | -94.68 | -11.85 | -106.53 |
| 12 | -66.14 | -17.23 | -83.37 |
| Compound | Length (Å) | Wide (Å) |
|---|---|---|
| 1 (kohamaic acid A) | 16.76 | 6.45 |
| 2 | 11.96 | 6.45 |
| 3 | 12.08 | 6.45 |
| 4 | 13.92 | 6.45 |
| 5 | 11.42 | 6.45 |
| 6 | 11.28 | 5.10 |
| 7 | 11.51 | 6.45 |
| 8 | 11.57 | 6.45 |
| 9 | 17.47 | 6.45 |
| 10 | 22.31 | 6.45 |
| 11 | 26.69 | 6.45 |
| 12 | 31.61 | 6.45 |
Experimental
General
Enzymes
DNA polymerase assays
Investigation of growth rate on cultured human cancer cells
Other enzyme assays
KA-A derivatives docking modeling
Acknowledgements
References and Notes
- Kokubo, S.; Yogi, K.; Uddin, M. J.; Inuzuka, T.; Suenaga, K.; Ueda, K.; Uemura, D. Kohamaic acids A and B, novel cytotoxic sesterterpenic acids, from the marine sponge Ircinia sp. Chem. Lett. 2001, 30, 176–177. [Google Scholar]
- Mizushina, Y.; Murakami, C.; Yogi, K.; Ueda, K.; Ishidoh, T.; Takemura, M.; Perpelescu, M.; Suzuki, M.; Oshige, M.; Yamaguchi, T.; Saneyoshi, M.; Yoshida, H.; Sakaguchi, K. Kohamaic acid A, a novel sesterterpenic acid, inhibits activities of DNA polymerases from deuterostomes. Biochim. Biophys. Acta 2003, 1648, 55–61. [Google Scholar]
- Hubscher, U.; Maga, G.; Spadari, S. Eukaryotic DNA polymerases. Ann. Rev. Biochem. 2002, 71, 133–163. [Google Scholar] [CrossRef]
- Chang, L. M. S. Phylogeny of DNA polymerase-β. Science 1976, 191, 1183–1185. [Google Scholar]
- Hobart, P. M.; Infante, A. A. A low molecular weight DNA polymerase β in the sea urchin Strongylocentrotus purpurantus. Partial purification, properties, and changes in development. J. Biol. Chem. 1978, 253, 8229–8238. [Google Scholar]
- Gustafson, T.; Wolpert, L. Cellular movement and contact in sea urchin morphogenesis. Biol. Rev. Camb. Philos. Soc. 1967, 42, 442–498. [Google Scholar] [CrossRef]
- De Petrocellis, B.; Filosa-Parisi, S.; Monroy, A.; Parisi, E. Cell and tissue interactions. Lash, J.W., Burger, M., Eds.; Raven Press: New York, USA, 1977. [Google Scholar]
- Parisi, E.; Filosa, S.; De Petrocellis, B.; Monroy, A. The pattern of cell division in the early development of the sea urchin, Paracentrotus lividus. Dev. Biol. 1978, 65, 38–49. [Google Scholar] [CrossRef]
- Katow, H.; Solursh, M. Ultrastructure of blastocoel material in blastulae and gastrulae of the sea urchin Lytechinus pictus. J. Exp. Zool. 1980, 213, 231. [Google Scholar] [CrossRef]
- Takikawa, H.; Kamatani, N.; Nakanishi, K.; Tashiro, T.; Sasaki, M.; Yoshida, H.; Mizushina, Y. Synthetic studies on kohamaic acids: Synthesis of structurally simplified analogs of kohamaic acid A. Biosci. Biotechnol. Biochem. 2008, 72, 3071–3074. [Google Scholar] [CrossRef]
- Bebenek, K.; Kunkel, T. A. DNA Repair and Replication. In Advances in Protein Chemistry; Yang, W., Ed.; Elsevier: San Diego, USA, 2004; Volume 69, pp. 137–165. [Google Scholar]
- Friedberg, E. C.; Feaver, W. J.; Gerlach, V. L. The many faces of DNA polymerases: strategies for mutagenesis and for mutational avoidance. Proc. Natl. Acad. Sci. USA 2000, 97, 5681–5683. [Google Scholar] [CrossRef]
- Burgers, P. M.; Koonin, E. V.; Bruford, E.; Blanco, L.; Burtis, K. C.; Christman, M. F.; Copeland, W. C.; Friedberg, E. C.; Hanaoka, F.; Hinkle, D. C.; Lawrence, C. W.; Nakanishi, M.; Ohmori, H.; Prakash, L.; Prakash, S.; Reynaud, C. A.; Sugino, A.; Todo, T.; Wang, Z.; Weill, J. C.; Woodgate, R. Eukaryotic DNA polymerases: proposal for a revised nomenclature. J. Biol. Chem. 2001, 276, 43487–43490. [Google Scholar] [CrossRef]
- Ohmori, H.; Friedberg, E. C.; Fuchs, R. P.; Goodman, M. F.; Hanaoka, F.; Hinkle, D.; Kunkel, T. A.; Lawrence, C. W.; Livneh, Z.; Nohmi, T.; Prakash, L.; Prakash, S.; Todo, T.; Walker, G. C.; Wang, Z.; Woodgate, R. The Y-family of DNA polymerases. Mol. Cell 2001, 8, 7–8. [Google Scholar] [CrossRef]
- Aravind, L.; Koonin, E. V. DNA polymerase β-like nucleotidyltransferase superfamily: identification of three new families, classification and evolutionary history. Nucleic Acids Res. 1999, 27, 1609–1618. [Google Scholar] [CrossRef]
- Sawaya, M. R.; Pelletier, H.; Kumar, A.; Wilson, S. H.; Kraut, J. Crystal structure of rat DNA polymerase β: evidence for a common polymerase mechanism. Science 1994, 264, 1930–1935. [Google Scholar]
- Pelletier, H.; Sawaya, M. R.; Kumar, A.; Wilson, S. H.; Kraut, J. Structures of ternary complexes of rat DNA polymerase β, a DNA template-primer, and ddCTP. Science 1994, 264, 1891–1903. [Google Scholar]
- Mizushina, Y.; Ohkubo, T.; Date, T.; Yamaguchi, T.; Saneyoshi, M.; Sugawara, F.; Sakaguchi, K. Mode analysis of a fatty acid molecule binding to the N-terminal 8-kDa domain of DNA polymerase β. A 1:1 complex and binding surface. J. Biol. Chem. 1999, 274, 25599–25607. [Google Scholar]
- Kornberg, A.; Baker, T. A. DNA replication, 2nd Ed.; Freeman W., H., Co., N.Y., Eds.; Freeman and Company: New York, NY, USA, 1992; Volume 6, pp. 197–225. [Google Scholar]
- Kumar, A.; Abbotts, J.; Karawya, E. M.; Wilson, S. H. Identification and properties of the catalytic domain of mammalian DNA polymerase β. Biochemistry 1990, 29, 7156–7159. [Google Scholar] [CrossRef]
- Kumar, A.; Widen, S. G.; Williams, K. R.; Kedar, P.; Karpel, R. L.; Wilson, S. H. Studies of the domain structure of mammalian DNA polymerase β. Identification of a discrete template binding domain. J. Biol. Chem. 1990, 265, 2124–2131. [Google Scholar]
- Liu, D.; DeRose, E. F.; Prasad, R.; Wilson, S. H.; Mullen, G. P. Assignments of 1H, 15N, and 13C resonances for the backbone and side chains of the N-terminal domain of DNA polymerase β Determination of the secondary structure and tertiary contacts. Biochemistry 1994, 33, 9537–9545. [Google Scholar] [CrossRef]
- Sawaya, M. R.; Prasad, R.; Wilson, S. H.; Kraut, J.; Pelletier, H. Crystal structures of human DNA polymerase β complexed with gapped and nicked DNA: evidence for an induced fit mechanism. Biochemistry 1997, 36, 11205–11215. [Google Scholar] [CrossRef]
- Liu, D.; Prasad, R.; Wilson, S. H.; DeRose, E. F.; Mullen, G. P. Three-dimensional solution structure of the N-terminal domain of DNA polymerase β and mapping of the ssDNA interaction interface. Biochemistry 1996, 35, 6188–6200. [Google Scholar] [CrossRef]
- Prasad, R.; Beard, W. A.; Chyan, J. Y.; Maciejewski, M. W.; Mullen, G. P.; Wilson, S. H. Functional analysis of the amino-terminal 8-kDa domain of DNA polymerase β as revealed by site-directed mutagenesis. DNA binding and 5'-deoxyribose phosphate lyase activities. J. Biol. Chem. 1998, 273, 11121–11126. [Google Scholar]
- Izuta, S.; Saneyoshi, M.; Sakurai, T.; Suzuki, M.; Kojima, K.; Yoshida, S. The 5'-triphosphates of 3'-azido-3'-deoxythymidine and 2', 3'-dideoxynucleosides inhibit DNA polymerase γ by different mechanisms. Biochem. Biophys. Res. Commun. 1991, 179, 776–783. [Google Scholar] [CrossRef]
- Tamai, K.; Kojima, K.; Hanaichi, T.; Masaki, S.; Suzuki, M.; Umekawa, H.; Yoshida, S. Structural study of immunoaffinity-purified DNA polymerase α-DNA primase complex from calf thymus. Biochim. Biophys. Acta 1988, 950, 263–273. [Google Scholar] [CrossRef]
- Date, T.; Yamaguchi, M.; Hirose, F.; Nishimoto, Y.; Tanihara, K.; Matsukage, A. Expression of active rat DNA polymerase β in Escherichia coli. Biochemistry 1988, 27, 2983–2990. [Google Scholar] [CrossRef]
- Umeda, S.; Muta, T.; Ohsato, T.; Takamatsu, C.; Hamasaki, N.; Kang, D. The D-loop structure of human mtDNA is destabilized directly by 1-methyl-4-phenylpyridinium ion (MPP+), a parkinsonism-causing toxin. Eur. J. Biochem. 2000, 267, 200–206. [Google Scholar] [CrossRef]
- Oshige, M.; Takeuchi, R.; Ruike, R.; Kuroda, K.; Sakaguchi, K. Subunit protein-affinity isolation of Drosophila DNA polymerase catalytic subunit. Protein Expr. Purif. 2004, 35, 248–256. [Google Scholar] [CrossRef]
- Masutani, C.; Kusumoto, R.; Iwai, S.; Hanaoka, F. Mechanisms of accurate translesion synthesis by human DNA polymerase η. EMBO J. 2000, 19, 3100–3109. [Google Scholar] [CrossRef]
- Tissier, A.; Frank, E. G.; McDonald, J. P.; Iwai, S.; Hanaoka, F.; Woodgate, R. Misinsertion and bypass of thymine-thymine dimers by human DNA polymerase ι. EMBO J. 2000, 19, 5259–5266. [Google Scholar] [CrossRef]
- Ohashi, E.; Ogi, T.; Kusumoto, R.; Iwai, S.; Masutani, C.; Hanaoka, F.; Ohmori, H. Error-prone bypass of certain DNA lesions by the human DNA polymerase κ. Genes Dev. 2000, 14, 1589–1594. [Google Scholar]
- Shimazaki, N.; Yoshida, K.; Kobayashi, T.; Toji, S.; Tamai, T.; Koiwai, O. Over-expression of human DNA polymerase λ in E. coli and characterization of the recombinant enzyme. Genes Cells 2000, 7, 639–651. [Google Scholar]
- Yamaguchi, T.; Saneyoshi, M.; Takahashi, H.; Hirokawa, S.; Amano, R.; Liu, X.; Inomata, M.; Maruyama, T. Synthetic Nucleoside and Nucleotides. 43. Inhibition of vertebrate telomerases by carbocyclic oxetanocin G (C.OXT-G) triphosphate analogues and influence of C.OXT-G treatment on telomere length in human HL60 cells. Nucleosides Nucleotides Nucleic Acids 2006, 25, 539–551. [Google Scholar] [CrossRef]
- Sakaguchi, K.; Hotta, Y.; Stern, H. Chromatin-associated DNA polymerase activity in meiotic cells of lily and mouse. Cell Struct. Funct. 1980, 5, 323–334. [Google Scholar] [CrossRef]
- Mizushina, Y.; Tanaka, N.; Yagi, H.; Kurosawa, T.; Onoue, M.; Seto, H.; Horie, T.; Aoyagi, N.; Yamaoka, M.; Matsukage, A.; Yoshida, S.; Sakaguchi, K. Fatty acids selectively inhibit eukaryotic DNA polymerase activities in vitro. Biochim. Biophys. Acta 1996, 1308, 256–262. [Google Scholar]
- Mizushina, Y.; Yoshida, S.; Matsukage, A.; Sakaguchi, K. The inhibitory action of fatty acids on DNA polymerase β. Biochim. Biophys. Acta 1997, 1336, 509–521. [Google Scholar]
- Ogawa, A.; Murate, T.; Suzuki, M.; Nimura, Y.; Yoshida, S. Lithocholic acid, a putative tumor promoter, inhibits mammalian DNA polymerase β. Jpn. J. Cancer Res. 1998, 89, 1154–1159. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Tamiya-Koizumi, K.; Murate, T.; Suzuki, M.; Simbulan, C. G.; Nakagawa, M.; Takamura, M.; Furuta, K.; Izuta, S.; Yoshida, S. Inhibition of DNA primase by sphingosine and its analogues parallels with their growth suppression of cultured human leukemic cells. Biochem. Mol. Biol. Int. 1997, 41, 1179–1189. [Google Scholar]
- Nakayama, C.; Saneyoshi, M. Inhibitory effects of 9-α-D-xylofuranosyladenine 5'-triphosphate on DNA-dependent RNA polymerase I and II from cherry salmon (Oncorhynchus masou). J. Biochem. (Tokyo) 1985, 97, 1385–1389. [Google Scholar]
- Soltis, D. A.; Uhlenbeck, O. C. Isolation and characterization of two mutant forms of T4 polynucleotide kinase. J. Biol. Chem. 1982, 257, 11332–11339. [Google Scholar]
- Lu, B. C.; Sakaguchi, K. An endo-exonuclease from meiotic tissues of the basidiomycete Coprinus cinereus: Its purification and characterization. J. Biol. Chem. 1991, 266, 21060–21066. [Google Scholar]
- Kurinov, I. V.; Myers, D. E.; Irvin, J. D.; Uckun, F. M. X-ray crystallographic analysis of the structural basis for the interactions of pokeweed antiviral protein with its active site inhibitor and ribosomal RNA substrate analogs. Protein Sci. 1999, 8, 1765–1772. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the synthesized KA-A compounds 2–12 are available from Dr. H. Takikawa (Kobe University).
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mizushina, Y.; Manita, D.; Takeuchi, T.; Sugawara, F.; Kumamoto-Yonezawa, Y.; Matsui, Y.; Takemura, M.; Sasaki, M.; Yoshida, H.; Takikawa, H. The Inhibitory Action of Kohamaic Acid A Derivatives on Mammalian DNA Polymerase β. Molecules 2009, 14, 102-121. https://doi.org/10.3390/molecules14010102
Mizushina Y, Manita D, Takeuchi T, Sugawara F, Kumamoto-Yonezawa Y, Matsui Y, Takemura M, Sasaki M, Yoshida H, Takikawa H. The Inhibitory Action of Kohamaic Acid A Derivatives on Mammalian DNA Polymerase β. Molecules. 2009; 14(1):102-121. https://doi.org/10.3390/molecules14010102
Chicago/Turabian StyleMizushina, Yoshiyuki, Daisuke Manita, Toshifumi Takeuchi, Fumio Sugawara, Yuko Kumamoto-Yonezawa, Yuki Matsui, Masaharu Takemura, Mitsuru Sasaki, Hiromi Yoshida, and Hirosato Takikawa. 2009. "The Inhibitory Action of Kohamaic Acid A Derivatives on Mammalian DNA Polymerase β" Molecules 14, no. 1: 102-121. https://doi.org/10.3390/molecules14010102