Trimethyl Lock: A Stable Chromogenic Substrate for Esterases
Abstract
:Introduction
Results and Discussion
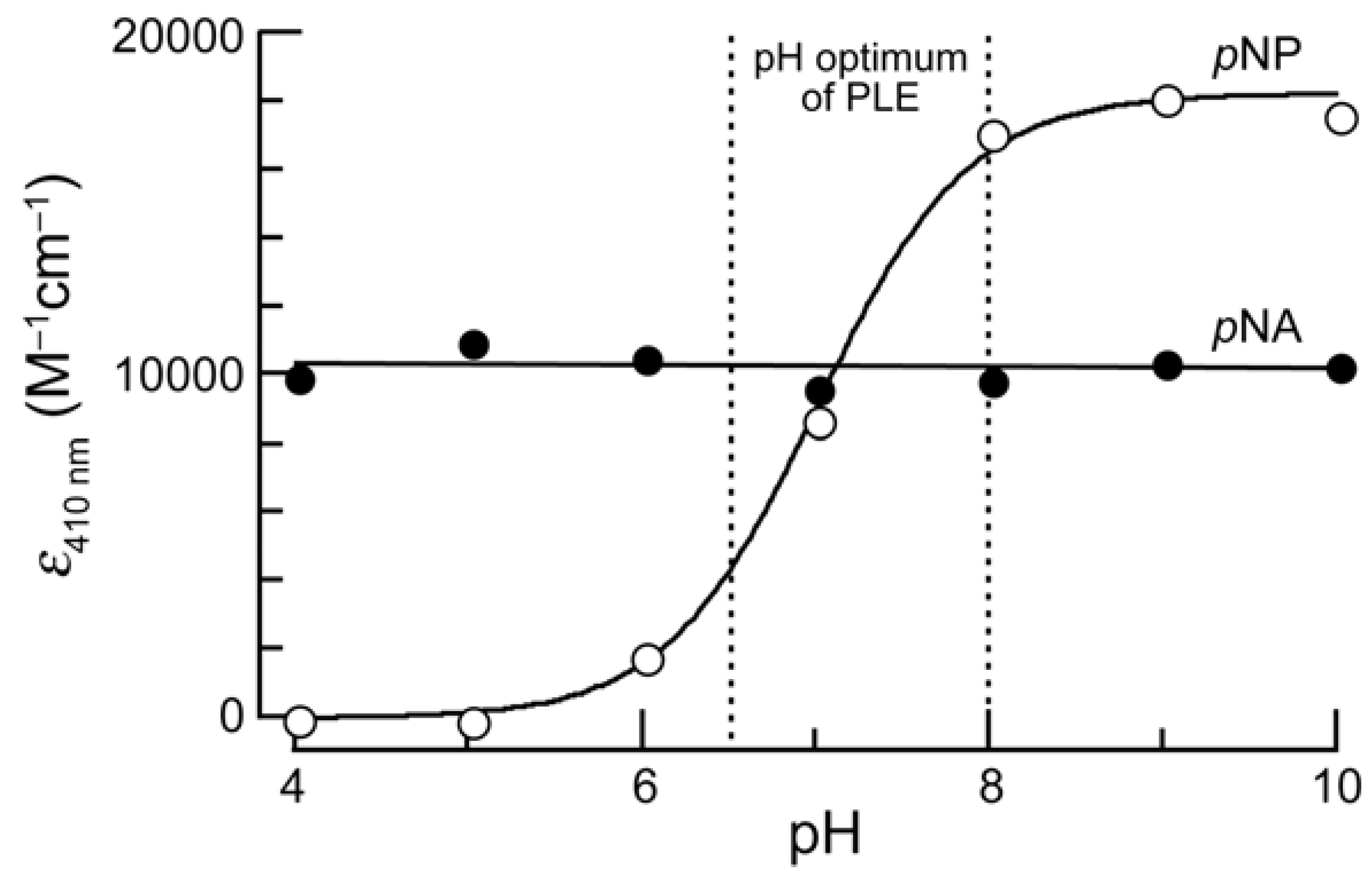
pH-Sensitivity of Chromophores
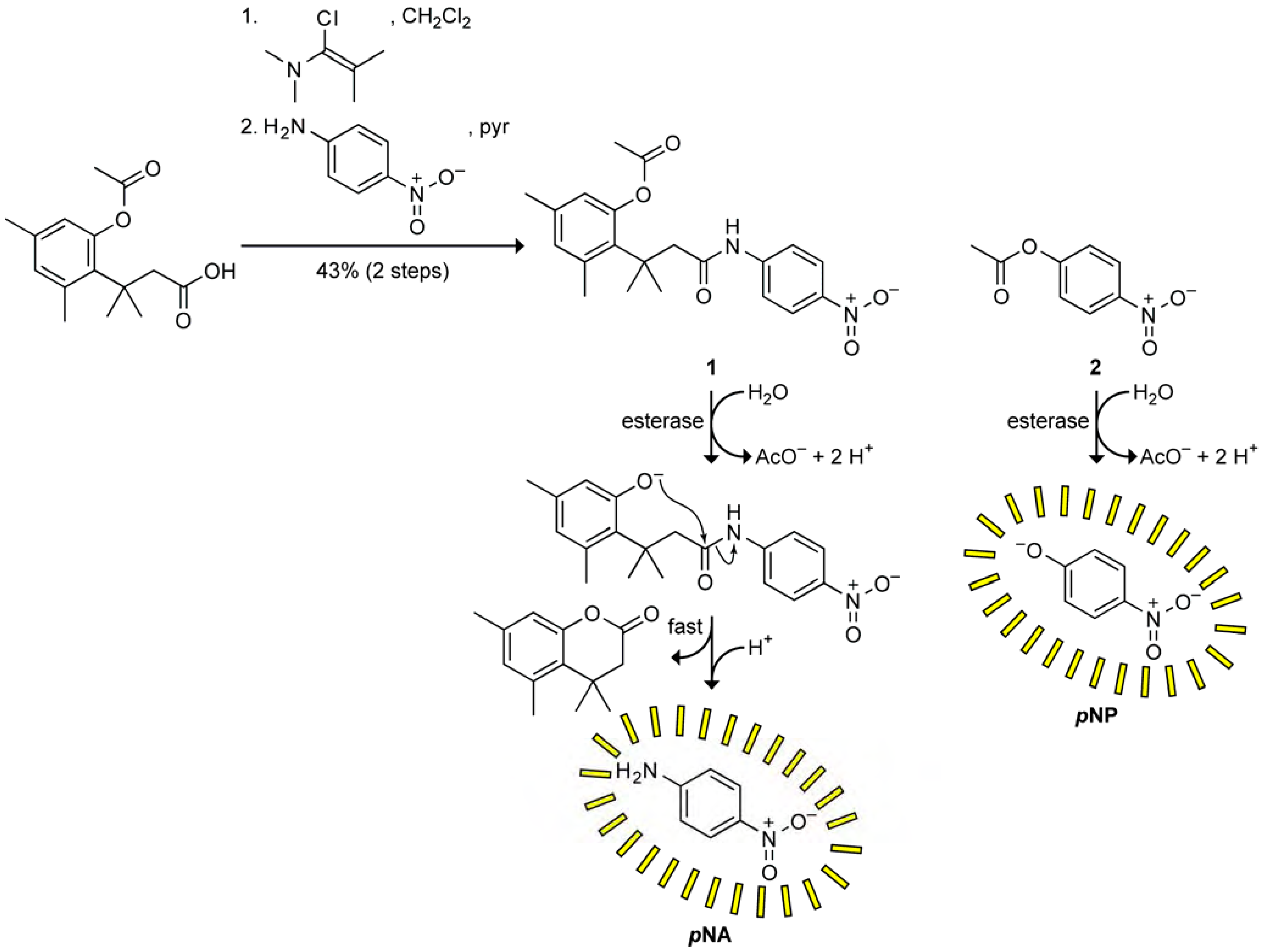
Synthesis of a Prochromophore 1
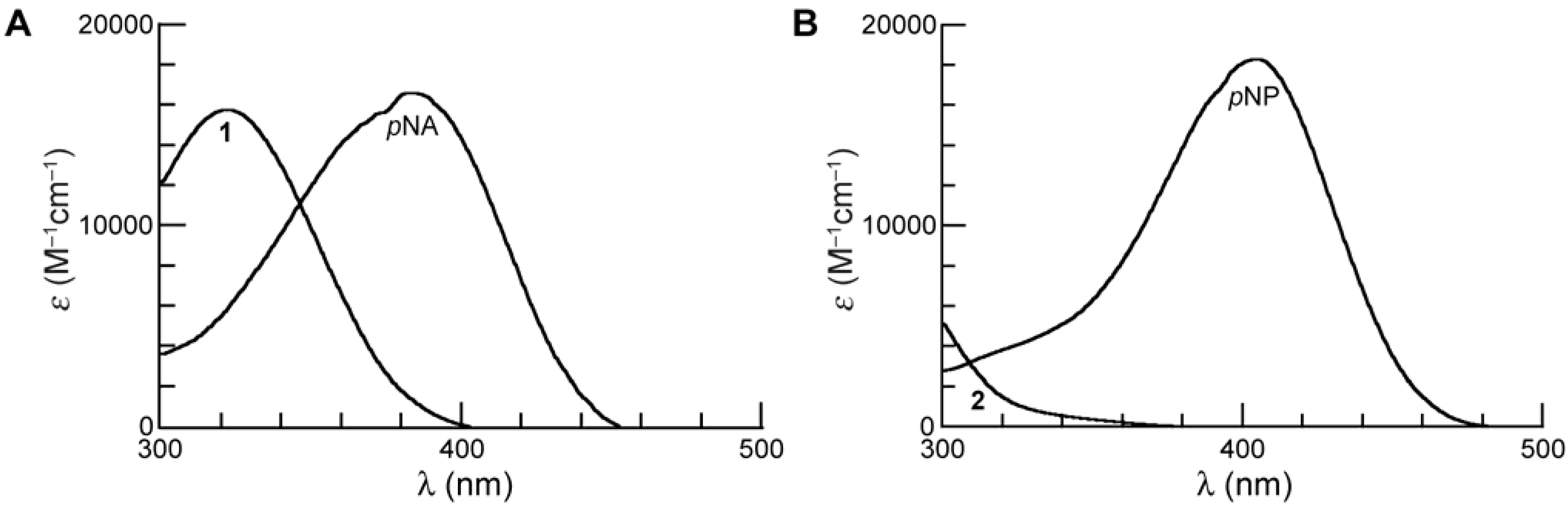
Spectroscopic Properties
Chemical Stability
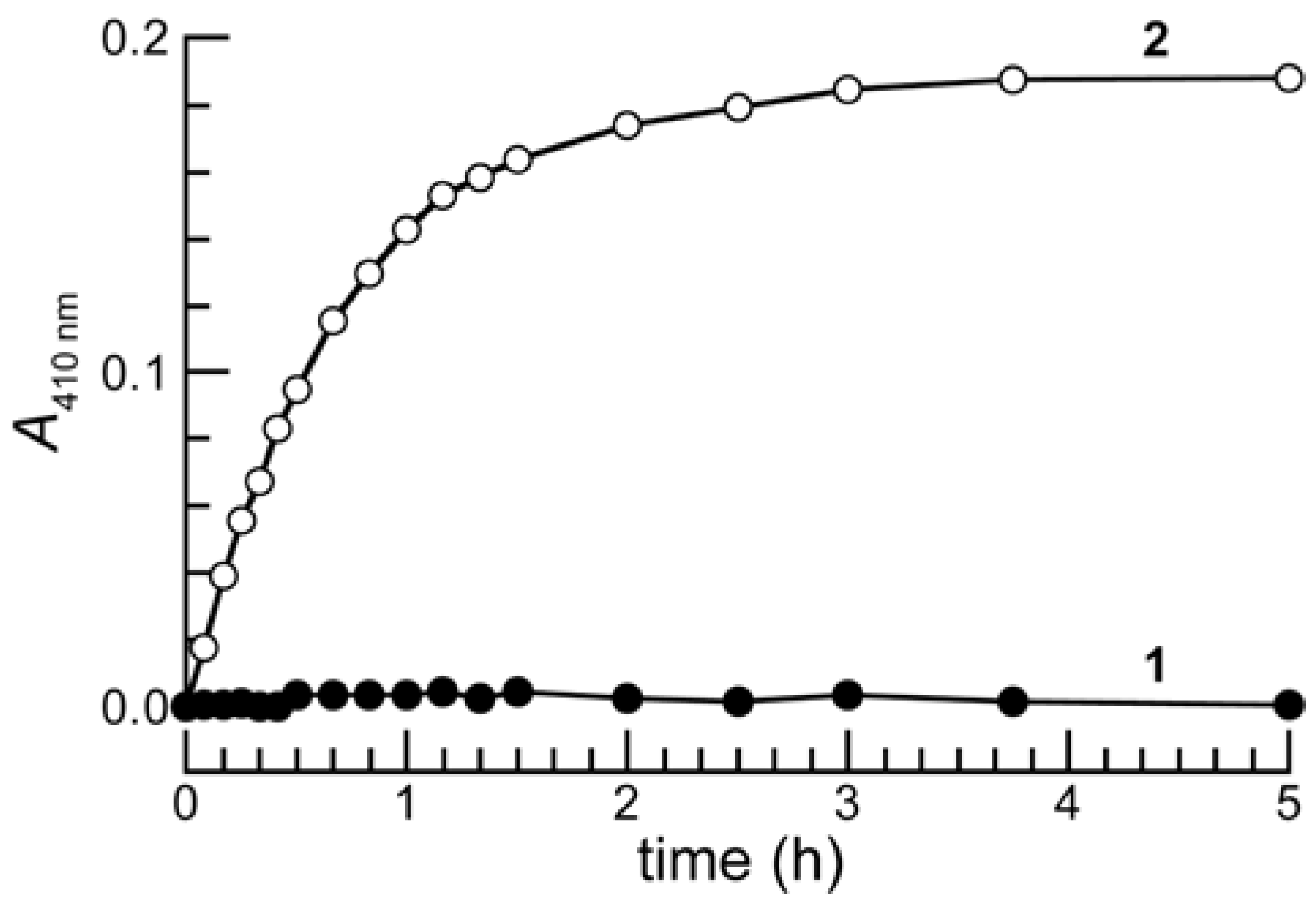
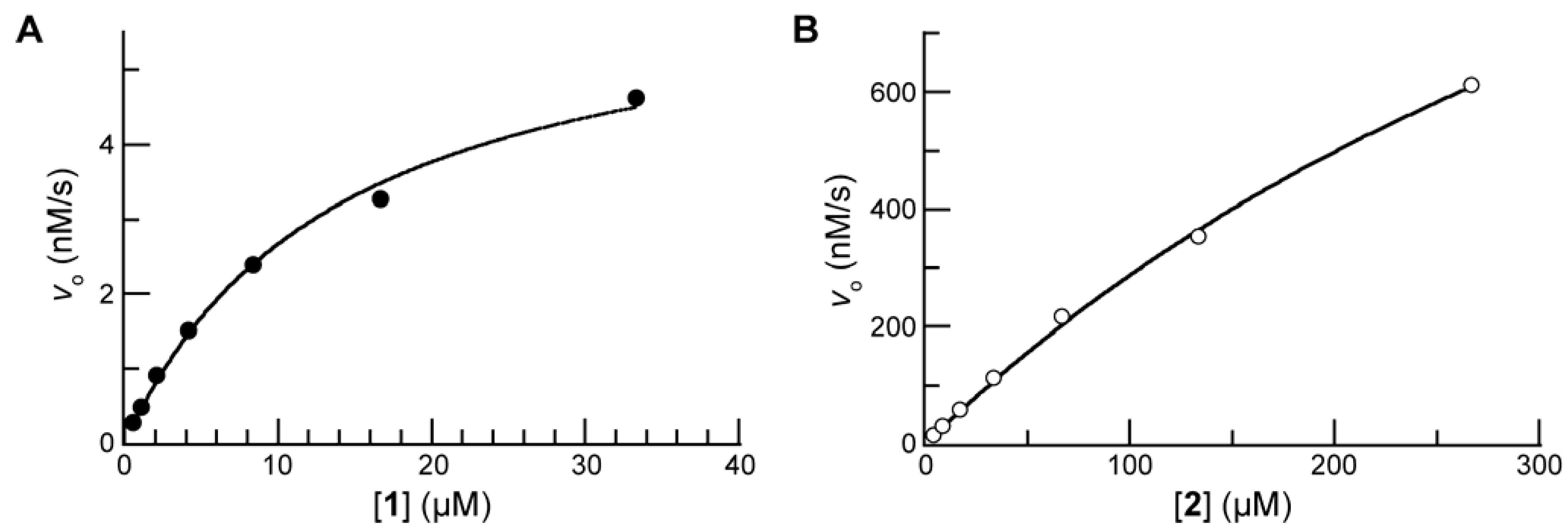
Enzymatic Reactivity
Conclusions
Experimental
General
Spectroscopy
Prochromophore 1
Acknowledgements
References and Notes
- Amsberry, K. L.; Borchardt, R. T. The lactonization of 2'-hydroxyhydrocinnamic acid amides: A potential prodrug for amines. J. Org. Chem. 1990, 55, 5867–5877. [Google Scholar] [CrossRef]
- Testa, B.; Mayer, J. M. Hydrolysis in Drug and Prodrug Metabolism: Chemistry, Biochemistry and Enzymology; Verlag Helvetica Chimica Acta: Zurich, Switzerland, 2003. [Google Scholar]
- Chandran, S. S.; Dickson, K. A.; Raines, R. T. Latent fluorophore based on the trimethyl lock. J. Am. Chem. Soc. 2005, 127, 1652–1653. [Google Scholar] [CrossRef] [PubMed]
- Lavis, L. D.; Chao, T. Y.; Raines, R. T. Fluorogenic label for biomolecular imaging. ACS Chem. Biol. 2006, 1, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Lavis, L. D.; Chao, T. Y.; Raines, R. T. Latent blue and red fluorophores based on the trimethyl lock. ChemBioChem 2006, 7, 1151–1154. [Google Scholar] [CrossRef] [PubMed]
- Milstein, S.; Cohen, L. A. Stereopopulation control. I. Rate enhancement in the lactonizations of o-hydroxyhydrocinnamic acids. J. Am. Chem. Soc. 1972, 94, 9158–9165. [Google Scholar]
- Borchardt, R. T.; Cohen, L. A. Stereopopulation control. II. Rate enhancement of intramolecular nucleophilic displacement. J. Am. Chem. Soc. 1972, 94, 9166–9174. [Google Scholar]
- Dillon, M. P.; Cai, H.; Maag, H. Application of the “trimethyl lock” to Ganciclovir, a pro-prodrug with increased oral bioavailability. Bioorg. Med. Chem. Lett. 1996, 6, 1653–1656. [Google Scholar] [CrossRef]
- Lavis, L.D.; Raines, R.T. Bright ideas for chemical biology. ACS Chem. Biol. 2008, 3. in press. [Google Scholar]
- Hartley, B. S.; Kilby, B. A. The reaction of p-nitrophenyl esters with chymotrypsin and insulin. Biochem. J. 1954, 56, 288–297. [Google Scholar] [PubMed]
- Menger, F. M.; Ladika, M. Origin of rate accelerations in an enzyme model: The p-nitrophenyl ester syndrome. J. Am. Chem. Soc. 1987, 109, 3145–3146. [Google Scholar] [CrossRef]
- Oldfield, C.; Robinson, B. H.; Freedman, R. B. Acid–base behaviour of 4-nitrophenol and 4-nitrophenyl-2-sulphonate in water-in-oil microemulsions stabilized by aerosol-OT. J. Chem. Soc. Faraday Trans. 1990, 86, 833–841. [Google Scholar] [CrossRef]
- Leroy, E.; Bensel, N.; Reymond, J.-L. A low background high-throughput screening (HTS) fluorescence assay for lipases and esterases using acyloxymethylethers of umbelliferone. Bioorg. Med. Chem. Lett. 2003, 13, 2105–2108. [Google Scholar] [CrossRef] [PubMed]
- Sicart, R.; Collin, M.-P.; Reymond, J.-L. Fluorogenic substrates for lipases, esterases, and acylases using a TIM-mechanism for signal release. Biotechnol. J. 2007, 2, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Lowe, G.; Yuthavong, Y. pH-Dependence and structure–activity relationships in the papain-catalysed hydrolysis of anilides. Biochem. J. 1971, 124, 117–122. [Google Scholar] [PubMed]
- Amsberry, K. L.; Gerstenberger, A. E.; Borchardt, R. T. Amine prodrugs which utilize hydroxy amide lactonization. II. A potential esterase-sensitive amide prodrug. Pharm. Res. 1991, 8, 455–461. [Google Scholar]
- Haveaux, B.; Dekoker, A.; Rens, M.; Sidani, A. R.; Toye, J.; Ghosez, L. Chloroenamines, reactive intermediates for synthesis: 1-Chloro-N,N,2-trimethylpropenylamine. Org. Synth. 1979, 59, 26–34. [Google Scholar] [CrossRef]
- Furstner, A.; Weintritt, H. Total synthesis of roseophilin. J. Am. Chem. Soc. 1998, 120, 2817–2825. [Google Scholar] [CrossRef]
- Lyublinskaya, L. A.; Belyaev, S. V.; Strongin, A. Y.; Matyash, L. F.; Levin, E. D.; Stepanov, V. M. A new chromogenic substrate for subtilisin. Anal. Biochem. 1974, 62, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Park, J. H.; Agnello, C. F.; Mathew, E. S→N Transfer and dual acetylation in the S-acetylation and N-acetylation of 3-phosphoglyceraldehyde dehydrogenase by substrates. J. Biol. Chem. 1966, 241, 769–771. [Google Scholar] [PubMed]
- Park, J. H.; Meriwether, B. P.; Clodfelder, P.; Cunningham, L. W. The hydrolysis of p-nitrophenyl acetate catalyzed by 3-phosphoglyceraldehyde dehydrogenase. J. Biol. Chem. 1961, 236, 136–141. [Google Scholar] [PubMed]
- Wolf, N. M.; Morisseau, C.; Jones, P. D.; Hock, B.; Hammock, B. D. Development of a high-throughput screen for soluble epoxide hydrolase inhibition. Anal. Biochem. 2006, 355, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Baumann, H.; Henke, E.; Knoarzycka-Bessler, M.; Bornscheuer, U. T. Directed evolution of lipases and esterases. Methods Enzymol. 2004, 388, 199–207. [Google Scholar] [PubMed]
- Schmidt, M.; Bornscheuer, U. T. High-throughput assays for lipases and esterases. Biomol. Eng. 2005, 22, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Hecht, M. H. Enzyme-like proteins from an unselected library of designed amino acid sequences. Protein Eng. Des. Sel. 2004, 17, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Hecht, M. H.; Das, A.; Go, A.; Bradley, L. H.; Wei, Y. De novo proteins from designed combinatorial libraries. Protein. Sci. 2004, 13, 1711–1723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sample Availability: Contact authors.
© 2008 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Levine, M.N.; Lavis, L.D.; Raines, R.T. Trimethyl Lock: A Stable Chromogenic Substrate for Esterases. Molecules 2008, 13, 204-211. https://doi.org/10.3390/molecules13020204
Levine MN, Lavis LD, Raines RT. Trimethyl Lock: A Stable Chromogenic Substrate for Esterases. Molecules. 2008; 13(2):204-211. https://doi.org/10.3390/molecules13020204
Chicago/Turabian StyleLevine, Michael N., Luke D. Lavis, and Ronald T. Raines. 2008. "Trimethyl Lock: A Stable Chromogenic Substrate for Esterases" Molecules 13, no. 2: 204-211. https://doi.org/10.3390/molecules13020204



