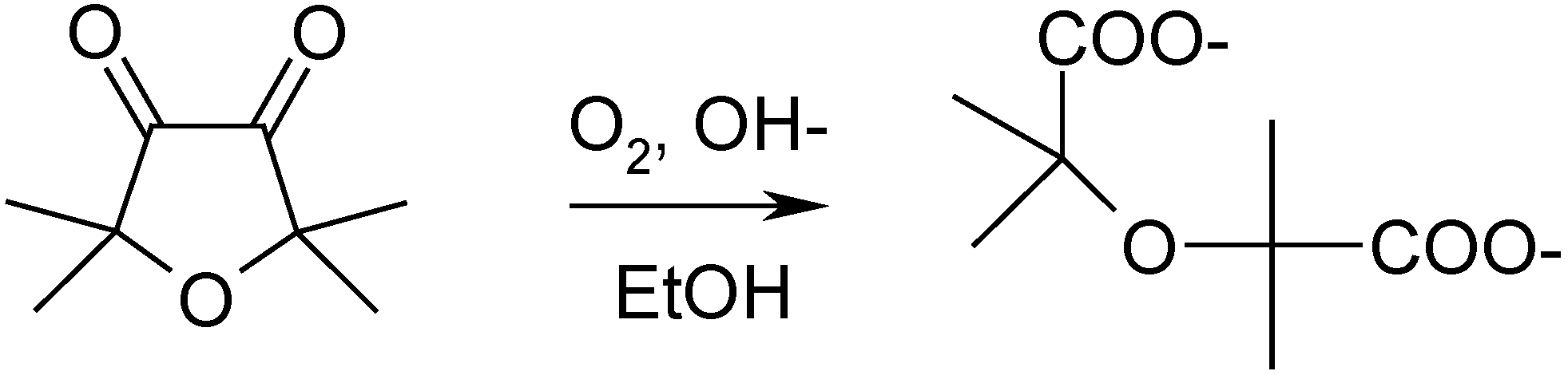Preliminary experiments
Easy oxidation of quercetin (
1) by air oxygen in the moderate-alkaline solutions at ambient temperature should be considered as one of most important chemical properties of flavonols. A simple experiment, namely the slow stepwise titration of
1 in 30 % water ethanol solution by sodium hydroxide within pH range from 2 to 10 (
Figure 1), confirms the universal character of this process.
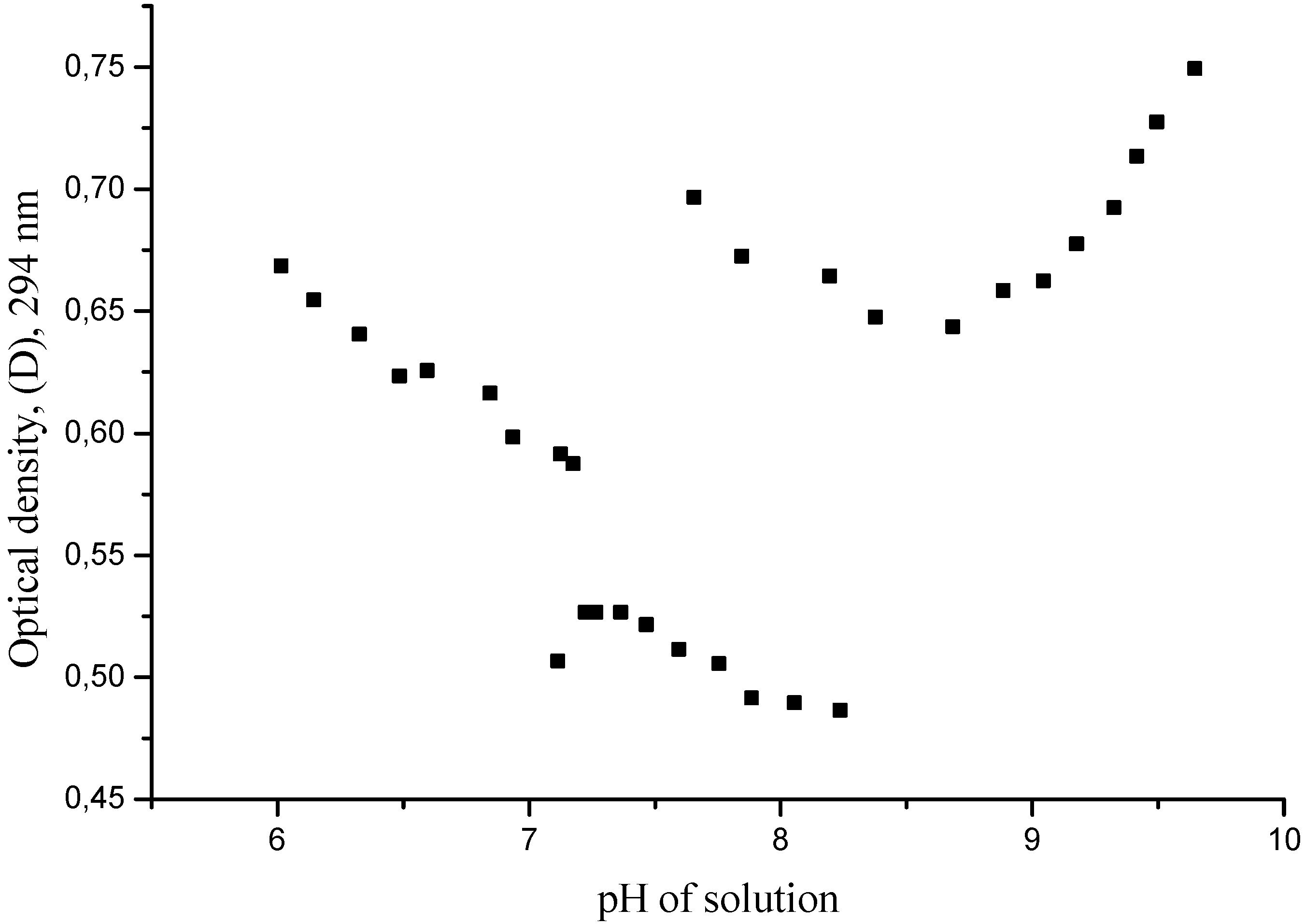
Figure 1.
Variations of optical densities of quercetin solution at wavelength 294 ± 2 nm in result of its slow stepwise titration by NaOH within the pH range 6-10. The anomaly at pH 7.2-7.1 is caused by the interruption of the process for ca. 30 min; the second one (at pH 8.2-7.7) was obtained after the storage of the solution during one week.
Figure 1.
Variations of optical densities of quercetin solution at wavelength 294 ± 2 nm in result of its slow stepwise titration by NaOH within the pH range 6-10. The anomaly at pH 7.2-7.1 is caused by the interruption of the process for ca. 30 min; the second one (at pH 8.2-7.7) was obtained after the storage of the solution during one week.
After addition of each portion of titrant, the pH of solution was measured and its UV-spectrum recorded. Surprisingly, any interruption of this uniform procedure leads to a gap in the smooth dependence of pH
vs. volume of titrant curve and disruptions in the UV-abundances at different wavelengths. These anomalies in the pH-D (294 nm) coordinates are clearly evident in
Figure 1.
The first anomalous area at pH ≈ 7.2-7.1 was caused by the storage of the solution under titration during
ca. 30 min; the second one (at pH ≈ 8.2-7.7) was caused by its storage for one week. The reproducibility of these effects permits us to exclude the influence of experimental errors and conclude the existence of an actual experimental feature. The complete UV-spectra of
1 during titration within the pH range 2-10 indicate anomalies as well. Some of them can be explained by the acid-base equilibrium ArOH ↔ ArO
- [рK
а(
1) = 7.3 ± 0.1 (ОН
7)] [
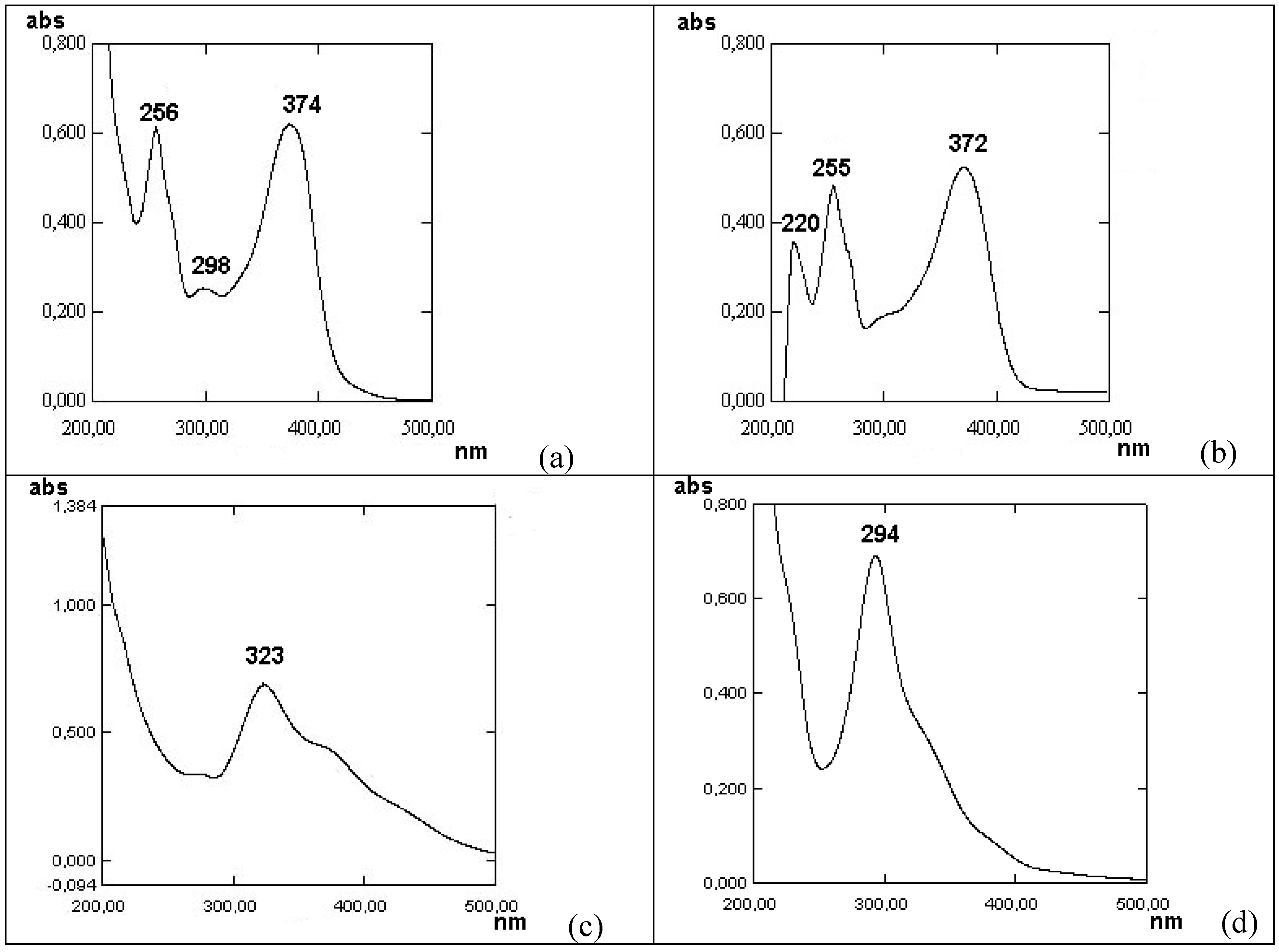
30]. However, the acidification of the basic solution (pH = 10) after slow stepwise titration did not restore its initial UV-spectrum (
Figure 2). At the same time, the quick reversible pH variation (in only two steps) in accordance with the scheme “acid → basic → acid” restores a UV-spectrum identical to that of initial solution.
Figure 2.
Variations of the UV-spectra of a water-ethanol solution of quercetin during its slow stepwise titration by NaOH within pH range 2-10, followed by the acidification up to pH = 4; (a) – UV-spectrum of quercetin in ethanol solution; (b) – at рН = 2; (c) – at рН = 10; (d) – UV-spectrum of solution after acidification to рН = 4.
Figure 2.
Variations of the UV-spectra of a water-ethanol solution of quercetin during its slow stepwise titration by NaOH within pH range 2-10, followed by the acidification up to pH = 4; (a) – UV-spectrum of quercetin in ethanol solution; (b) – at рН = 2; (c) – at рН = 10; (d) – UV-spectrum of solution after acidification to рН = 4.
All the mentioned effects can be explained by the instability of quercetin (
1) towards the oxidation by air oxygen during slow titration and all other operations involving its dilute solutions at ambient temperature. The oxygen ground state is the triplet one. This means that the O
2 molecule exists as a reactive biradical [
.O-O
.]. The solubility of oxygen in the water at ambient conditions (760 torr, 20 °C) is about 8.8-9.4 mg/L [
31,
32] or about 0.29 mmol/L. This reference value can be accepted without additional verification and it should remain constant when air is bubbling through aqueous reaction media. For comparison, the concentration of
1 in the solution used for recording of UV-spectrum was
ca. 20 mg/L, or 0.06 mmol/L. That is, there was at least a 4.5-fold molar excess of oxygen in this solution.
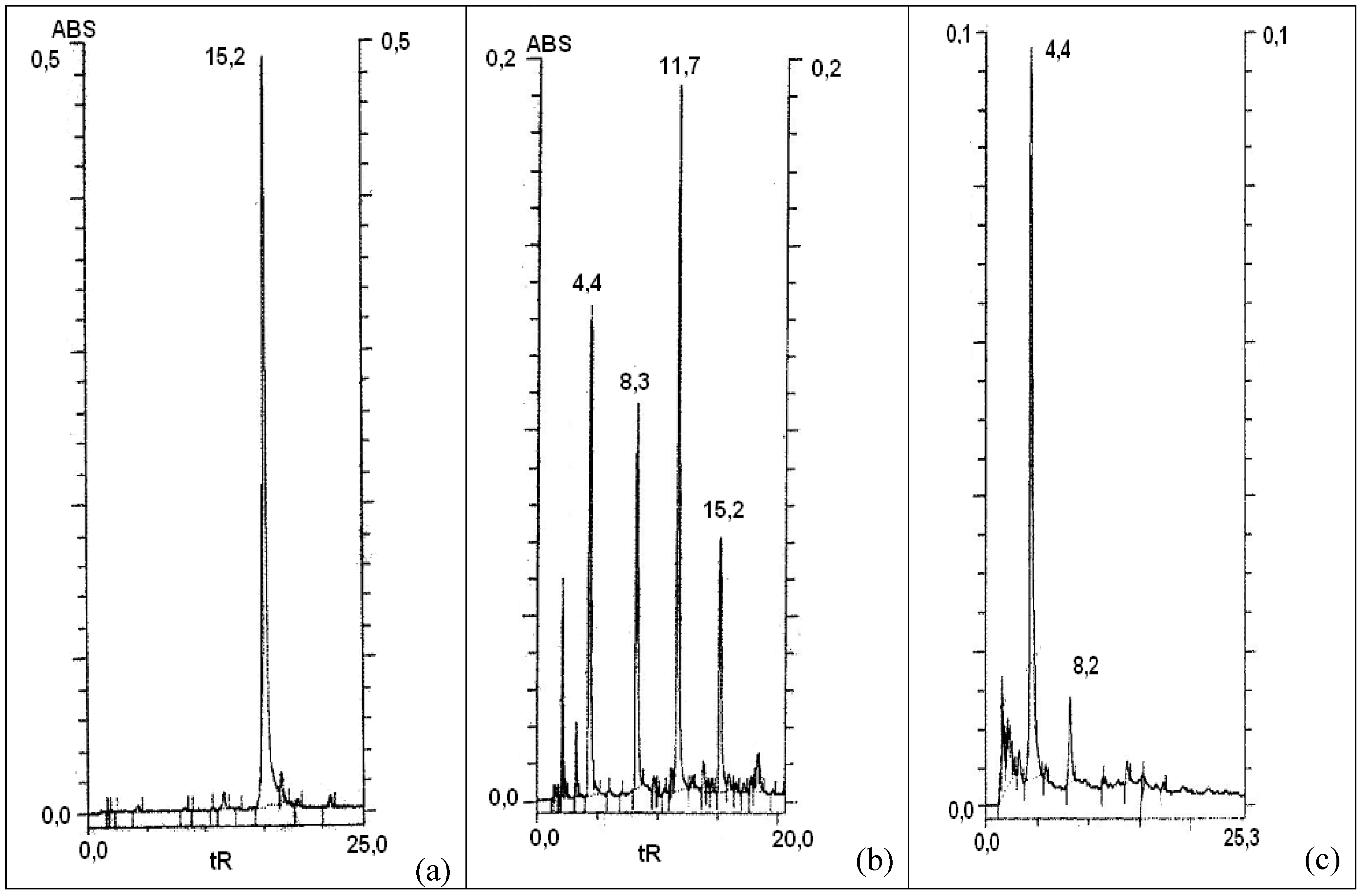
Figure 3.
HPLC chromatograms (regime 1, detection wavelength 254 nm) (a) – initial solution of quercetin (tR 15.2 min); (b) – the same solution after ~ 1 h of air bubbling at ambient temperature; (c) – the same solution after termination of the process (air bubbling during more than 3-3.5 h).
Figure 3.
HPLC chromatograms (regime 1, detection wavelength 254 nm) (a) – initial solution of quercetin (tR 15.2 min); (b) – the same solution after ~ 1 h of air bubbling at ambient temperature; (c) – the same solution after termination of the process (air bubbling during more than 3-3.5 h).
Experimental oxidation of
1 by air was carried out using a simple procedure. Air was bubbled through water-ethanol solutions (pH ≈ 8-10) of
1 at ambient temperature during up to 3.5 h with periodical HPLC analyses of the reaction medium at different times. The chromatograms of the initial quercetin solution (individual compound with t
R 15.2 min, regime 1) after 1 h of air bubbling and that after termination of the process (3.5 h) are compared in
Figure 3. No peak of
1 is observed on the final chromatogram; only the signal of a hydrophilic compound(s) with t
R ca. 4.4 min is registered. No additional changes have been detected with the increase of the contact time of this solution with air. The same transformations after a similar period are observed when the reaction medium was cooled down to 0 °C, which is typical for radical processes. Oxidation of
1 by air oxygen is accompanied by the appearance of an intense red-brown coloration of the solution. The dry product isolated from this solution after evaporation of the solvents possesses the same color.
Analysis of oxidation products
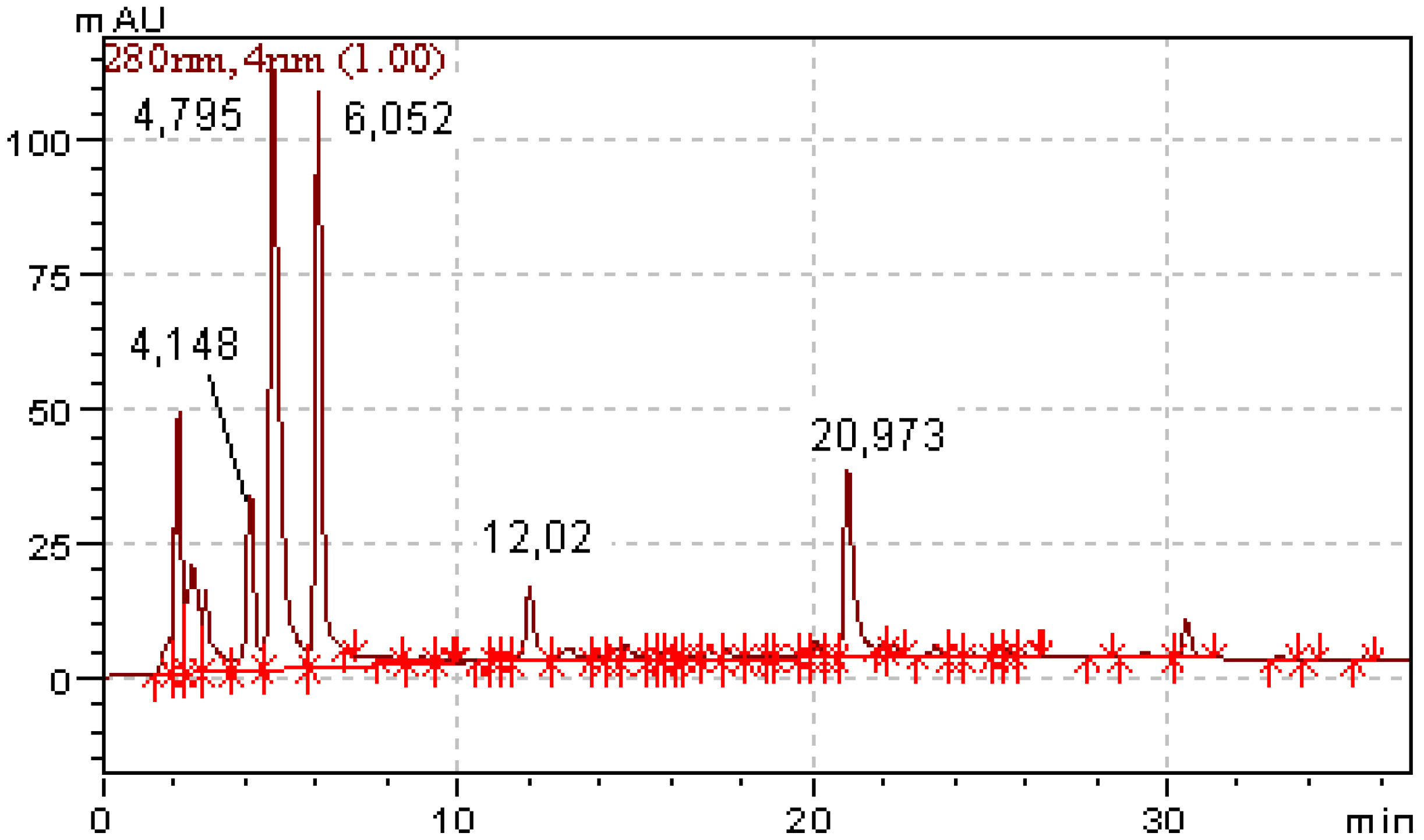
HPLC analysis of dry mixture of oxidation products (regime 2) indicate the presence of four principal products with retention times of 4.15, 4.80, 6.05 and 20.97 min (initial quercetin with t
R 25.7 min is absent in this mixture), as shown in
Figure 4. By analogy with literature data it seems reasonable to believe that oxidation products of
1 possess simpler chemical structures than that of the initial compound. So far as quercetin itself can be analyzed in the form of its trimethylsilyl (TMS) derivatives (see their RI values in
Table 1), GC-MS analysis of TMS-derivatives of oxidation products can be used as the method for their identification. Three principal compounds accounting for
ca. 96 % among semi-volatile substances were found in the dry mixture of oxidation products (
Table 3).
Figure 4.
HPLC chromatogram (regime 2) of dried quercetin oxidation products (initial compound with tR 25.68 min is absent). Component with tR 4.15 min – phloroglucinol; 4.890 min – 2,4,6-trihydroxybenzoic acid; 6.05 min – 3,4-dihydroxybenzoic acid; 12.02 – intermediate depside (2); 20.97 min – unidentified compounds. Each peak is 100 % pure as there is no co-elution of compounds.
Figure 4.
HPLC chromatogram (regime 2) of dried quercetin oxidation products (initial compound with tR 25.68 min is absent). Component with tR 4.15 min – phloroglucinol; 4.890 min – 2,4,6-trihydroxybenzoic acid; 6.05 min – 3,4-dihydroxybenzoic acid; 12.02 – intermediate depside (2); 20.97 min – unidentified compounds. Each peak is 100 % pure as there is no co-elution of compounds.
Table 3.
| tR, min | Relative content, % | MS identification | Reference RI values |
|---|
| 25.48 | 10 | Phloroglucinol, tris-TMS (A) | 1656 |
| 27.71 | 1 | Ethyl-3,4-dihydroxybenzoate, bis-TMS | - |
| 28.25 | 53 | 3,4-Dihydroxybenzoic acid, tris-TMS (B) | 1830 |
| 30.72 | 33 | 2,3,4-Trihydroxybenzoic acid, tetrakis-TMS (C) | 1932 (2,3,4-isomer);
2003 (2,4,6-isomer) |
| 32.34 | 2 | Unidentified | - |
Components A and B can be identified ambiguously with the use of the Wiley 138.L MS database: they are TMS-derivatives of 1,3,5-trihydroxybenzene (phloroglucinol, A) and 3,4-dihydroxybenzoic (protocatechuic) acid (B). The third component C was tentatively identified as the TMS derivative of 2,3,4-trihydroxybenzoic acid, but this is not consistent with the absence of a structural fragment with this arrangement of hydroxyl groups in the molecule of 1. A more reliable assignment of the structure of this oxidation product is 2,4,6-trihydroxybenzoic (phloroglucinic) acid, which is in agreement with the position of substituents on ring A of the initial molecule. The erroneous GC-MS identification of this compound is likely caused by the absence of its mass spectrum in the Wiley MS database; as a result it is recognized as an isomer. The value of GC retention index of this TMS-derivative (1996) is in the better agreement with reference RI value for TMS derivative of 2,4,6-trihydroxybenzoic acid (2003 ± 9) than with that for 2,3,4-trihydroxybenzoic acid (a single reference value 1932 is available).
The comparison of GC-MS results with the data of HPLC analysis (
Figure 4) permits us to identify 3,4-dihydroxybenzoic acid (
B) by the coincidence of its retention time (6.05 min) and UV-spectrum (λ
max 216, 259 and 293 nm) with a reference sample. More hydrophilic components with retention times 4.15 and 4.80 min are phloroglucinol (
A) and 2,4,6-trihydroxybenzoic acid (
C), respectively. The relatively hydrophobic compounds with retention times 12.02 and 20.97 min have no matches in the GC-MS analysis results and therefore cannot be identified this way. One of them (with t
R 8.3 min in regime 1 and 12.02 min in regime 2) is the intermediate depside (ester) that is unstable to hydrolysis in a basic media.
The retention indices of compounds
B and
C in reversed phase HPLC (with the use of
n-alkyl phenyl ketones as reference compounds) have been determined first time; both of them are in the accordance with the previously reported RI value of phloroglucinol [
11], because the ascending order of RI values reflects the order of chromatographic elution (
Table 4). The same products were isolated in the result of quercetin oxidation by hydrogen peroxide under the same pH conditions.
Table 4.
| Compound | RI |
|---|
| Phloroglucinol (A) | 582 [11] |
| 2,4,6-Trihydroxybenzoic acid (C) | 606 |
| 3,4-Dihydroxybenzoic acid (B) | 618 |
The formation of the aforementioned products is in complete agreement with literature data on the oxidation of quercetin under different conditions [
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27]. To date the oxidative decarbonylation of
1 was considered the likely process which could take place preferably in non-aqueous solvents in the presence of strong bases and often at above-ambient temperatures (in pyridine during 14 h [
14], in dimethylformamide with additives of potassium
tert-butoxide at 37 °C during 3 h [
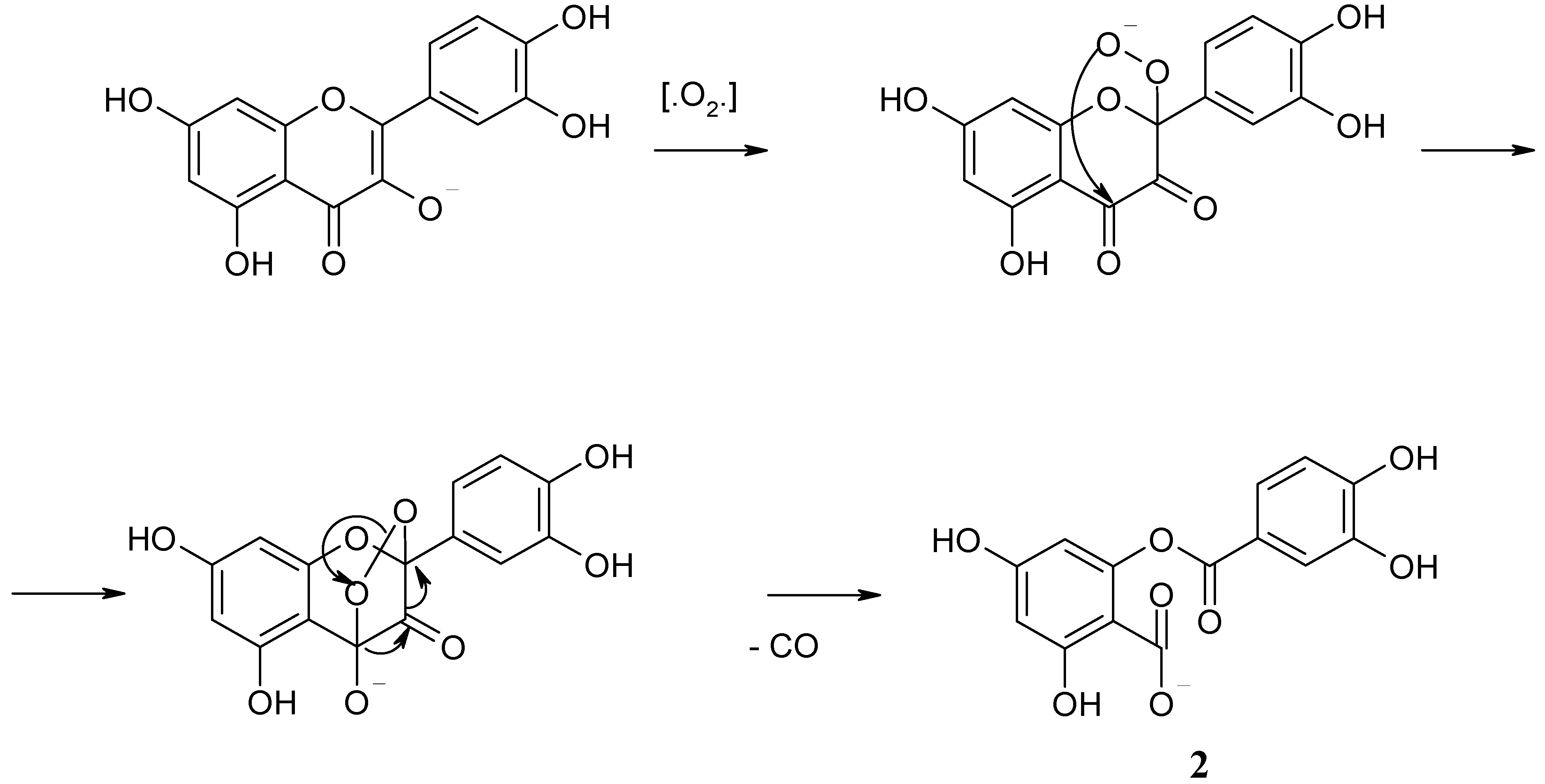
16], etc.). The accepted mechanism of this reaction (
Scheme 1) includes the attack of carbon atom C
2 in ring C by oxygen with formation of a hydroperoxide anion. The cyclization of this anion implies the formation of a new O-C
4 bond, followed by elimination of the C
3 carbon atom in the form of carbon monoxide (CO). The resulting primary product is 4,6-dihydroxy-2-(3,4-dihydroxybenzoyloxy)benzoic acid (depside,
2, CAS # 30048-34-1); its subsequent hydrolysis in basic media gives salts of 3,4-dihydroxy- and 2,4,6-trihydroxybenzoic acids. Compound
2 has been identified as one of the intermediate products of quercetin oxidation (component with t
R 8.3 min and 12.02 min on the chromatograms presented in
Figure 3 and
Figure 4, correspondingly).
Scheme 1.
Possible mechanism of quercetin oxidative decarbonylation by oxygen [
16].
Scheme 1.
Possible mechanism of quercetin oxidative decarbonylation by oxygen [
16].
This mechanism was partially confirmed by isotope labeling using of
18O
2 for oxidation [
16]. The isotope labels were found distributed in a 1:1 ratio in both acids formed after hydrolysis of depside (
2).
Nevertheless, the oxidative decarbonylation of quercetin presented in
Scheme 1 seems to not be the only possible mechanism. First, as reported in the publications of Matsuura
et al. [
14,
15], carbon monoxide may not be the sole gaseous product of this reaction; the formation of carbon dioxide was detected as well. The CO
2:CO ratio was about 0.55 if the reaction media (pyridine solution) was irradiated by a tungsten lamp, but it exceeds 1 under UV-irradiation (when a high-pressure mercury lamp with Pyrex filter was used). In accordance with
Scheme 1, the ratio of reacted oxygen and formed carbon monoxide should be 1:1, i.e., if the oxidation of
1 is carried out in a closed system, the total gas pressure should remain constant.
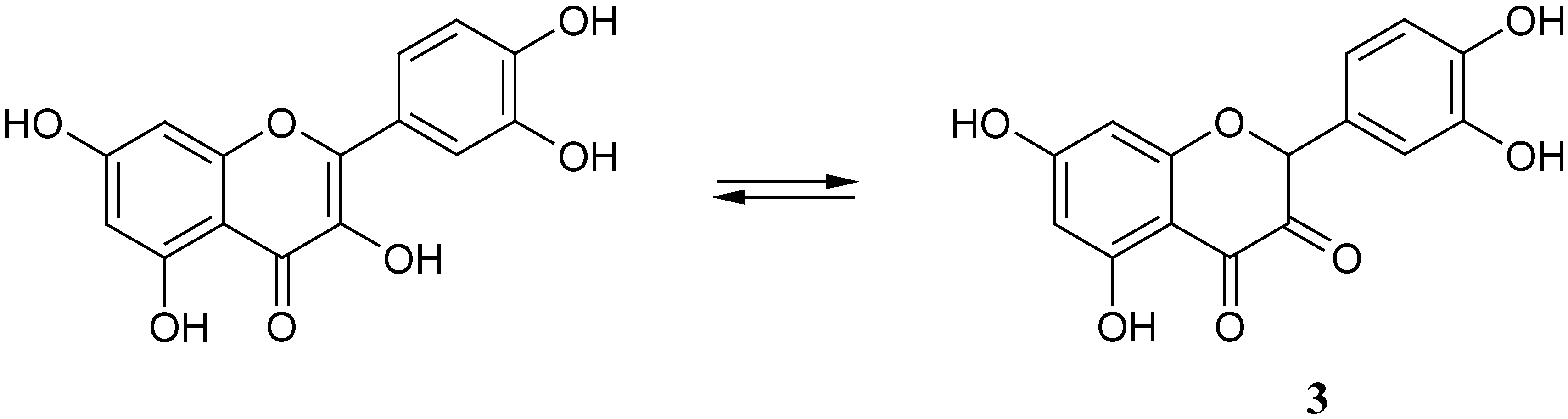
Quercetin (like other flavonoids) with a γ-pyrone ring in the molecule (ring C) can exist in two prototropic forms (
Scheme 2); the first of them is well-known enolic structure the γ-pyrone molecular fragment, while the second one is the tautomeric diketoform
3 (the ratio of tautomers is unknown and it can depend on the pH of the solution)
Scheme 2.
Keto-enol tautomerisation of quercetin.
Scheme 2.
Keto-enol tautomerisation of quercetin.
The existence of tautomer
3 was taken into account in the quantum chemical modeling of the oxidative properties of
1 [
33]. It is interesting to note that one of the principal chemical features of α-diketones is their easy oxidation in basic media by air oxygen (
Scheme 3). This reaction goes via intermediate ketyl anion-radicals [
34] and gives two carboxylic acids in result of C-C bond cleavage [
35,
36]. It was characterized in details on the example of 2,2,5,5-tetramethylfuranidin-3,4-dione [
35]:
Scheme 3.
Example of the oxidative cleavage of α-diketones.
Scheme 3.
Example of the oxidative cleavage of α-diketones.
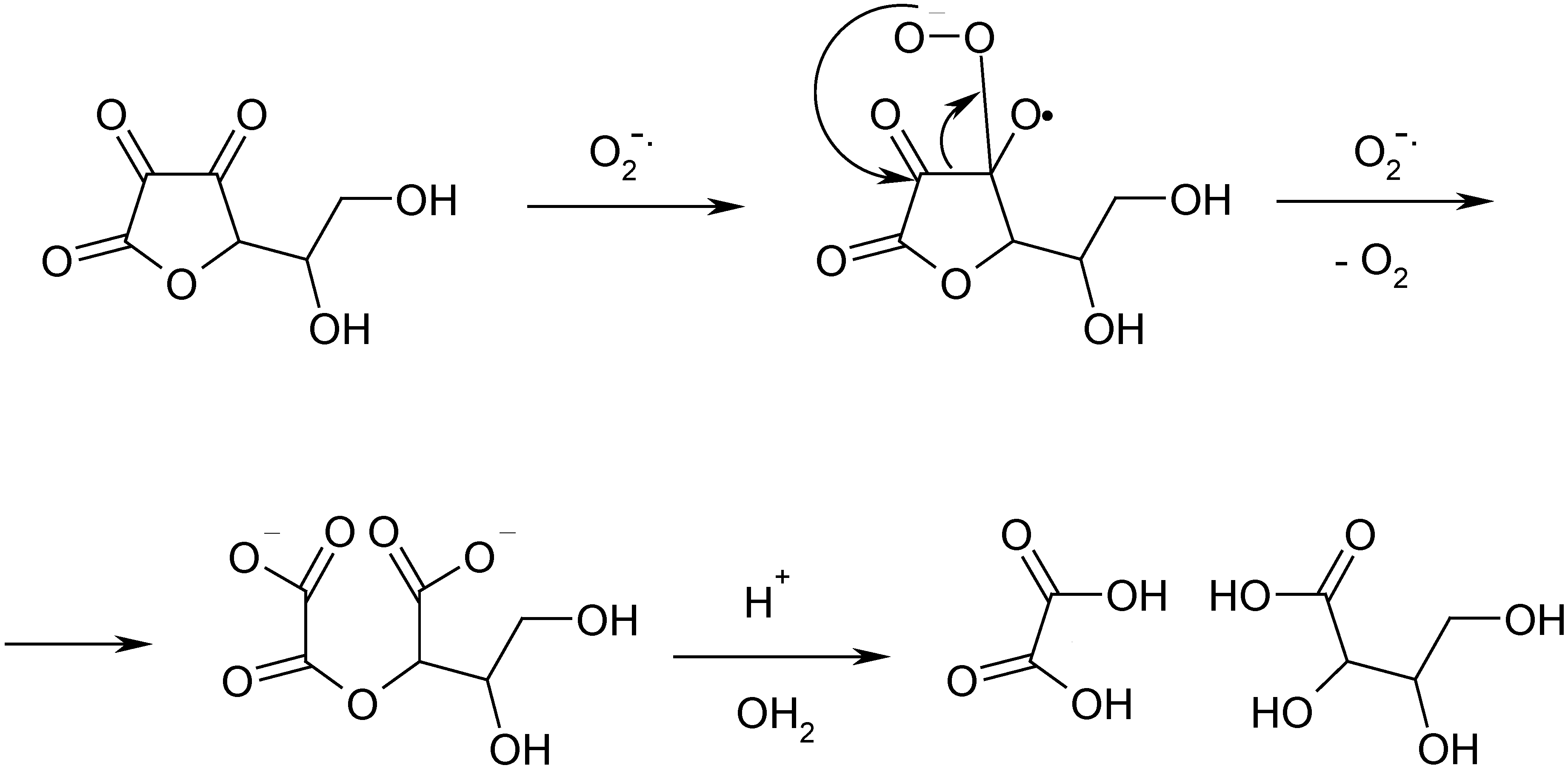
A similar scheme for oxidation of another α-diketo compound (dehydroascorbic acid) by superoxide anion-radicals (O
2-. – one of the forms of active oxygen in chemical reactions) with the cleavage of C-C bong and formation of two carboxylic acids was proposed by Frimer and Gilinsky-Sharon in 1995 [
37] (
Scheme 4).
Scheme 4.
Oxidative cleavage of dehydroascorbic acid by O2-.
Scheme 4.
Oxidative cleavage of dehydroascorbic acid by O2-.
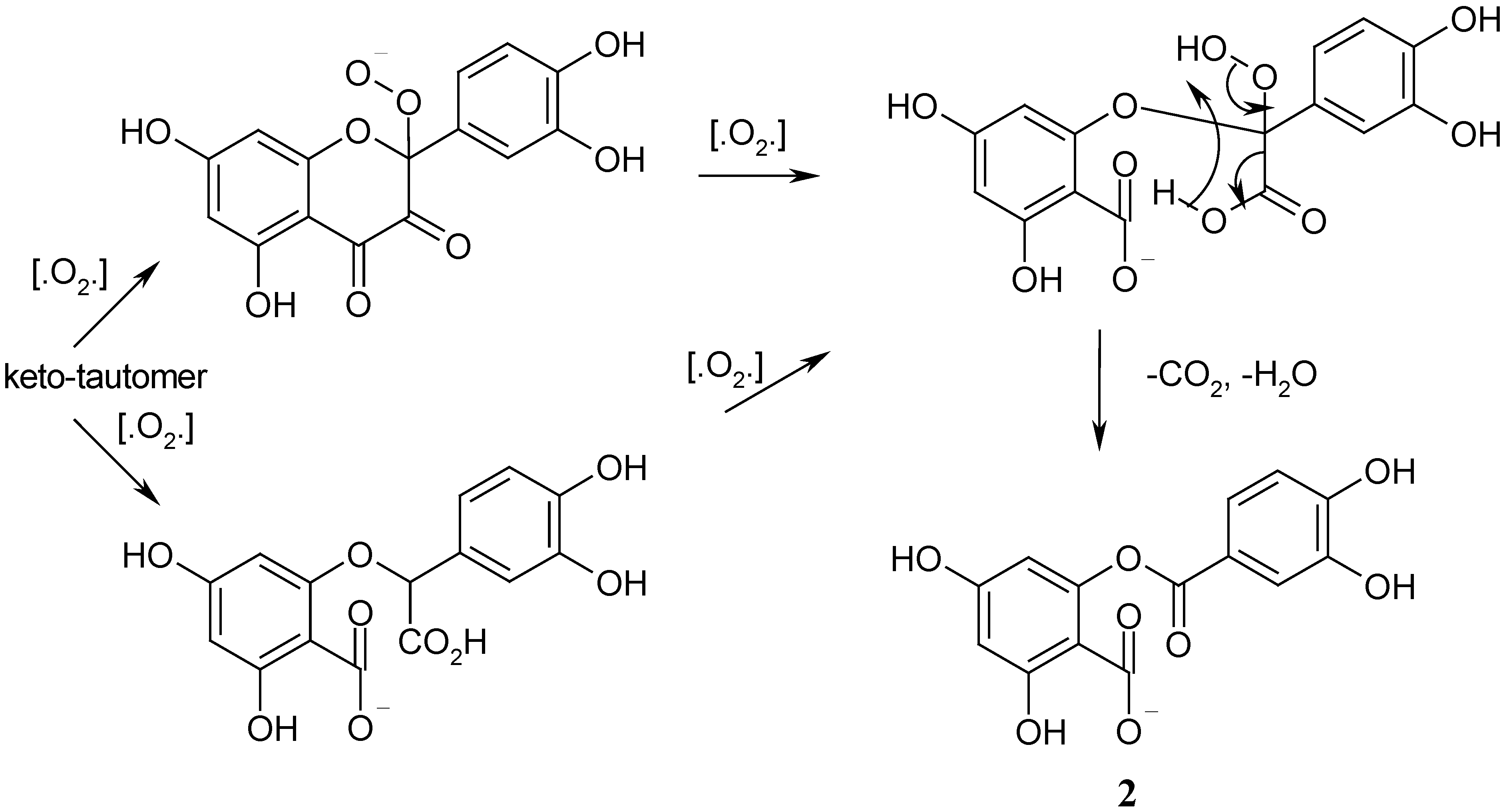
Keeping in mind these properties of α-diketones and applying them to the quercetin oxidation permits us to propose the alternative mechanism of the process with the formation of the same depside
2 shown in
Scheme 5.
In comparison with
Scheme 1 this is not oxidative decarbonylation, but rather what has taken place is a decarboxylation (accompanied by CO
2 formation). Therefore, more than one equivalent of oxygen is required for the formation of one equivalent of carbon dioxide. As far as this process takes place in basic media, the carbon dioxide formed immediately converts into CO
32- ions in the solution; hence no elimination of gases from the reaction media should be observed. The radical character of this reaction is confirmed by the absence of changes in its rate as a result of the cooling of the quercetin solution.
Scheme 5.
Alternative mechanism of quercetin oxidative decarboxylation by oxygen.
Scheme 5.
Alternative mechanism of quercetin oxidative decarboxylation by oxygen.
It is important to note that
Scheme 5 should be in the agreement with the observed distribution of
18O isotope labels among products as
Scheme 1 as well. The primary product of this reaction is the same depside
2; its subsequent hydrolysis in basic media gives two hydroxybenzoic acids. Besides that, the hydrolysis of
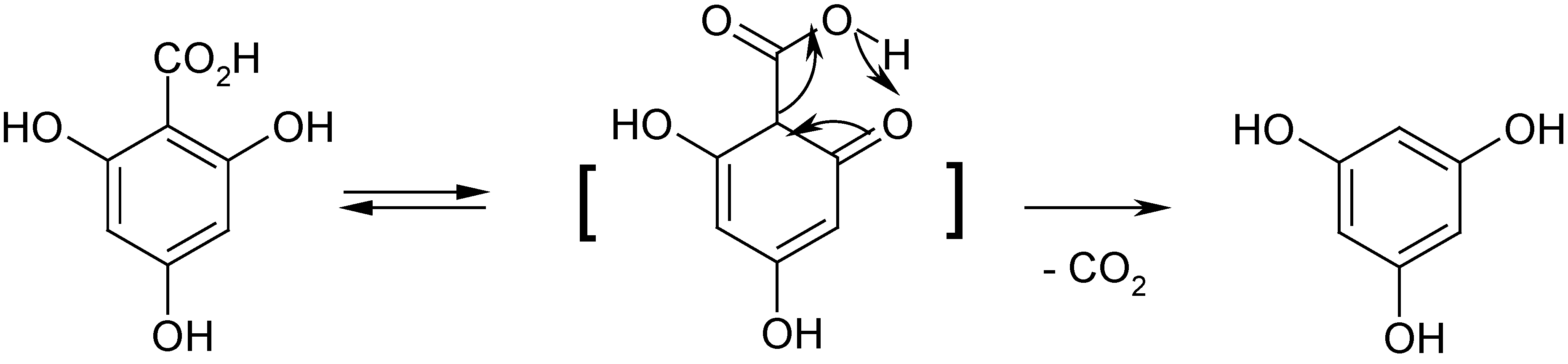
2 in water-ethanol mixtures gives small amounts of an ethyl ester (ethyl-3,4-dihydroxybenzoate); this compound was detected with GC-MS analysis of the mixture of reaction products. The presence of variable amounts of phloroglucinol (
A) among the isolated quercetin oxidation products can be explained by the easy decarboxylation of 2,4,6-trihydroxybenzoic acid (
B) during drying of reaction mixtures (at the temperature
ca. 100 °C) (
Scheme 6).
Scheme 6.
Formation of phloroglucinol in the result of decarboxylation of 2,4,6-trihydroxybenzoic acid.
Scheme 6.
Formation of phloroglucinol in the result of decarboxylation of 2,4,6-trihydroxybenzoic acid.
Unfortunately, the chemical origin of the compound(s) responsible for the appearance of intensive color of reaction media and isolated dry products remains unknown. It is reasonable to assume that these compound(s) should include benzoquinone-like molecular fragments (chromophores), but its (their) content may be small. The screening of UV-spectra of all components detected during HPLC analysis with diode array detector in relation to the absorbance in the visible region gave no results. Both
Scheme 1 and
Scheme 5 confirm that the principal structural fragment of the quercetin molecule that is responsible for the easy oxidation of this compound by air oxygen is the hydroxyl group at C
3. The substitution of this group by glycoside fragments completely suppresses the oxidation. For example, rutin becomes stable, while another flavonol (3,5,7,4′-tetrahydroxyflavon, kaempferol) oxidizes easily under the same conditions. This fact is in accordance with the literature data, whereby 3-O-methylated flavonols gave no oxidation products in the presence of radical initiators [
18].
Therefore, the selection of the reliable mechanism of quercetin oxidation by air oxygen seems impossible without direct analysis of gaseous products (CO or CO
2) formed in this process in the gas phase contacting with reaction media (so-called head-space analysis, HSA [
37]). This experiment was carried out in closed system containing 1 L of water quercetin solution (2.1 g, 6.2 mmol/L, pH ~ 11, basified by NaOH) and 2 L of air and a digital manometer for pressure measurements. The analysis of gas phase was conducted with the use of gas chromatograph equipped by two packed columns placed in parallel and thermo conductivity detector (carrier gas was hydrogen). The first column (with non-polar polymer sorbent Porapak Q) was used for separation of air (no separation for O
2 and N
2) and carbon dioxide (signals of positive polarity); the second column was used for separation of O
2, N
2 and carbon monoxide (signals of negative polarity). Both sets of signals were summarized on the same chromatogram.
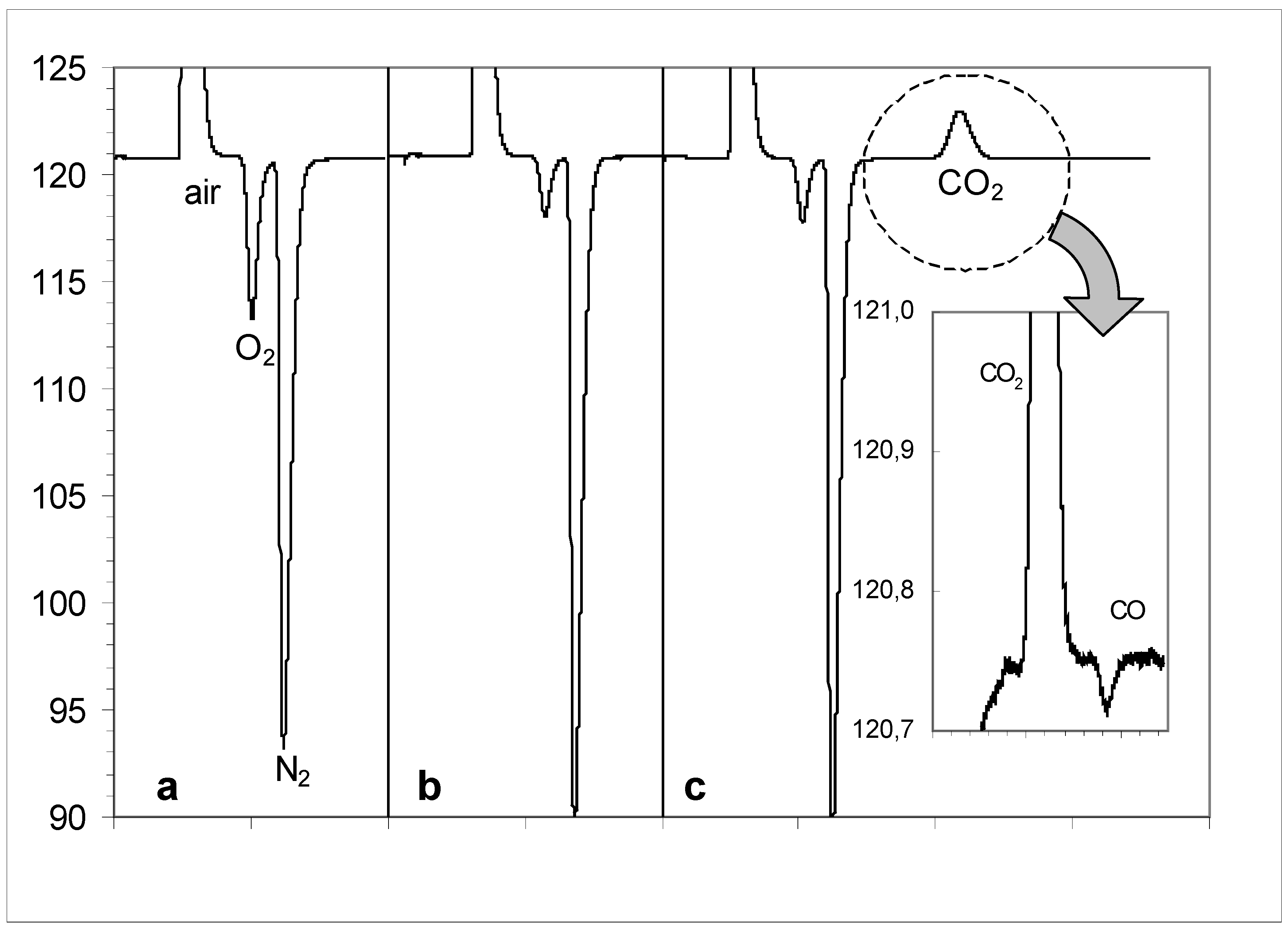
Figure 5.
Gas chromatograms of gas phase under quercetin solution during its oxidation by air oxygen in a closed system: (a) – under initial quercetin suspension in the water (pH = 6.56); (b) – under basic solution of quercetin (pH ~ 11) after 4 days of contacting with air; (c) – after acidification of basic solution up to pH ~ 1.
Figure 5.
Gas chromatograms of gas phase under quercetin solution during its oxidation by air oxygen in a closed system: (a) – under initial quercetin suspension in the water (pH = 6.56); (b) – under basic solution of quercetin (pH ~ 11) after 4 days of contacting with air; (c) – after acidification of basic solution up to pH ~ 1.
Figure 5a presents the gas phase chromatogram of a quercetin suspension in water at pH = 6.56, where the ratio of peak areas of O
2 and N
2 are equal to the content of these gases in the air (1:4). The chromatogram of the gas phase of an alkalinized solution of
1 in the water at pH ~ 11 after 4 days of the contact with air in the closed system is shown in
Figure 5b; the content of oxygen is visibly less, but no other gases (CO or CO
2) are detected. It is interesting that the total gas pressure in this system during the same time decreased from 1022 up to 930 mbar (- 9 %), which corresponds to the decrease of oxygen contents in the gas phase without formation of any volatile reaction products. Just this fact is enough to reject the previously proposed scheme for oxidative decarbonylation of quercetin [
16].
Figure 5c presents the chromatogram of the gas phase after acidification of the solution of
1 with concentrated HCl up to pH ~ 1; a carbon dioxide peak has appeared that is the direct confirmation of alternative mechanism of oxidative decarboxylation of quercetin (
Scheme 5). However, the variations of the recorder scale allow us to reveal the presence of small amounts of carbon monoxide in the gas phase (negative signal with higher retention time than that of CO
2). The peak areas of these two carbon oxides can be recalculated from the relative content of these reaction products in the gas phase with the use of coefficient
k = 2.96 (
Table 5, see Experimental).
Table 5.
| Gas phase component | Peak areas, S×10-3, mV×ms | Average content in gas phase, % |
|---|
| CO2 | 81.6 | 83.9 | 84.9 | 96.3 ± 0.3 |
| CO | 1.18 | 1.04 | 1.06 | 3.7 ± 0.3 |
Therefore, different mechanisms of quercetin transformation occur during its oxidation by air oxygen in moderately-basic water and water-ethanol solutions at ambient temperature. Under the mildest conditions of quercetin oxidation by air oxygen (at ambient temperature in moderately-basic media) the contribution of oxidative decarboxylation exceeds that of oxidative decarbonylation approximately by 26 times.