Poly[3-(3,4-dihydroxyphenyl)glyceric Acid], A New Biologically Active Polymer from Symphytum Asperum Lepech. and S.Caucasicum Bieb. (Boraginaceae)
Abstract
:Introduction
Results and Discussion
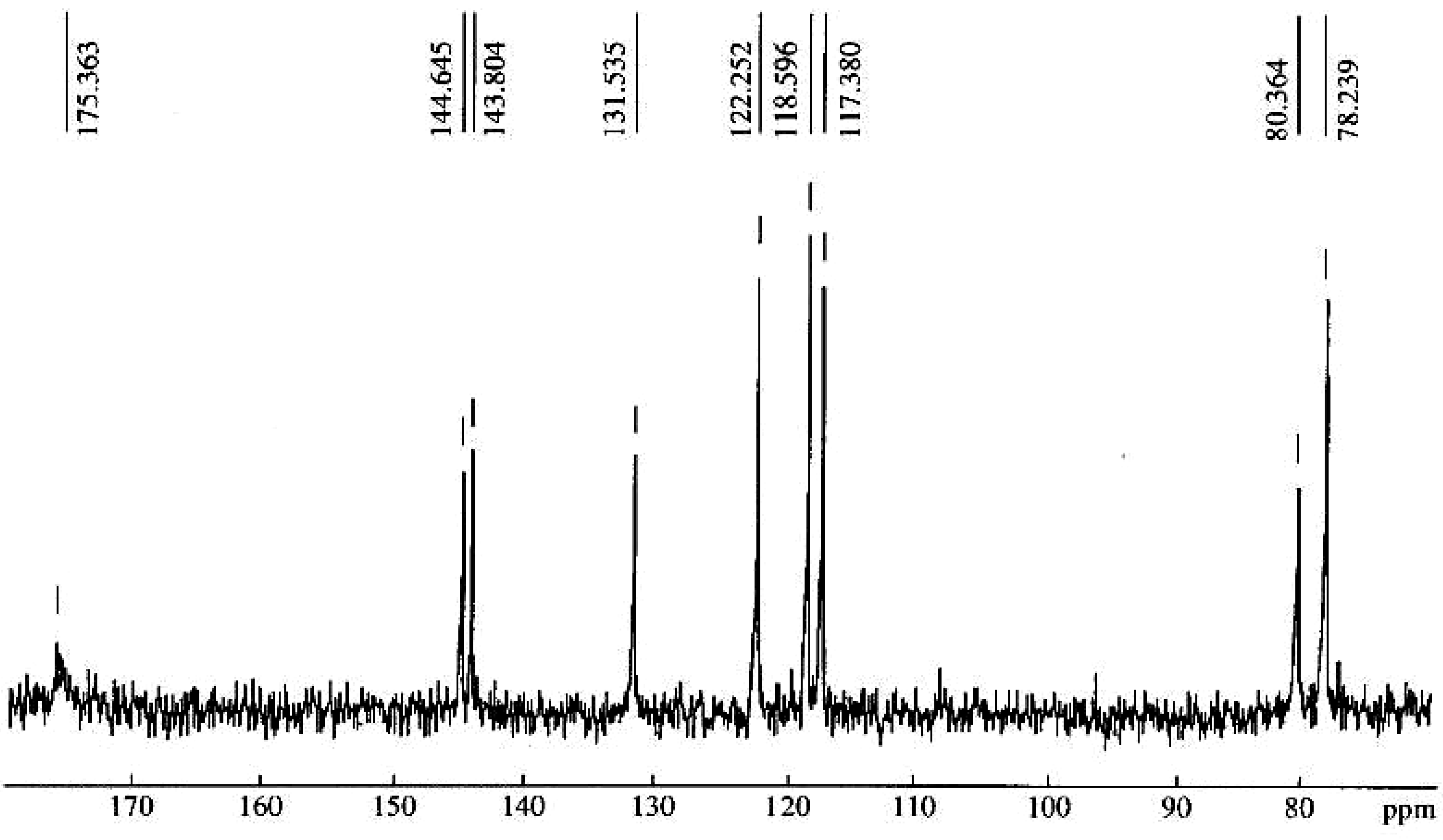

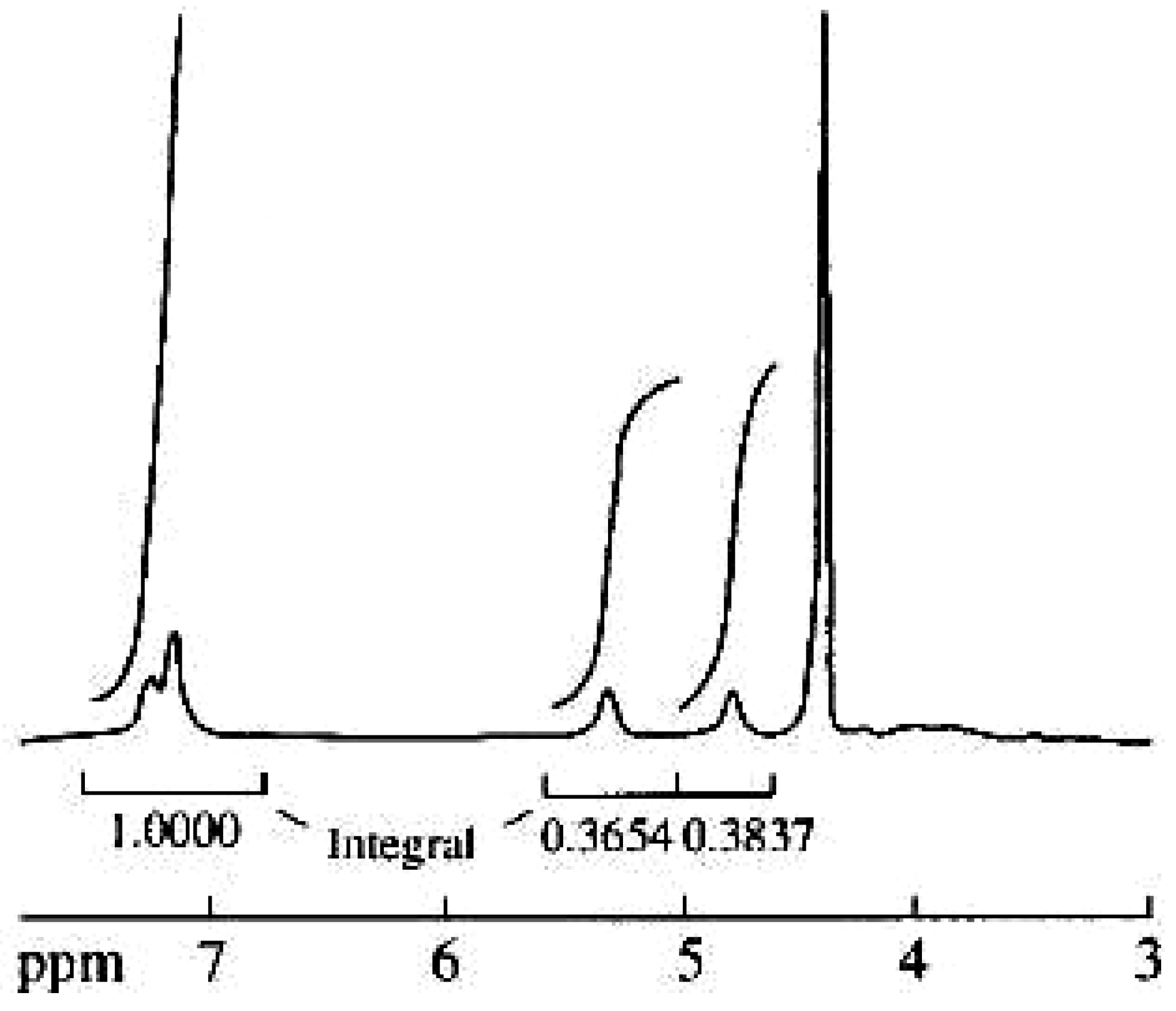
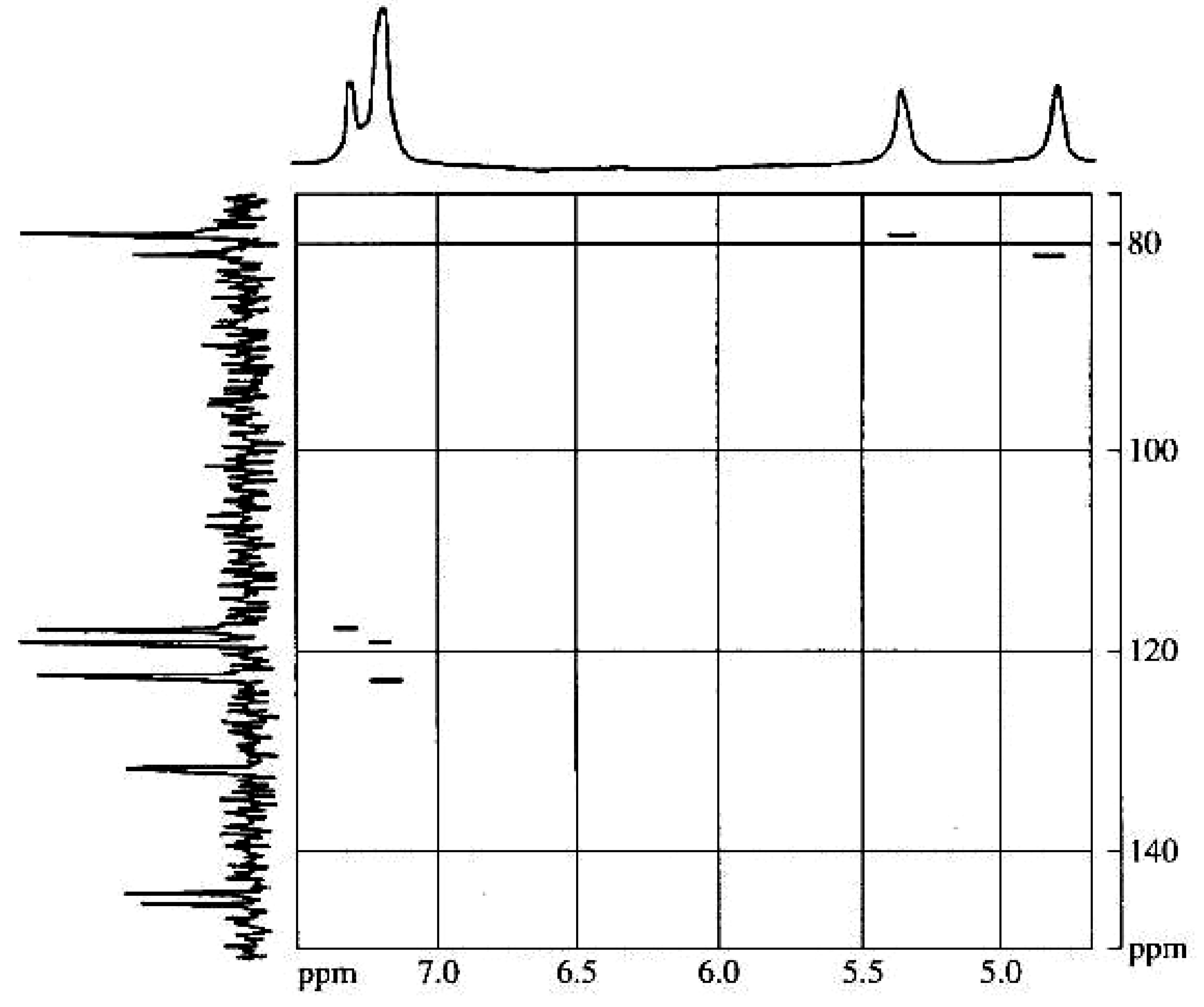
| Repeating unit | Atom no. | 13C chemical shift,δ, ppm | 1H chemical shift,δ, ppm |
 | 1' | 175.4 | 5.33 |
| 1 | 78.2 | ||
| 2 | 80.4 | 4.88 | |
| 1'' | 131.5 | ||
| 2'' | 117.4 | 7.24 | |
| 3'' | 144.6 | ||
| 4'' | 143.8 | ||
| 5'' | 118.6 | 7.13 | |
| 6'' | 122.3 | 7.13 |
Conclusions
Experimental
General
References
- Barbakadze, V.V.; Kemertelidze, E.P.; Usov, A.I.; Kroes, B.H.; Quarles van Ufford, H.C.; Van den Worm, E.; Beukelman, C.J.; Van den Berg, A.J.J.; Labadie, R.P. Evaluation of Immunomodulatory Activity of Some Plant Polysaccharides. Proc. Georg. Acad. Sci., Biol. Ser. 1999, 25, 207–216. [Google Scholar]
- Barbakadze, V.V.; Kemertelidze, E.P.; Dekanosidze, H.E.; Beruchashvili, T.G.; Usov, A.I. investigation of Glucofructans from Roots of Two Species of Comfrey Symphytum asperum Lepech. and S. caucasicum Bieb. Bioorg. Khim. 1992, 18, 671–679. [Google Scholar]
- Barbakadze, V.V.; Kemertelidze, E.P.; Shashkov, A.S.; Usov, A.I.; Kroes, B.H.; C.J.; Van den Berg, A.J.J.; Labadie, R.P. Partial Characterization of a New Anticomplementary Dihydroxycinnamate- Derived Polymer from Symphytum asperum Lepech. Proc. Georg. Acad. Sci., Biol. Ser. 2000, 25, 207–216. [Google Scholar]
- Barbakadze, V.V.; Kemertelidze, E.P.; Shashkov, A.S.; Usov, A.I. Structure of a New Anticomplementary Dihydroxycinnamate-Derived Polymer from Symphytum asperum (Boraginaceae). Mendeleev Commun. 2000, 10, 148–149. [Google Scholar] [CrossRef]
- Dyer, M.A. Applications of Absorption Spectroscopy of Organic Compounds; Prentice-Hall Inc.: Englewood Cliffs, NY, 1965. [Google Scholar]
- Patt, S.L.; Schoolery, J.N. Attached Proton Test for Carbon-13 NMR. J. Magn. Reson. 2000, 46, 535–539. [Google Scholar] [CrossRef]
- Higuchi, T. Encyclopedia of Plant Physiology: Plant Carbohydrates II: Extracellular Carbohydrates; Tanner, W., Loewus, F.A., Eds.; Springer-Verlag: Berlin, Heidelberg, New York, 1981; Vol. 13B, Chapter 9; pp. 194–224. [Google Scholar]
- Lewis, N.G. a 20(th) Century Roller Coaster Ride: a Short Account of Lignification. Curr. Opin. Plant Biol. 1999, 2, 153–162. [Google Scholar] [CrossRef]
- Bernards, M.A.; Lewis, N.G. The Macromolecular Aromatic Domain in Suberized Tissue; a Changing Paradigm. Phytochemistry 1998, 47, 915–933. [Google Scholar] [CrossRef]
- Bernards, M.A.; Lopez, M.L.; Zajicek, J.; Lewis, N.G. Hydroxycinnamic Acid-Derived Polymers Constitute the Polyaromatic Domain of Suberin. J. Biol. Chem. 1995, 270, 7382–7386. [Google Scholar] [PubMed]
- Lapierre, C.; Pollet, B.; Negrel, J. The Phenolic Domain of Potato Suberin: Structural Comparison With Lignin. Phytochemistry 1996, 42, 949–953. [Google Scholar] [CrossRef]
- Zeier, J.; Schreiber, L. Chemical Composition of Hypodermal and Endodermal Cell Walls and Xylem Vessels Isolated from Clivia mimata. Plant Physiol. 1997, 113, 1223–1231. [Google Scholar] [PubMed]
- Eberhardt, T.L.; Bernards, M.A.; He, L.; Davin, L.B.; Wooten, J.B.; Lewis, N.G. Lignification in Cell Suspension Cultures of Pinus Taeda. In situ Characterization of a Gymnosperm Lignin. J. Biol. Chem. 1993, 268, 21088–21096. [Google Scholar] [PubMed]
- Stark, R.E.; Sohn, W.; Pacchiano, R.A.; Al-Bashir, M.; Garbow, J.R. Following Suberization in Potato Wound Periderm By Histochemical and Solid-State 13C Nuclear Magnetic Resonance Methods. Plant Physiol. 1994, 104, 527–533. [Google Scholar] [PubMed]
- Kelley, C.J.; Harruff, R.C.; Carmack, M. The Polyphenolic Acids of Lithospermum Ruderale. II. Carbon-13 Nuclear Magnetic Resonance of Lithospermic and Rosmarinic Acids. J. Org. Chem. 1976, 41, 449–455. [Google Scholar] [CrossRef]
- Tezuka, Y.; Kasimu, R.; Li, J.X.; Basnet, P.; Tanaka, K.; Namba, T.; Kadota, S. Constituent of Roots of Salvia deserta Schang. (Xinjiang-Danshen). Chem. Pharm. Bull. 1998, 46, 107–112. [Google Scholar] [CrossRef]
- Ralf, J.; Helm, R.F.; Quideau, S.J. Lignin-Feruloyl Ester Cross-Links in Grasses. Part 2. Model Compound Syntheses. J. Chem. Soc. Perkin Trans.1 1992, 2971–2980. [Google Scholar]
- Barthomeuf, C.M.; Debiton, E.; Barbakadze, V.V.; Kemertelidze, E.P. Evaluation of the Dietetic andTherapeutic Potential of a High Molecular Weight Hydroxycinnamate-Derived Polymer from Symphytum asperum Lepech. Regarding its Antioxidant, Antilipoperoxidant, Antiinflammatory, and Cytotoxic Properties. J. Agric. Food Chem. 2001, 49, 3942–3946. [Google Scholar] [CrossRef] [PubMed]
- Halkes, S.B.A.; Van den Berg, A.J.J.; Hoekstra, M.J.; Du Point, J.S.; Kreis, R.W. The Use of Tannic Acid in the Local Treatment of Burn Wounds: intriguing Old and New Perspectives. Wounds 2001, 13, 144–158. [Google Scholar]
- Latha, B.; Babu, M. The involvement of Free Radicals in Burn injury: a review. Burns 2001, 27, 309–317. [Google Scholar] [CrossRef]
- Van den Worm, E.; Beukelman, C.J.; Van den Berg, A.J.J.; Kroes, B.H.; Labadie, R.P.; Van Dijk, H. Effects of Methoxylation of Apocynin and Analogs on the inhibition of Reactive Oxygen Species Production By Stimulated Human Neutrophils. Eur. J. Pharmacol. 2001, 433, 225–230. [Google Scholar] [CrossRef]
- Mattsson, E.; Van Dijk, H.; Van Kessel, K.; Verhoef, J.; Fleer, A.; Rollof, J. intracellular Pathways involved in Tumor Necrosis Factor-Alpha Release by Human Monocytes on Stimulation With Lipopolysaccharide Or Staphylococcal Peptidoglycan Are Partly Similar. J. Infect. Dis. 1996, 173, 212–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kardava, L.; Gabunia, Kh.; Tevzadze, M.; Barbakadze, V.; Ghirdaladze, D.; Iosava, G. Dihydroxycinnamate-Derived Polymer from Symphytum asperum increases Spontaneous in vitro Apoptosis of β-Chronic Lymphocytic Leukaemia Cells. Bull. Georg. Acad. Sci. 2000, 162, 47–50. [Google Scholar]
- Kardava, L.; Kulikova, N.; Tevzadze, M.; Gabunia, Kh.; Barbakadze, V.; Ghirdaladze, D.; Iosava, G.; Porakishvili, N. The Cell Cycle Progression of β-Chronic Lymphocytic Leukaemia Cells in vitro. Proc. Georg. Acad. Sci. Biol. Ser. 2001, 27, 465–470. [Google Scholar]
- Barbakadze, V.V.; Gakhokidze, R.A.; Shengelia, Z.S.; Usov, A. Preliminary investigation of Water- Soluble Polysaccharides from Georgian Plants. Khim. Prir. Soedin. 1989, 3, 330–335, [Chem. Nat. Compd. (Eng. Transl.). 1989, 25, 281-286]. [Google Scholar]
© 2005 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Barbakadze, V.; Kemertelidze, E.; Targamadze, I.; Mulkijanyan, K.; Shashkov, A.; Usov, A. Poly[3-(3,4-dihydroxyphenyl)glyceric Acid], A New Biologically Active Polymer from Symphytum Asperum Lepech. and S.Caucasicum Bieb. (Boraginaceae). Molecules 2005, 10, 1135-1144. https://doi.org/10.3390/10091135
Barbakadze V, Kemertelidze E, Targamadze I, Mulkijanyan K, Shashkov A, Usov A. Poly[3-(3,4-dihydroxyphenyl)glyceric Acid], A New Biologically Active Polymer from Symphytum Asperum Lepech. and S.Caucasicum Bieb. (Boraginaceae). Molecules. 2005; 10(9):1135-1144. https://doi.org/10.3390/10091135
Chicago/Turabian StyleBarbakadze, V., E. Kemertelidze, I. Targamadze, K. Mulkijanyan, A. Shashkov, and A. Usov. 2005. "Poly[3-(3,4-dihydroxyphenyl)glyceric Acid], A New Biologically Active Polymer from Symphytum Asperum Lepech. and S.Caucasicum Bieb. (Boraginaceae)" Molecules 10, no. 9: 1135-1144. https://doi.org/10.3390/10091135




