Pd-catalyzed Synthesis of β-Biarylacryl Ferrocenes via Suzuki Cross-coupling
Abstract
:Introduction
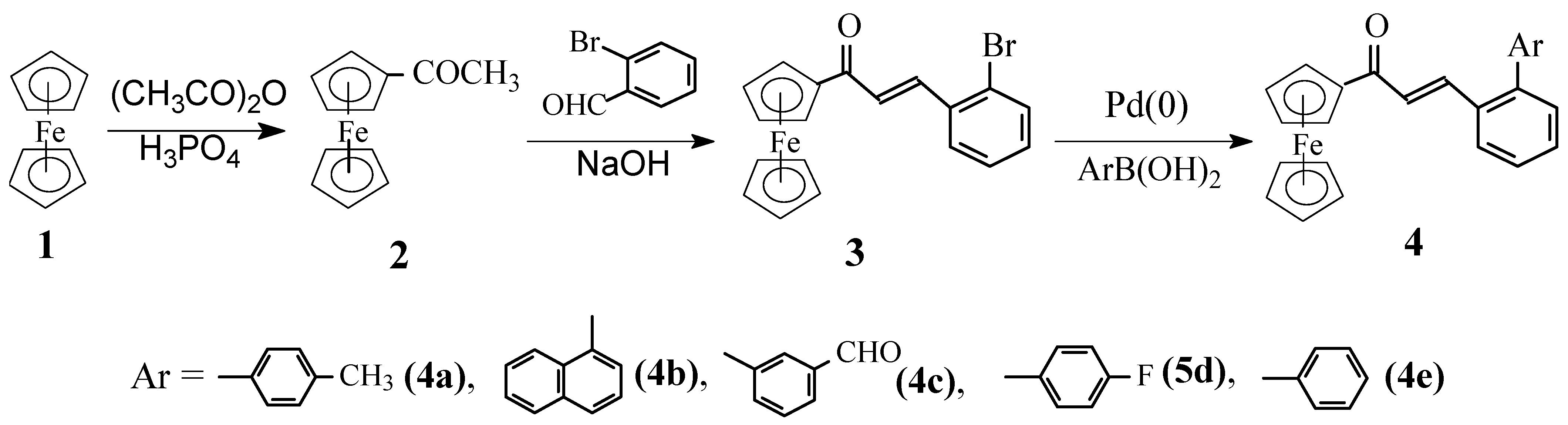
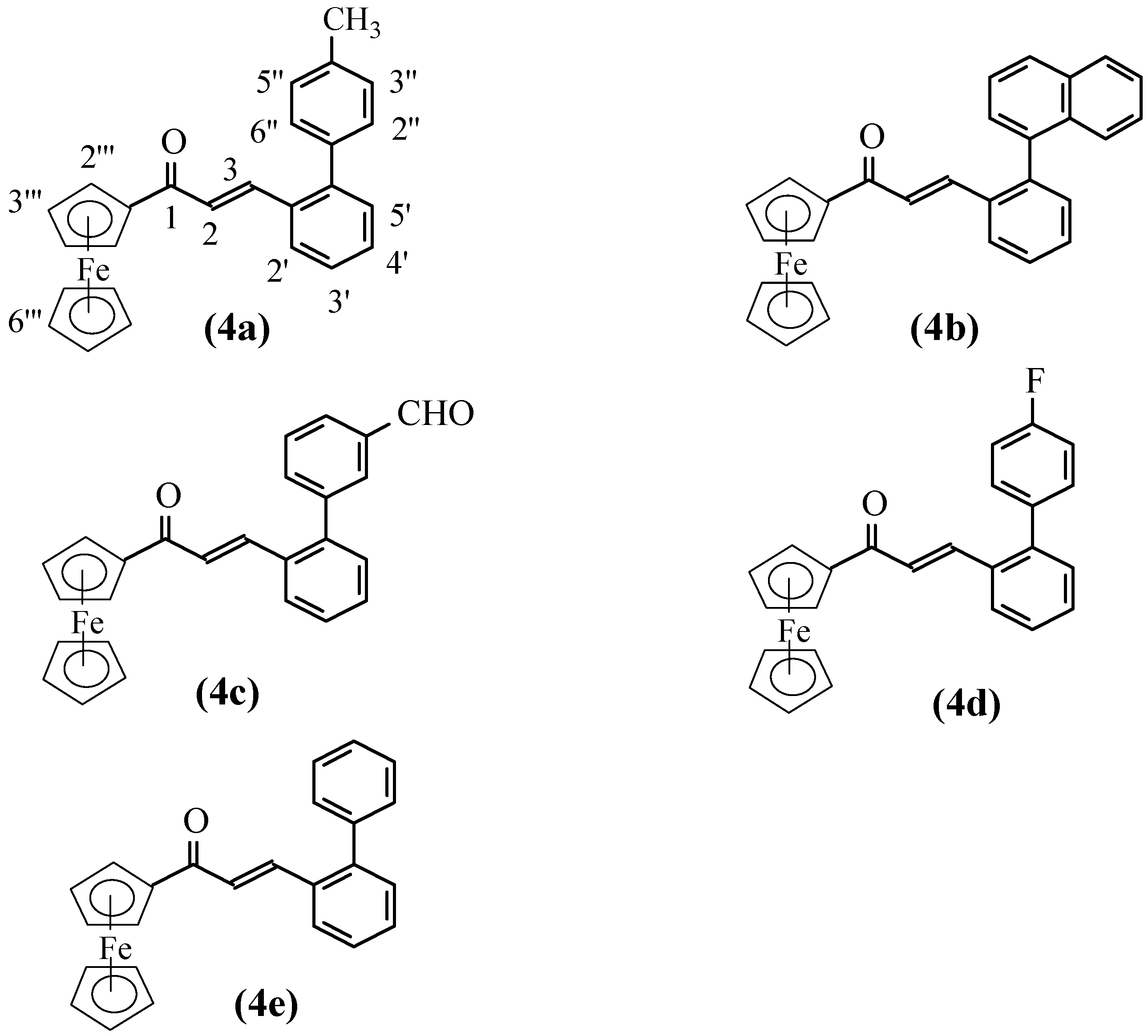
Results and Discussion
Conclusions
Experimental
General
β-(2-Bromophenyl)acrylferrocene (3).
General procedure for preparation of the title compounds 4a-e.
References
- Green, M. L. H.; Marder, S. R.; Thompson, M. E.; Bandy, J. A.; Bloor, D.; Kolinsky, P. V.; Jones, R. J. Synthesis and Structure of cis-1-Ferrocenyl- 2-(4-nitrophenyl) Ethylene, An Organo- transition Metal Compound with A Large Second-order Optical Non-linearity. Nature 1987, 330, 360. [Google Scholar] [CrossRef]
- Kott, K. L.; Higgins, D. A.; McMahon, R. J.; Corn, R. C. Observation of Photo-induced Electron Transfer at A Liquid-liquid Interface by Optical Second Harmonic Generation. J. Am. Chem. Soc. 1993, 115, 542. [Google Scholar]
- Calabrese, J. C.; Cheng, L.-T.; Green, J. C.; Marder, S. R.; Tam, W. Molecular Second-order Optical Nonlinearities of Metallocenes. J. Am. Chem. Soc. 1991, 13, 7227. [Google Scholar] [CrossRef]
- Yuan, Z.; Stringer, G.; Jobe, I. R.; Kreller, D.; Scot, K.; Koch, L.; Taylor, N. J.; Marder, T. B. Synthesis and Characterization of Ferrocenyl and Bis (ferrocenyl) Alkynes and Polynes: Crystal Structure of 1,4-Bis(ferrocenyl) Butadiyne and Third-order Nonlinear Optical Properties of 1,8-Bis(ferrocenyl) Octatetrayne. J. Organomet. Chem. 1993, 452, 115. [Google Scholar] [CrossRef]
- Long, N. J. Organometallic Compounds for Nonlinear Optics–The Search for En-light-enment. Angew. Chem. Int. Ed. Engl. 1995, 34, 21. [Google Scholar]
- Song, Q-B; Li, X-N; Shen, T-H; Yang, S-D; Qiang, G-R; Wu, X-L.; Ma, Y-X. Synthesis of Novel Chalcone Analogues of Ferrocene Biarenes. Synth. Commun. 2003, 33, 3935. [Google Scholar]
- Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457. [Google Scholar]
- Graham, P. J.; Lindsey, R. V.; Parshall, G.; Peterson, M. L.; Whitman, G. M. Some Acyl Ferrocenes and their Reactions. J. Am. Chem. Soc. 1957, 79, 3416. [Google Scholar] Rosenblum, M.; Woodward, R. B. The Structure of Chemistry of Ferrocene III. Evidence Pertaining to the Ring Rotational Barrier. J. Am. Chem. Soc. 1958, 80, 5443. [Google Scholar]
- Nagy, A. G.; Marton, J.; Sohar, P. Spectroscopic Investigation of Chalcone- analogous Ferrocenes Ortho-substituted in the Aromatic ring. II. Ferrocenyl- COCH=CH-aryl Type Compounds. Acta Chim. Hung. 1991, 128, 961. [Google Scholar]
- Hawkins, R. T.; Lennarr, W. T.; Snyder, H. R. Arylboronic Acids (V) Methyl-substituted Boronic Acids, and Aryl Borons. J. Am. Chem. Soc. 1960, 82, 3053. [Google Scholar]
- Feulner, H.; Linti, G.; Noth, H. Boron Chemistry 206. Preparation and Structural Charaterization of p-Formylphenylboronic Acid. Chem. Ber. 1990, 123, 1841. [Google Scholar]
- Rodriguez, J.G.; Pleite, S. Synthesis of Controlled π-Extended Conjugate Nanostructures of 1,1’-Ferrocene. J. Organomet. Chem. 2001, 637-639, 230. [Google Scholar]
- Coulson, D. R. Tetrakis(triphenylphosphine)palladlum. Inorg. Synth. 1972, 13, 121. [Google Scholar]
© 2005 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Song, Q.; Lin, R.; Yang, Z.; Qi, C. Pd-catalyzed Synthesis of β-Biarylacryl Ferrocenes via Suzuki Cross-coupling. Molecules 2005, 10, 634-639. https://doi.org/10.3390/10060634
Song Q, Lin R, Yang Z, Qi C. Pd-catalyzed Synthesis of β-Biarylacryl Ferrocenes via Suzuki Cross-coupling. Molecules. 2005; 10(6):634-639. https://doi.org/10.3390/10060634
Chicago/Turabian StyleSong, Q., R. Lin, Z. Yang, and C. Qi. 2005. "Pd-catalyzed Synthesis of β-Biarylacryl Ferrocenes via Suzuki Cross-coupling" Molecules 10, no. 6: 634-639. https://doi.org/10.3390/10060634



