The Case for Tetrahedral Oxy-subhydride (TOSH) Structures in the Exclusion Zones of Anchored Polar Solvents Including Water
Abstract
: We hypothesize a mechanistic model of how negatively-charged exclusion zones (EZs) are created. While the growth of EZs is known to be associated with the absorption of ambient photonic energy, the molecular dynamics giving rise to this process need greater elucidation. We believe they arise due to the formation of oxy-subhydride structures (OH−)(H2O)4 with a tetrahedral (sp3) (OH−)(H2O)3 core. Five experimental data sets derived by previous researchers were assessed in this regard: (1) water-derived EZ light absorbance at specific infrared wavelengths, (2) EZ negative potential in water and ethanol, (3) maximum EZ light absorbance at 270 nm ultraviolet wavelength, (4) ability of dimethyl sulphoxide but not ether to form an EZ, and (5) transitory nature of melting ice derived EZs. The proposed tetrahedral oxy-subhydride structures (TOSH) appear to adequately account for all of the experimental evidence derived from water or other polar solvents.1. Introduction
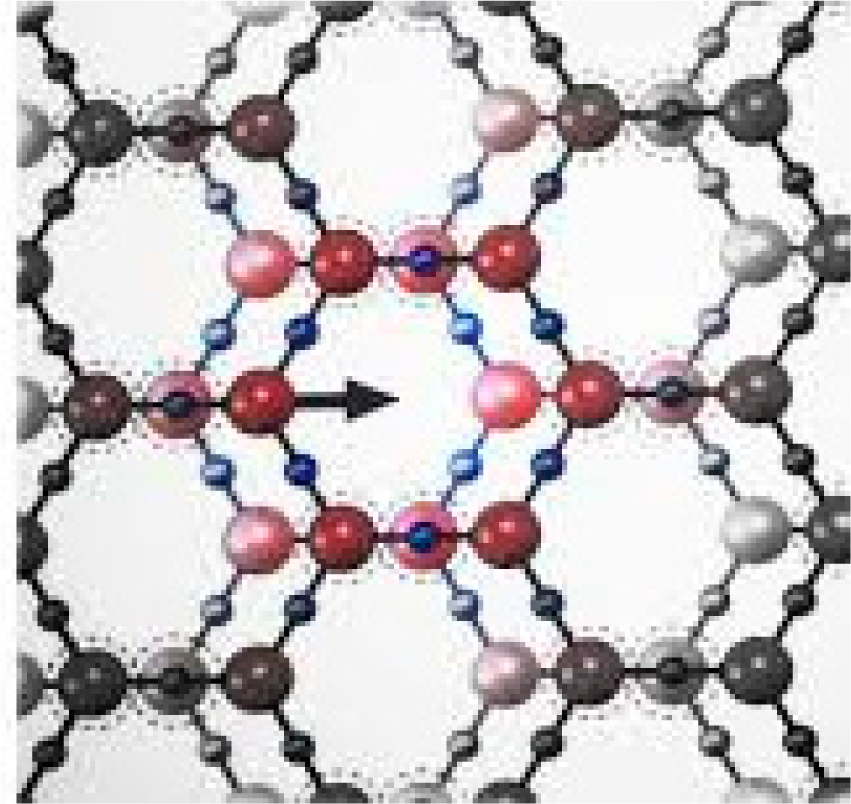
In 2003, Zheng and Pollack stated that “solute-solute interactions in aqueous solutions are generally thought to occur on a scale in nanometers” [1]. They further presented evidence that attractive and repulsive forces exhibited at this scale could extend to distances on the order of 100 μm. Subsequent research by Zheng et al. demonstrated the exclusion zones (EZs) these forces generated could extend to 360 ± 50 μm [2]. Existing literature does not adequately explain how or why negatively-charged EZs form at all [2–8]. Although Pollack has offered what might be termed a trigonal “sp2 bond-type offset hexagonal sheet model” as a possible explanation for EZ structures, as illustrated in Figure 1, this model is problematic in several regards.
It does not offer an irrefutable account for: (1) enhanced absorbance of infrared light at ~3436 nm and 2907 nm (equivalent to wavenumbers ~2919 and ~3430 cm−1), (2) EZ negative potential of ~ −160 to −200 mV in Nafion®-water or Nafion®-ethanol, (3) maximum light absorbance at 270 nm ultraviolet wavelength in water, (4) presence of EZs in dimethyl sulphoxide but not ether, and (5) the transitory occurrence of EZs in melting ice. We are unaware of any other researchers who have proposed models that fully address these issues. Thus arose a need for an explanatory model that could attempt to account for all historical experimental data related to EZs.
2. EZ Growth Model
We propose an EZ growth model for water, partially melted ice, or polar solvents adjacent to solid or gel anchors containing O− or OH functional groups able to hydrogen bond to water or polar solvents. Molecules containing OH functional groups include the amino acids serine, threonine and tyrosine, components of rabbit muscle protein, plus polyvinyl alcohol shown to form EZs [1,2]. Molecules containing O− functional groups include sulphonate in Nafion® [3]. Each of these three proposed EZ systems will now be discussed separately in detail.
2.1 Water EZs
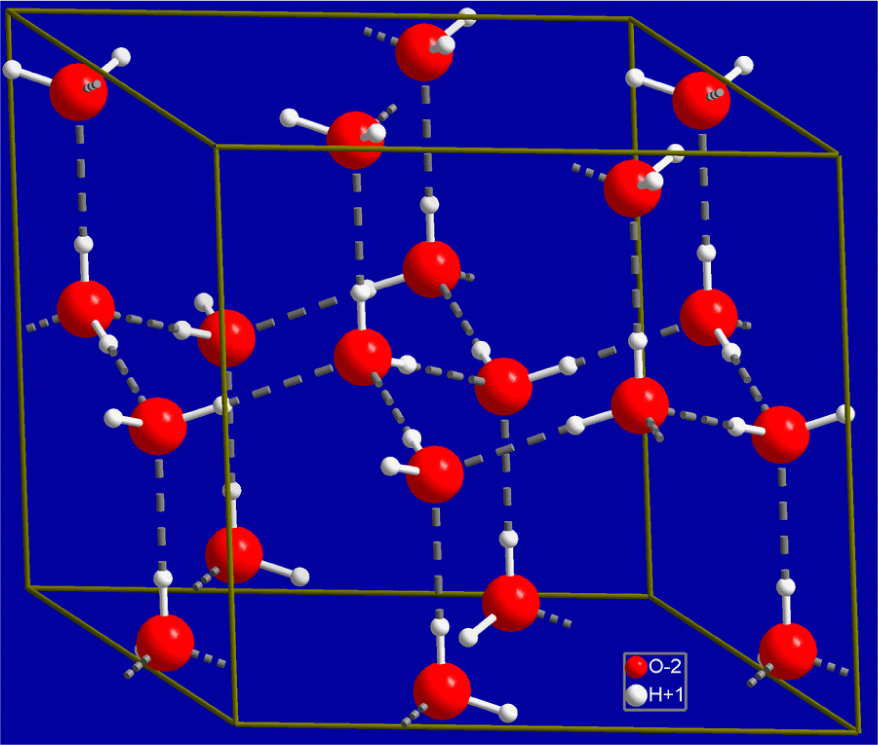
Figure 2 illustrates a proposed structure for ice [9]. Exclusion zones derived from water have been shown to have negative potentials (charge). Thus it follows that melting ice would also likely exhibit transitory exclusion zones with a net negative charge and would also expel protons (H+) to create such a negative charge.
To illustrate this process, we refer again to Figure 2. The following may occur:
An ambient, incident photon (e.g., in the ultraviolet wavelength region), strikes and ruptures a non-hydrogen bonded hydrogen atom in a water molecule on the top surface of a block of ice. Note that H+ and OH- exist in water free of incident radiation. The addition of specific wavelengths of infrared, visible light or ultraviolet light at varying amplitudes to water is expected to shift the HOH auto-dissociation in favour of additional proton formation [10,11].
In our example, we use the topmost central water molecule in Figure 2 as it has 2 hydrogen bonds plus 2 hydrogen atoms which are non-hydrogen bonded to other water molecules. We believe this configuration is the most susceptible to rupture and formation of H+. Rupture at this site should occur prior to the water molecule directly below it, to which it is hydrogen bonded and which has 3 hydrogen bonds, since it should take more energy to rupture a combination O-H-HOH bond than a single O-H bond.
The OH− functional group remaining after H+ ejection then forms a hydrogen bond with a new water molecule to make it more stable via a fourth tetrahedral bond.
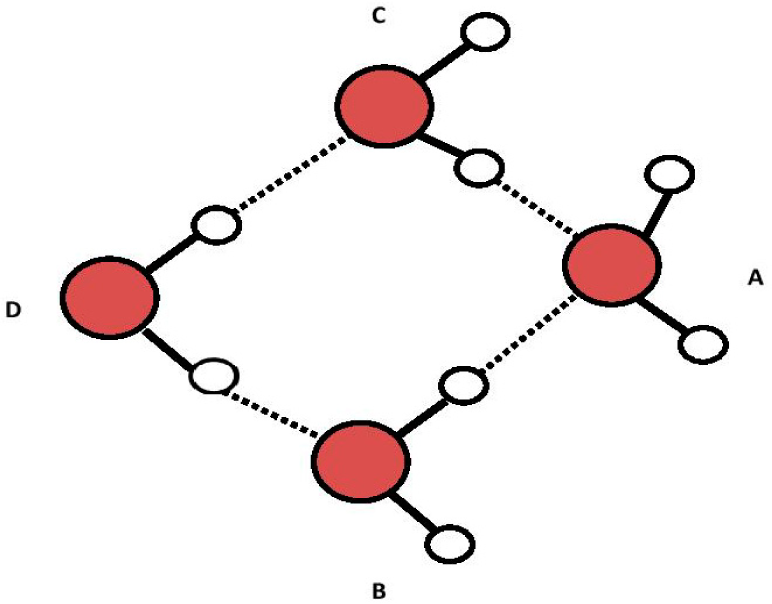
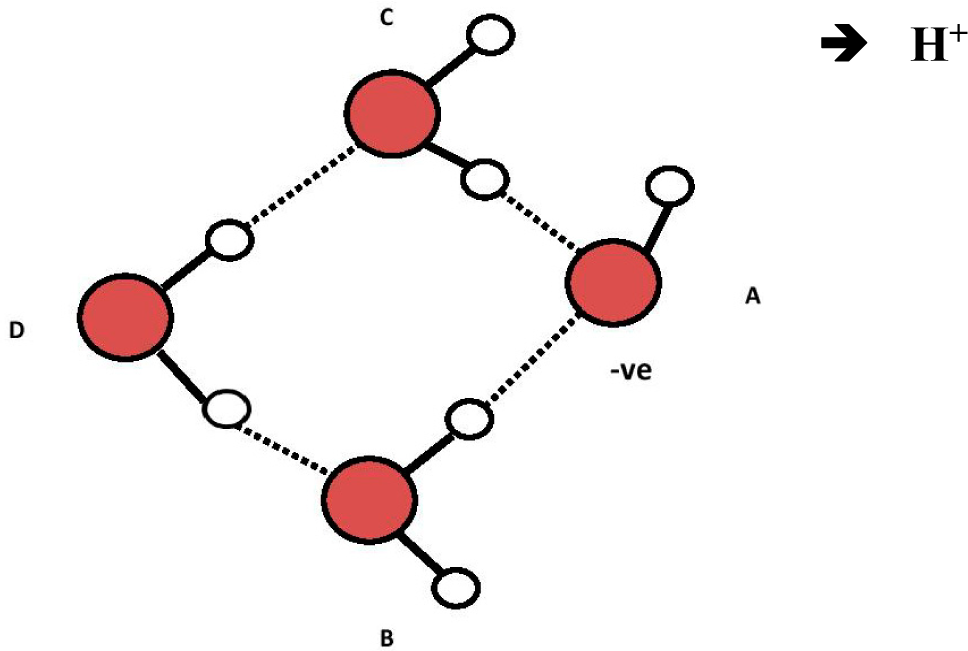
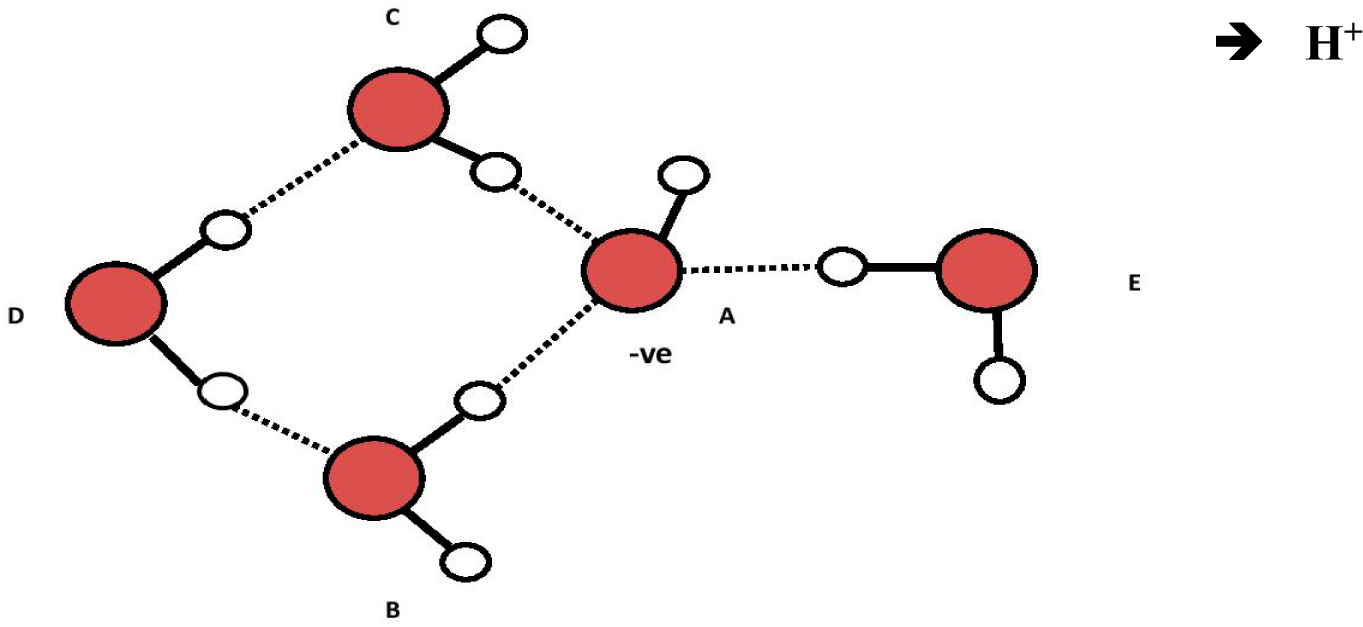
This hypothesized two-step process is illustrated in Figures 3 to 5 below:
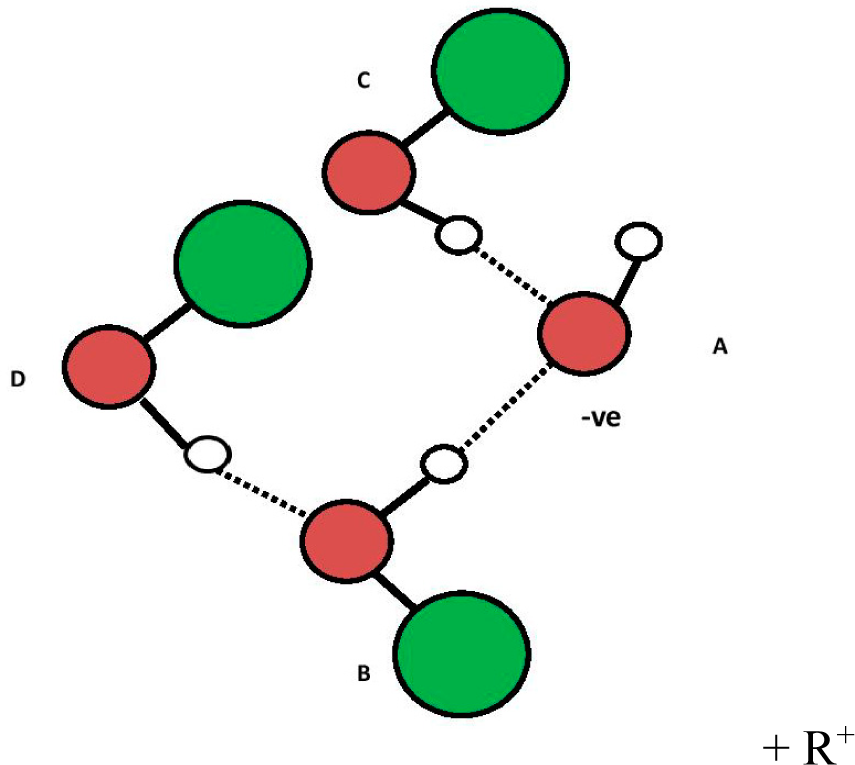
The proposed ABCE structure of Figure 5 precisely matches the hydroxyl-tetrawater cluster designated “OHW4I” (a), containing a tetrahedral (sp3) (OH−) (H2O)3 core, shown by Chaudhuri et al. [12] (p. 1164). This particular structure highly absorbs infrared light at both ~3436 nm and 2907 nm (i.e., 2910 and 3440 cm−1), which closely matches the ~3426 nm and 2914 nm (i.e., 2919 and 3432 cm−1) for water-derived EZs [3]. Chaudhuri shows that different isomers of (OH−)(H2O)4 have different infrared spectra. What is of additional significance is that Novoa et al. describe and show under “2” that the infrared absorbance peaks in the structures Chaudhuri identified as OHW4I happen to be the most stable of all the possible variants for (OH−)(H2O)4 [13] (p. 7845). It is therefore likely that water-derived EZs contain a negatively-charged specific hydroxy-tetrawater isomer containing a tetrahedral (sp3) (OH−)(H2O)3 core with an H4O− sub-core.
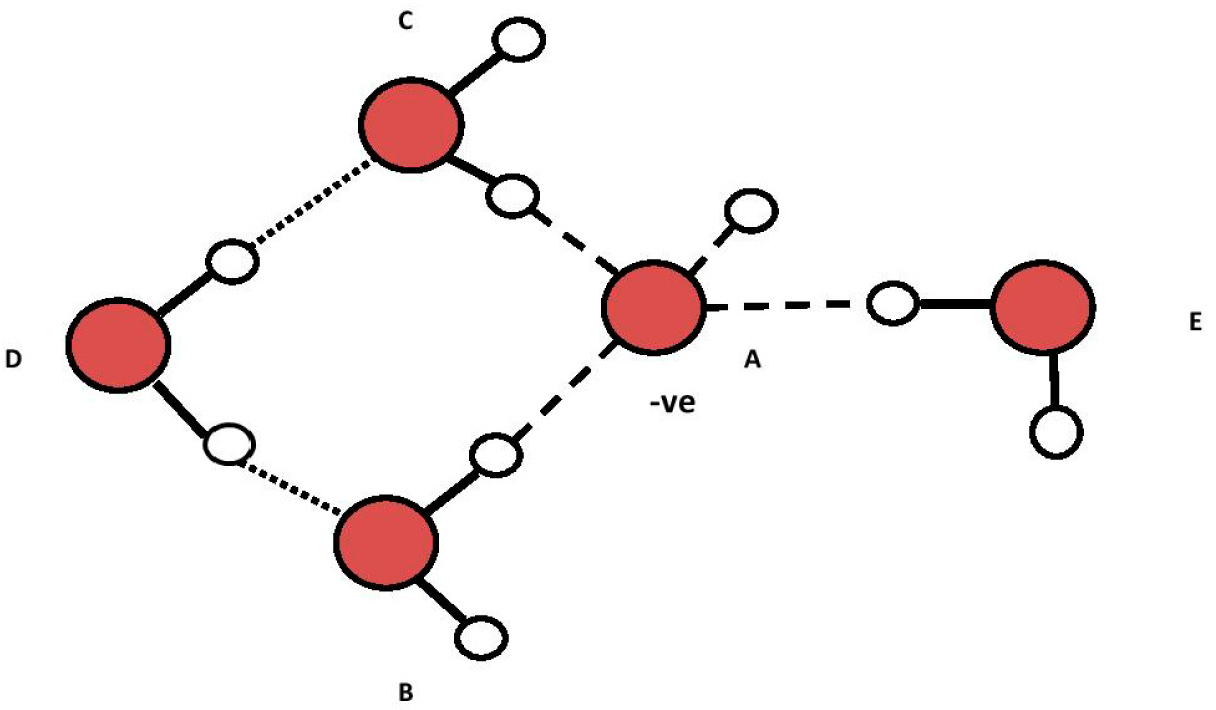
Another possible structure for Figure 5 is shown in Figure 6 in which the three hydrogen bonds from water molecules “B”, “C” and “E” to the oxygen in “A” and the hydrogen to oxygen bond in “A” undergo resonance to make them equivalent (dashed lines from “A” oxygen). Such resonance, if it occurs, could establish TOSH structures as EZ anchors inside the EZ field due to enhanced bond strength of three of the four TOSH O to H bonds (see Figure 5 vs. Figure 6).
The electrical potential for a single hydride ion (1s2)/single hydrogen atom (1s1) couple is estimated at −0.83 ± 0.11 V (i.e., 0.83 ± 0.11 V for the H-/H one electron reaction couple) [14]. If the four hydrogen atoms attached to oxygen “A” as H4O− (oxytetrasubhydride) equally share the one electron of OH− in a pseudo 1s2 orbital (i.e., less swollen than a normal 1s2 orbital), they should have a potential of −0.208 ± 0.028 V, ¼ of 0.83 ± 0.11 V, which overlaps the potentials ~ −0.16 to ~ −0.20 V measured in EZs in water next to Nafion® by Zheng et al. [2,8]. The redox couple for this potential could be given by the following reaction:
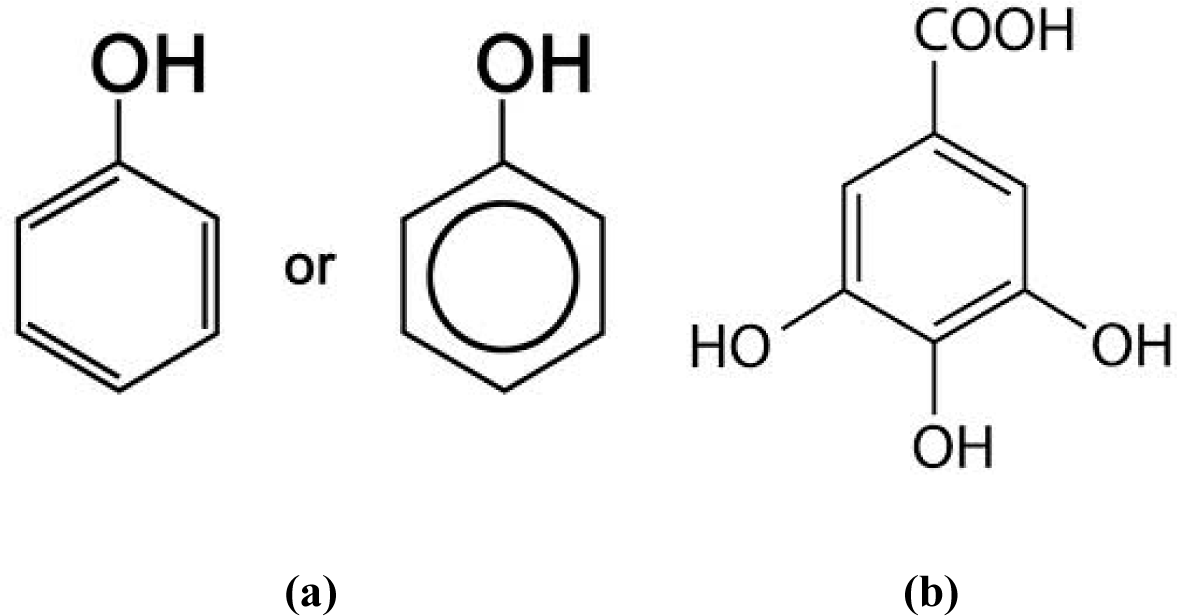
Figure 7 illustrates phenol and gallic acid, respectively. Both of these molecules and water-derived EZs show maximum UV absorbance at 270 nm [15,16].
The resonant frequency of un-hybridized and un-bonded electrons (designated “n”→π*) has been estimated as 270 nm [17]. There is one un-hybridized and un-bonded “n” type 2pz electron in each carbon of a C-O phenol and gallic acid sub-cluster. There is also one un-hybridized and un-bonded “n” type 1s2 electron in the four hydrogen atoms of an HO4− oxytetrasubhydride structure (TOSH). Identical numbers of additional “n” electrons are contained in the oxygen atoms of C-O and H4O−. The resonant ultraviolet frequencies of C-O in phenol and gallic acid and TOSH structures are therefore expected to match.
2.2. Non-Water EZs
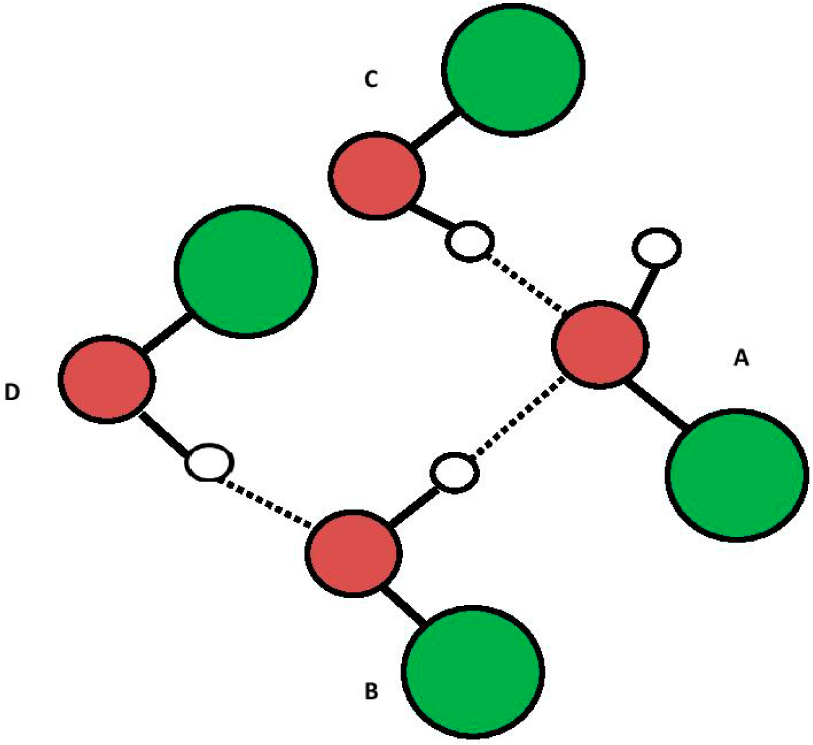
Experimental data on heavy water and non-aqueous polar solvent (e.g., ethanol) EZs suggests oxytetrasubhydride structures are analogous to those in EZs suggested for water. Figures 8 to 10 are the ROH polar solvent analogues of Figures 3–5 (e.g., R shown in green is C2H5 and, R+ is C2H5+ for ethanol). Further credence to the oxytetrasubhydride structure hypothesis is suggested by their super-imposable electrode potentials of ~ −160 to ~ −200 mV.
Figure 11 shows that dimethyl sulphoxide, unlike diethyl ether, can form a ROH isomer and a subsequent EZ via:
2.2. EZ Anchors
It is believed that EZ water containing tetrahedral oxy-subhydride sub-clusters is stabilized by the presence of solid or gel-like non-billowable anchors containing OH (e.g., polyvinyl alcohol, polyacrylic acid, protein, amino acids, O− [Nafion®] functional groups). Once the solid ice OH functional group anchors melt with or without additional photonic energy inputs, they cease to be anchors. We believe this is responsible for the subsequent destruction of the EZ fields as evidenced by their lack of UV absorbance at 270 nm. This would suggest that intermediate TOSH anchors are of insufficient strength to sustain an EZ field in the absence of a stronger anchor such as Nafion®, polyacrylic acid, polyvinyl alcohol or amino acids in protein (e.g., rabbit muscle).
4. Conclusions
The following experimentally-derived empirical data sets from previous investigators have been assessed to validate the proposition that tetrahedral H4O− oxy-subhydride (TOSH) structures account for the properties observed in water, heavy water or polar solvent derived EZs:
Water-derived EZ light absorbance at specific infrared wavelengths.
EZ negative potential in water and ethanol.
Maximum EZ light absorbance at 270 nm ultraviolet wavelength.
Ability of dimethyl sulphoxide but not ether to form an EZ.
Transitory nature of melting ice derived EZs.
The mechanism of formation of the suggested TOSH structures has been described in detail. The likelihood of the proposition being valid is high since correlations with a wide variety of experimental data are good.
Acknowledgments
The authors wish to thank Carol Oehr for her careful edits and preparation of certain figures, Gerald Pollack for his encouragement in submitting this paper, and the unknown referees of this paper.
PACS Codes: 32,33,36,61,63,68,73,79,81,82
Author Contributions
Paul H. LeMay introduced Klaus Oehr to Gerald Pollack’s concepts of EZs, helped formulate the concepts of this paper as well as edit its contents. Klaus Oehr carried out all of the calculations and supporting literature searches necessary to prepare the paper. Both authors have read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zheng, J.; Pollack, G.H. Long-range forces extending from polymer-gel surfaces. Phys. Rev. E 2003, 68, 031408. [Google Scholar]
- Zheng, J.; Chin, W.C.; Khijniak, E.; Khijniak, E., Jr.; Pollack, G.H. Surfaces and interfacial water: Evidence that hydrophilic surfaces have long-range impact. Adv. Colloid Interface Sci. 2006, 127, 19–27. [Google Scholar]
- Elia, V.; Ausanio, G.; De Ninno, A.; Gentile, F.; Germano, R.; Napoli, E.; Niccoli, M. Experimental evidence of stable aggregates of water at room temperature and normal pressure after iterative contact with a Nafion® polymer membrane. Water 2013, 5, 16–26. [Google Scholar]
- Chai, B.; Yoo, H.; Pollack, G.H. Effect of radiant energy on near surface water. J. Phys. Chem. B 2009, 113, 12953–13958. [Google Scholar]
- Chai, B.; Pollack, G.H. Solute free interfacial zones in polar liquids. J. Phys. Chem. B 2010, 114, 5371–5375. [Google Scholar]
- So, E.; Stahlberg, R.; Pollack, G.H. Exclusion zone as intermediate between ice and water. In Water and Society; Pepper, D.W., Brebbia, C.A., Eds.; WIT Press: Southampton, UK, 2012; pp. 3–11. [Google Scholar]
- Das, R.; Pollack, G.H. Charge based forces at the Nafion-water interface. Langmuir 2013, 29, 2651–2658. [Google Scholar]
- Zheng, J.; Pollack, G.H. Solute exclusion and potential distribution near hydrophilic surfaces. In Water and the Cell; Pollack, G.H., Cameron, I.L., Wheatley, D.N., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 165–174. [Google Scholar]
- Ice, Available online: http://en.wikipedia.org/wiki/Ice accessed on 1 August 2014.
- Bakker, H.J.; Nienhuys, H.-K. Delocalization of protons in liquid water. Science 2002, 297, 587–590. [Google Scholar]
- Photon Energies and the Electromagnetic Spectrum, Available online: http://cnx.org/content/m42563/latest/?collection=col11406/latest accessed on 1 August 2014.
- Chaudhuri, C.; Wang, Y.-S.; Jiang, J.C.; Lee, Y.T.; Chang, H.-C.; Niedner-Schatteburg, G. Infrared spectra and isomeric structures of hydroxide ion water clusters OH−(H2O)1–5: A comparison with H3O(H2O)1–5. Mol. Phys. 2001, 99, 1161–1173. [Google Scholar]
- Novoa, J.J.; Mota, F.; del Valle, C.P.; Planas, M. Structure of the first solvation shell of the hydroxide anion. A model study using OH−(H2O)n (n = 4,5,6,7,11,17) clusters. J. Phys. Chem. A 1997, 101, 7842–7853. [Google Scholar]
- Kelly, C.A.; Rosseinsky, D.R. Estimates of hydride ion stability in condensed systems: Energy of formation and solvation in aqueous and polar organic solvents. Phys. Chem. Chem. Phys. 2001, 3, 2086–2090. [Google Scholar]
- Phenols, Available online: http://en.wikipedia.org/wiki/Phenols accessed on 19 July 2014.
- Nucleic acid quantitation, Available online: http://en.wikipedia.org/wiki/Nucleic_acid_quantitation accessed on 19 July 2014.
- Ultraviolet Spectroscopy. KFUPM Open Courseware, Lecture Notes of CHEM 303, Available online: http://opencourseware.kfupm.edu.sa/colleges/cs/chem/chem303/files%5C3-Lecture_Notes_CHEM-303_UV_Spectroscopy.pdf accessed on 3 November 2014.

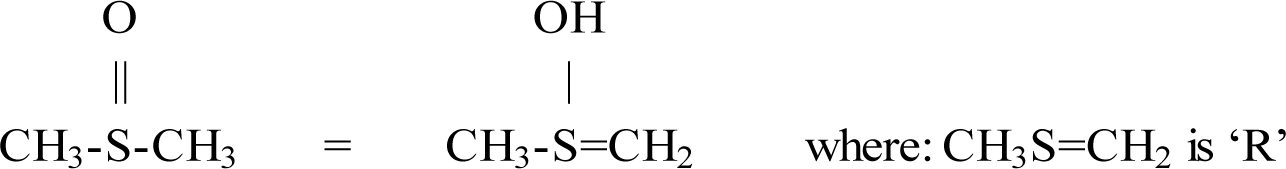
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oehr, K.; LeMay, P.H. The Case for Tetrahedral Oxy-subhydride (TOSH) Structures in the Exclusion Zones of Anchored Polar Solvents Including Water. Entropy 2014, 16, 5712-5720. https://doi.org/10.3390/e16115712
Oehr K, LeMay PH. The Case for Tetrahedral Oxy-subhydride (TOSH) Structures in the Exclusion Zones of Anchored Polar Solvents Including Water. Entropy. 2014; 16(11):5712-5720. https://doi.org/10.3390/e16115712
Chicago/Turabian StyleOehr, Klaus, and Paul H. LeMay. 2014. "The Case for Tetrahedral Oxy-subhydride (TOSH) Structures in the Exclusion Zones of Anchored Polar Solvents Including Water" Entropy 16, no. 11: 5712-5720. https://doi.org/10.3390/e16115712
APA StyleOehr, K., & LeMay, P. H. (2014). The Case for Tetrahedral Oxy-subhydride (TOSH) Structures in the Exclusion Zones of Anchored Polar Solvents Including Water. Entropy, 16(11), 5712-5720. https://doi.org/10.3390/e16115712



