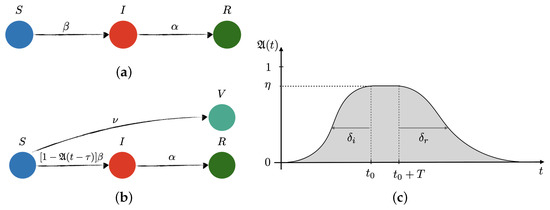
Vaccines 2021, 9(7), 744; https://doi.org/10.3390/vaccines9070744 - 5 Jul 2021
Cited by 20 | Viewed by 5555
Abstract
Due to the rapid transmission of the coronavirus disease 2019 (COVID-19) causing serious public health problems and economic burden, the development of effective vaccines is a high priority for controlling the virus spread. Our group has previously demonstrated that the plant-produced receptor-binding domain
[...] Read more.
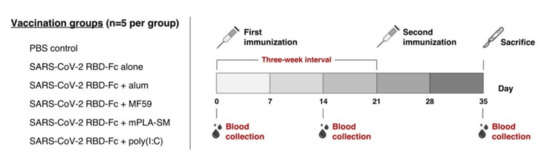
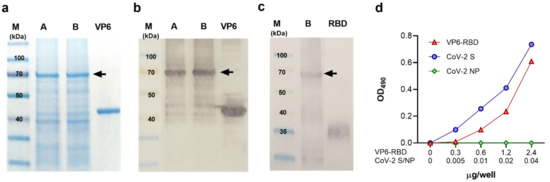
Due to the rapid transmission of the coronavirus disease 2019 (COVID-19) causing serious public health problems and economic burden, the development of effective vaccines is a high priority for controlling the virus spread. Our group has previously demonstrated that the plant-produced receptor-binding domain (RBD) of SARS-CoV-2 fused with Fc of human IgG was capable of eliciting potent neutralizing antibody and cellular immune responses in animal studies, and the immunogenicity could be improved by the addition of an alum adjuvant. Here, we performed a head-to-head comparison of different commercially available adjuvants, including aluminum hydroxide gel (alum), AddaVax (MF59), monophosphoryl lipid A from Salmonella minnesota R595 (mPLA-SM), and polyinosinic-polycytidylic acid (poly(I:C)), in mice by combining them with plant-produced RBD-Fc, and the differences in the immunogenicity of RBD-Fc with different adjuvants were evaluated. The specific antibody responses in terms of total IgG, IgG1, and IgG2a subtypes and neutralizing antibodies, as well as vaccine-specific T-lymphocyte responses, induced by the different tested adjuvants were compared. We observed that all adjuvants tested here induced a high level of total IgG and neutralizing antibodies, but mPLA-SM and poly (I:C) showed the induction of a balanced IgG1 and IgG2a (Th2/Th1) immune response. Further, poly (I:C) significantly increased the frequency of IFN-γ-expressing cells compared with control, whereas no significant difference was observed between the adjuvanted groups. This data revealed the adjuvants’ role in enhancing the immune response of RBD-Fc vaccination and the immune profiles elicited by different adjuvants, which could prove helpful for the rational development of next-generation SARS-CoV-2 RBD-Fc subunit vaccines. However, additional research is essential to further investigate the efficacy and safety of this vaccine formulation before clinical trials.
Full article
(This article belongs to the Special Issue Recombinant Vaccines Produced in Emerging Expression Systems for Human and Animal Health)
►
Show Figures