Urinary Metabolomics in Pediatric Obesity and NAFLD Identifies Metabolic Pathways/Metabolites Related to Dietary Habits and Gut-Liver Axis Perturbations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.1.1. Anthropometric Measurements and Lifestyle Evaluation
2.1.2. Imaging Investigations and Biochemical Parameters
2.2. Untargeted Metabolomics Analysis
2.2.1. Metabolite Extraction and Derivatization
2.2.2. GC-MS Analysis
2.3. Statistical Analysis
2.3.1. Monovariate Analysis
2.3.2. Multivariate Data Analysis
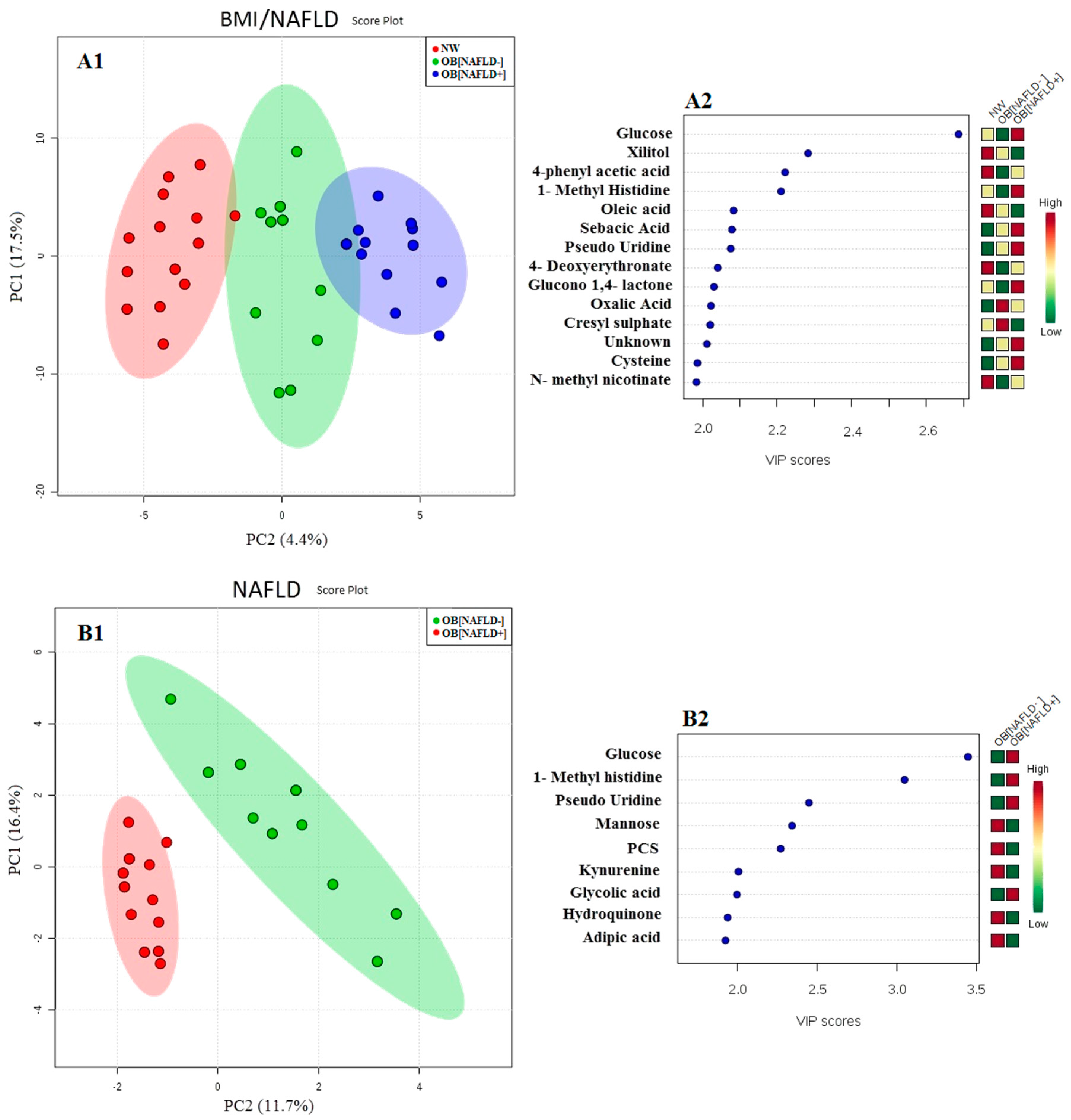
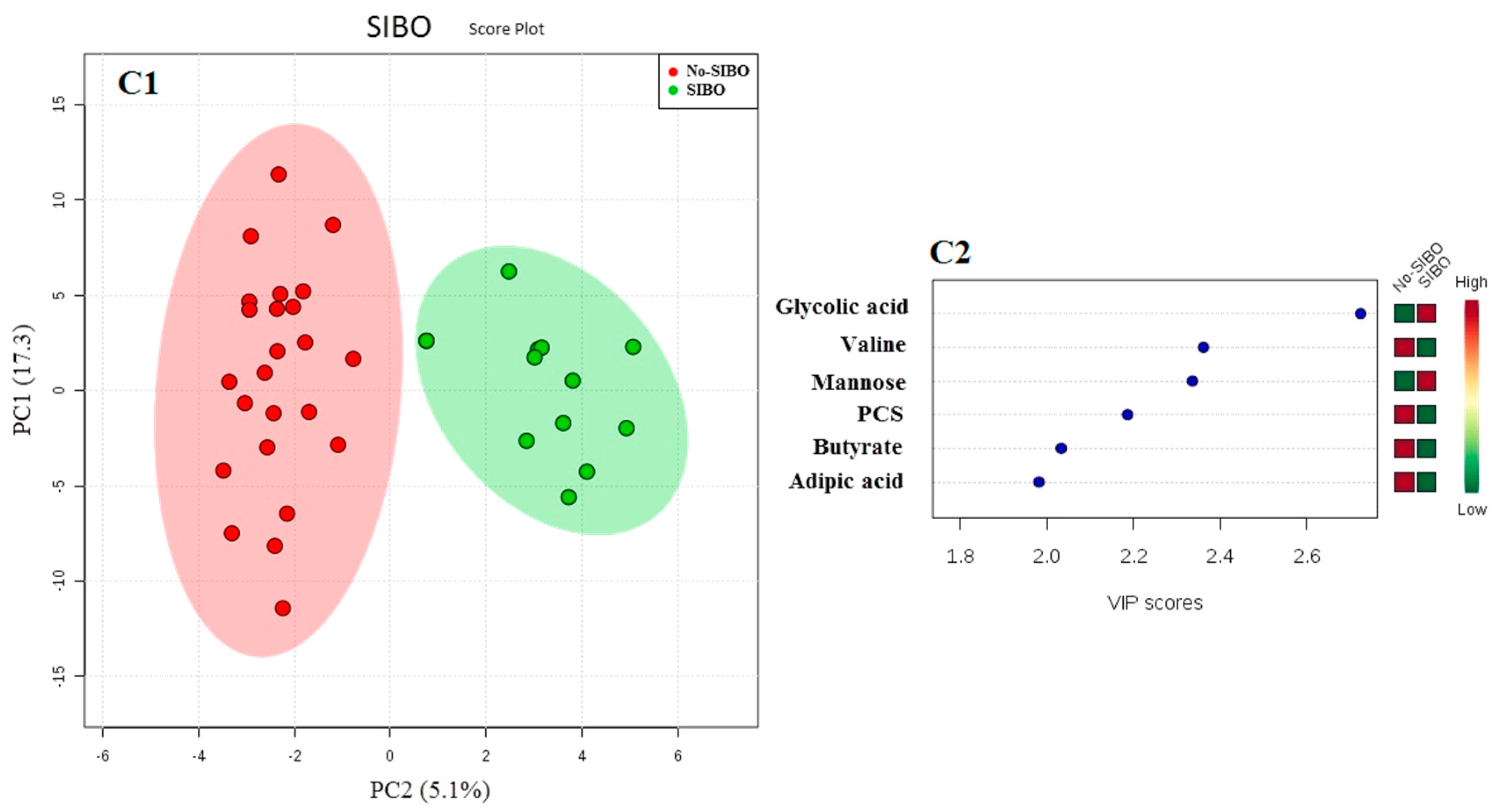
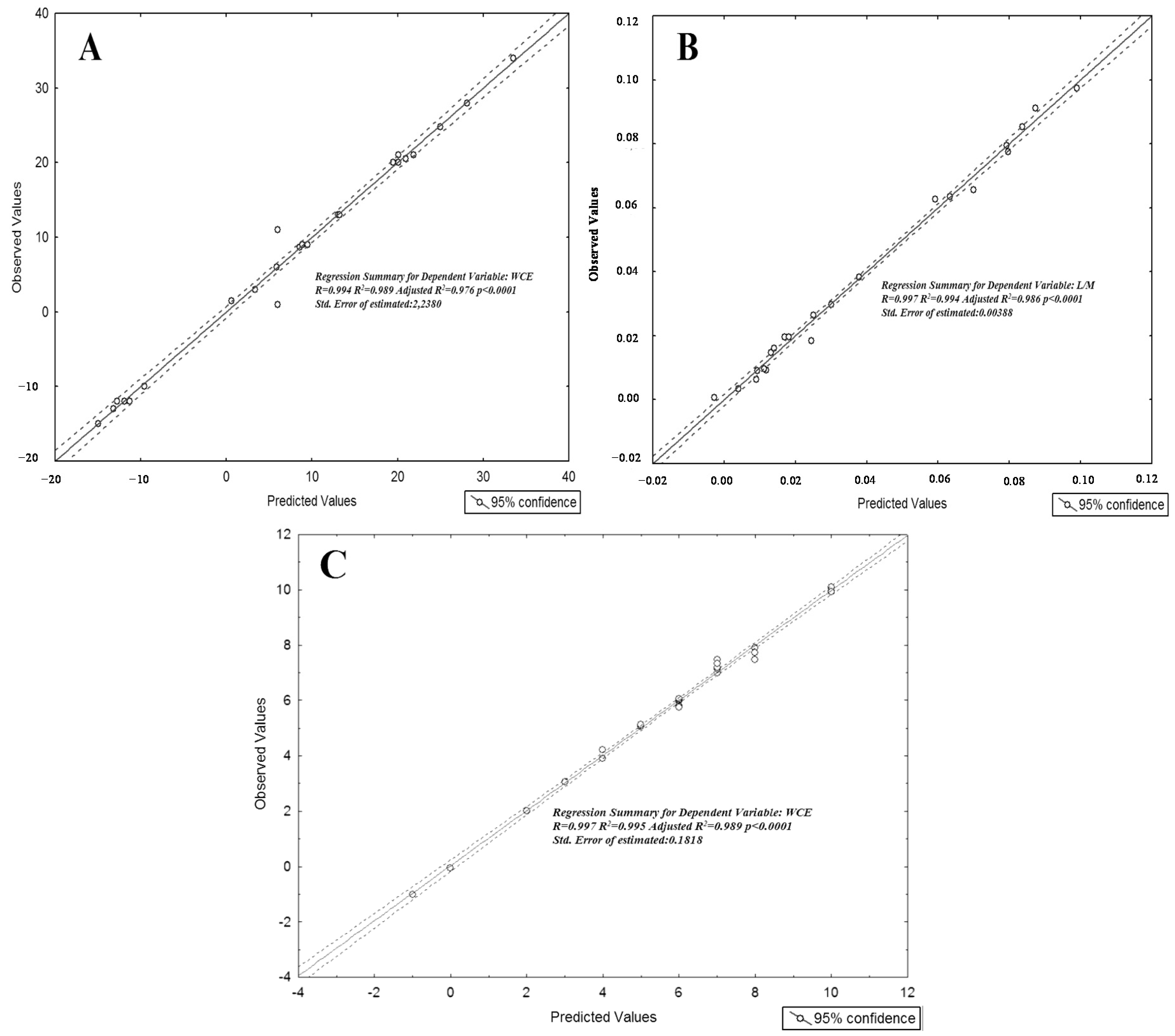
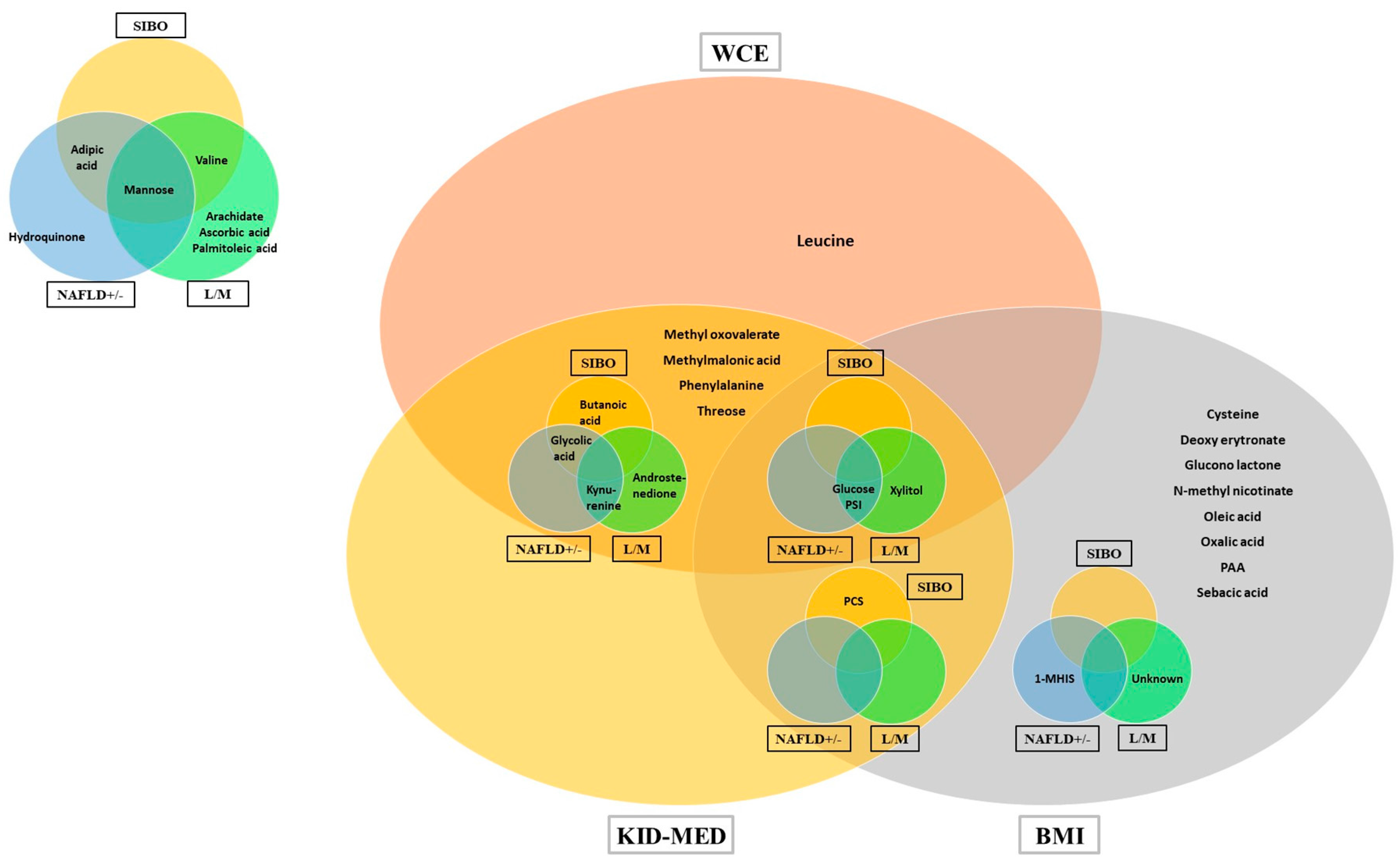
3. Results
4. Discussion
4.1. Obesity
4.2. NAFLD and Gut-Liver Axis Disturbances
4.3. Study Limitations
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Clemente, M.G.; Mandato, C.; Poeta, M.; Vajro, P. Pediatric non-alcoholic fatty liver disease: Recent solutions, unresolved issues, and future research directions. World J. Gastroenterol. 2016, 22, 8078–8093. [Google Scholar] [CrossRef] [PubMed]
- Koot, B.G.; van der Baan-Slootweg, O.H.; Vinke, S.; Bohte, A.E.; Tamminga-Smeulders, C.L.; Jansen, P.L.; Stoker, J.; Benninga, M.A. Intensive lifestyle treatment for non-alcoholic fatty liver disease in children with severe obesity: Inpatient versus ambulatory treatment. Int. J. Obes. 2016, 40, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Guercio Nuzio, S.; Di Stasi, M.; Pierri, L.; Troisi, J.; Poeta, M.; Bisogno, A.; Belmonte, F.; Tripodi, M.; Di Salvio, D.; Massa, G.; et al. Multiple gut-liver axis abnormalities in children with obesity with and without hepatic involvement. Pediatr. Obes. 2016. [Google Scholar] [CrossRef] [PubMed]
- Paolella, G.; Vajro, P. Childhood obesity, breastfeeding, intestinal microbiota, and early exposure to antibiotics: What is the link? JAMA Pediatr. 2016, 170, 735–737. [Google Scholar] [CrossRef] [PubMed]
- Vajro, P.; Paolella, G.; Fasano, A. Microbiota and gut-liver axis: Their influences on obesity and obesity-related liver disease. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Paolella, G.; Mandato, C.; Pierri, L.; Poeta, M.; Di Stasi, M.; Vajro, P. Gut-liver axis and probiotics: Their role in non-alcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 15518–15531. [Google Scholar] [CrossRef] [PubMed]
- Miccheli, A.; Capuani, G.; Marini, F.; Tomassini, A.; Pratico, G.; Ceccarelli, S.; Gnani, D.; Baviera, G.; Alisi, A.; Putignani, L.; et al. Urinary (1)h-nmr-based metabolic profiling of children with NAFLD undergoing VSL#3 treatment. Int. J. Obes. 2015, 39, 1118–1125. [Google Scholar]
- Wiklund, P.K.; Pekkala, S.; Autio, R.; Munukka, E.; Xu, L.; Saltevo, J.; Cheng, S.; Kujala, U.M.; Alen, M.; Cheng, S. Serum metabolic profiles in overweight and obese women with and without metabolic syndrome. Diabetol. Metab. Syndr. 2014, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wurtz, P.; Makinen, V.P.; Soininen, P.; Kangas, A.J.; Tukiainen, T.; Kettunen, J.; Savolainen, M.J.; Tammelin, T.; Viikari, J.S.; Ronnemaa, T.; et al. Metabolic signatures of insulin resistance in 7098 young adults. Diabetes 2012, 61, 1372–1380. [Google Scholar] [CrossRef] [PubMed]
- Um, S.H.; D’Alessio, D.; Thomas, G. Nutrient overload, insulin resistance, and ribosomal protein s6 kinase 1, s6k1. Cell Metab. 2006, 3, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Butte, N.F.; Liu, Y.; Zakeri, I.F.; Mohney, R.P.; Mehta, N.; Voruganti, V.S.; Goring, H.; Cole, S.A.; Comuzzie, A.G. Global metabolomic profiling targeting childhood obesity in the Hispanic population. Am. J. Clin. Nutr. 2015, 102, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Yde, C.C.; Arnberg, K.; Molgaard, C.; Michaelsen, K.F.; Larnkjaer, A.; Bertram, H.C. NMR-based metabolomic profiling of overweight adolescents: An elucidation of the effects of inter-/intraindividual differences, gender, and pubertal development. BioMed. Res. Int. 2014, 2014, 10. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Moon, J.S.; Kang, J.H.; Jang, H.B.; Lee, H.J.; Park, S.I.; Yu, K.S.; Cho, J.Y. Combined untargeted and targeted metabolomic profiling reveals urinary biomarkers for discriminating obese from normal-weight adolescents. Pediatr. Obes. 2017, 12, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.E.; Larson, M.G.; Ghorbani, A.; Cheng, S.; Chen, M.-H.; Keyes, M.; Rhee, E.P.; Clish, C.B.; Vasan, R.S.; Gerszten, R.E.; et al. Metabolomic profiles of body mass index in the Framingham heart study reveal distinct cardiometabolic phenotypes. PLoS ONE 2016, 11, e0148361. [Google Scholar] [CrossRef] [PubMed]
- Association, W.M. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar]
- Cacciari, E.; Milani, S.; Balsamo, A.; Spada, E.; Bona, G.; Cavallo, L.; Cerutti, F.; Gargantini, L.; Greggio, N.; Tonini, G.; et al. Italian cross-sectional growth charts for height, weight and BMI (2 to 20 years). J. Endocrinol. Investig. 2006, 29, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Nagy, P.; Kovacs, E.; Moreno, L.A.; Veidebaum, T.; Tornaritis, M.; Kourides, Y.; Siani, A.; Lauria, F.; Sioen, I.; Claessens, M.; et al. Percentile reference values for anthropometric body composition indices in European children from the Idefics study. Int. J. Obes. 2014, 38 (Suppl. 2), S15–S25. [Google Scholar] [CrossRef] [PubMed]
- D’Aniello, R.; Troisi, J.; D’Amico, O.; Sangermano, M.; Massa, G.; Moccaldo, A.; Pierri, L.; Poeta, M.; Vajro, P. Emerging pathomechanisms involved in obesity. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Serra-Majem, L.; Ribas, L.; Ngo, J.; Ortega, R.M.; Garcia, A.; Perez-Rodrigo, C.; Aranceta, J. Food, youth and the Mediterranean diet in Spain. Development of KidMed, Mediterranean diet quality index in children and adolescents. Public Health Nutr. 2004, 7, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Vajro, P.; Lenta, S.; Pignata, C.; Salerno, M.; D’Aniello, R.; De Micco, I.; Paolella, G.; Parenti, G. Therapeutic options in pediatric non alcoholic fatty liver disease: Current status and future directions. Ital. J. Pediatr. 2012, 38, 55. [Google Scholar] [CrossRef] [PubMed]
- Schwenzer, N.F.; Springer, F.; Schraml, C.; Stefan, N.; Machann, J.; Schick, F. Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J. Hepatol. 2009, 51, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Järvinen, K.M.; Konstantinou, G.N.; Pilapil, M.; Arrieta, M.-C.; Noone, S.; Sampson, H.A.; Meddings, J.; Nowak-Węgrzyn, A. Intestinal permeability in children with food allergy on specific elimination diets. Pediatr. Allergy Immunol. 2013, 24, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, U.C. How to interpret hydrogen breath tests. J. Neurogastroenterol. Motil. 2011, 17, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Schwimmer, J.B.; Dunn, W.; Norman, G.J.; Pardee, P.E.; Middleton, M.S.; Kerkar, N.; Sirlin, C.B. Safety study: Alanine aminotransferase cutoff values are set too high for reliable detection of pediatric chronic liver disease. Gastroenterology 2010, 138, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Sysi-Aho, M.; Katajamaa, M.; Yetukuri, L.; Orešič, M. Normalization method for metabolomics data using optimal selection of multiple internal standards. BMC Bioinform. 2007, 8, 93. [Google Scholar] [CrossRef] [PubMed]
- Mevik, B.-H.; Wehrens, R. The PLS package: Principal component and partial least squares regression in R. J. Stat. Softw. 2007, 18, 23. [Google Scholar] [CrossRef]
- Kuhn, M. Building predictive models in R using the caret package. J. Stat. Softw. 2008, 28. [Google Scholar] [CrossRef]
- Bijlsma, S.; Bobeldijk, I.; Verheij, E.R.; Ramaker, R.; Kochhar, S.; Macdonald, I.A.; van Ommen, B.; Smilde, A.K. Large-scale human metabolomics studies: A strategy for data (pre-) processing and validation. Anal. Chem. 2006, 78, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Karnovsky, A.; Weymouth, T.; Hull, T.; Tarcea, V.G.; Scardoni, G.; Laudanna, C.; Sartor, M.A.; Stringer, K.A.; Jagadish, H.V.; Burant, C.; et al. Metscape 2 bioinformatics tool for the analysis and visualization of metabolomics and gene expression data. Bioinformatics 2012, 28, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Ono, K.; Kanaya, S.; Takahashi, K. Keggscape: A cytoscape app for pathway data integration. F1000Research 2014, 3, 144. [Google Scholar] [CrossRef] [PubMed]
- Amo, K.; Arai, H.; Uebanso, T.; Fukaya, M.; Koganei, M.; Sasaki, H.; Yamamoto, H.; Taketani, Y.; Takeda, E. Effects of xylitol on metabolic parameters and visceral fat accumulation. J. Clin. Biochem. Nutr. 2011, 49, 1–7. [Google Scholar] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Sabelli, H.C.; Fawcett, J.; Gusovsky, F.; Javaid, J.; Edwards, J.; Jeffriess, H. Urinary phenyl acetate: A diagnostic test for depression? Science 1983, 220, 1187–1188. [Google Scholar] [CrossRef] [PubMed]
- Muhlig, Y.; Antel, J.; Focker, M.; Hebebrand, J. Are bidirectional associations of obesity and depression already apparent in childhood and adolescence as based on high-quality studies? A systematic review. Obes. Rev. 2016, 17, 235–249. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Ji, G.; Jia, W.; Li, H. Gut microbiota and nonalcoholic fatty liver disease: Insights on mechanism and application of metabolomics. Int. J. Mol. Sci. 2016, 17, 300. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; Migaud, M.; Glastonbury, C.A.; Beaumont, M.; Nikolaou, A.; Small, K.S.; Brosnan, M.J.; Mohney, R.P.; Spector, T.D.; Valdes, A.M. Metabolomic profiling to dissect the role of visceral fat in cardiometabolic health. Obesity 2016, 24, 1380–1388. [Google Scholar] [CrossRef] [PubMed]
- Urakami, T.; Morimoto, S.; Nitadori, Y.; Harada, K.; Owada, M.; Kitagawa, T. Urine glucose screening program at schools in Japan to detect children with diabetes and its outcome-incidence and clinical characteristics of childhood type 2 diabetes in japan. Pediatr. Res. 2007, 61, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Elliott, P.; Posma, J.M.; Chan, Q.; Garcia-Perez, I.; Wijeyesekera, A.; Bictash, M.; Ebbels, T.M.; Ueshima, H.; Zhao, L.; van Horn, L.; et al. Urinary metabolic signatures of human adiposity. Sci. Trans. Med. 2015, 7, 285ra262. [Google Scholar] [CrossRef] [PubMed]
- Mandato, C.; Lucariello, S.; Licenziati, M.R.; Franzese, A.; Spagnuolo, M.I.; Ficarella, R.; Pacilio, M.; Amitrano, M.; Capuano, G.; Meli, R.; et al. Metabolic, hormonal, oxidative, and inflammatory factors in pediatric obesity-related liver disease. J. Pediatr. 2005, 147, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Ofengand, J. Ribosomal RNA pseudouridines and pseudouridine synthases. FEBS Lett. 2002, 514, 17–25. [Google Scholar] [CrossRef]
- Charette, M.; Gray, M.W. Pseudouridine in RNA: What, where, how, and why. IUBMB Life 2000, 49, 341–351. [Google Scholar] [PubMed]
- Topp, H.; Fusch, G.; Schoch, G.; Fusch, C. Noninvasive markers of oxidative DNA stress, RNA degradation and protein degradation are differentially correlated with resting metabolic rate and energy intake in children and adolescents. Pediatr. Res. 2008, 64, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Sabaté, J.-M.; Jouët, P.; Harnois, F.; Mechler, C.; Msika, S.; Grossin, M.; Coffin, B. High prevalence of small intestinal bacterial overgrowth in patients with morbid obesity: A contributor to severe hepatic steatosis. Obes. Surg. 2008, 18, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Boursier, J.; Mueller, O.; Barret, M.; Machado, M.; Fizanne, L.; Araujo-Perez, F.; Guy, C.D.; Seed, P.C.; Rawls, J.F.; David, L.A.; et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology 2016, 63, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Kirk, M.C.; Coward, L.U.; Jackson, P.; Whitaker, J.N. P-cresol sulfate is the dominant component of urinary myelin basic protein like material. Arch. Biochem. Biophys. 2000, 377, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.P.; Luo, F.J.; Plummer, N.S.; Hostetter, T.H.; Meyer, T.W. The production of p-cresol sulfate and indoxyl sulfate in vegetarians versus omnivores. Clin. J. Am. Soc. Nephrol. 2012, 7, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Banton, S.; Tran, V.T.; Konomi, J.V.; Li, S.; Jones, D.P.; Vos, M.B. Amino acid metabolism is altered in adolescents with nonalcoholic fatty liver disease-an untargeted, high resolution metabolomics study. J. Pediatr. 2016, 172, 14–19.e15. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012, 15, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Pastore, A.; Alisi, A.; di Giovamberardino, G.; Crudele, A.; Ceccarelli, S.; Panera, N.; Dionisi-Vici, C.; Nobili, V. Plasma levels of homocysteine and cysteine increased in pediatric NAFLD and strongly correlated with severity of liver damage. Int. J. Mol. Sci. 2014, 15, 21202–21214. [Google Scholar] [CrossRef] [PubMed]
- Kalhan, S.C.; Guo, L.; Edmison, J.; Dasarathy, S.; McCullough, A.J.; Hanson, R.W.; Milburn, M. Plasma metabolomic profile in nonalcoholic fatty liver disease. Metabolism 2011, 60, 404–413. [Google Scholar] [CrossRef] [PubMed]
- von Schonfels, W.; Patsenker, E.; Fahrner, R.; Itzel, T.; Hinrichsen, H.; Brosch, M.; Erhart, W.; Gruodyte, A.; Vollnberg, B.; Richter, K.; et al. Metabolomic tissue signature in human non-alcoholic fatty liver disease identifies protective candidate metabolites. Liver Int. 2015, 35, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Wang, X.J. Induction of the keap1/nrf2/are pathway by oxidizable diphenols. Chem. Biol. Interact. 2011, 192, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Sookoian, S.; Castano, G.O.; Scian, R.; Fernandez Gianotti, T.; Dopazo, H.; Rohr, C.; Gaj, G.; San Martino, J.; Sevic, I.; Flichman, D.; et al. Serum aminotransferases in nonalcoholic fatty liver disease are a signature of liver metabolic perturbations at the amino acid and Krebs cycle level. Am. J. Clin. Nutr. 2016, 103, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Dumas, M.E.; Kinross, J.; Nicholson, J.K. Metabolic phenotyping and systems biology approaches to understanding metabolic syndrome and fatty liver disease. Gastroenterology 2014, 146, 46–62. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Control (NW) (N = 14) | Obese without NAFLD (N = 10) | Obese with NAFLD (N = 12) | All Obese (N = 22) |
|---|---|---|---|---|
| Gender (F/M) | 5/9 | 5/5 | 4/8 | 9/13 |
| Age (years) | 11.25 ± 2.26 | 11.43 ± 2.32 | 11.66 ± 2.03 | 11.55 ± 2.12 |
| Weight (kg) | 37.48 ± 10.39 | 63.23 ± 17.97 * | 65.05 ± 20.34 * | 64.18 ± 18.79 * |
| Height (cm) | 145.58 ± 15.21 | 150.71 ± 16.27 | 151.11 ± 15.62 | 150.92 ± 15.53 |
| BMI (kg/cm2) | 17.27 ± 2.09 | 27.46 ± 4.37 * | 27.78 ± 4.93 * | 27.63 ± 4.56 * |
| BMI Percentile | 43.25 ± 25.28 | 98.60 ± 0.70 * | 98.36 ± 0.92 * | 98.48 ± 0.81 * |
| SDS BMI | −0.14 ± 0.82 | 2.50 ± 0.44 * | 2.47 ± 0.50 * | 2.48 ± 0.46 * |
| Waist Circumference (cm) | 62.83 ± 8.61 | 81.36 ± 10.74 * | 84.72 ± 11.24 * | 83.12 ± 10.87 * |
| Waist Circumference percentile | 35.83 ± 29.14 | 88.50 ± 4.74 * | 88.64 ± 4.52 * | 88.57 ± 4.51 * |
| Cm exceeding 90th percentile Waist Circumference | −7.69 ± 5.62 | 11.86 ± 6.83 * | 14.26 ± 9.87 * | 13.12 ± 8.44 * |
| WHtR | 0.43 ± 0.04 | 0.54 ± 0.05 * | 0.56 ± 0.06 * | 0.55 ± 0.05 * |
| Hips Circumference (cm) | 73.00 ± 11.21 | 93.75 ± 9.60 * | 95.04 ± 14.16 * | 94.42 ± 11.92 * |
| Systolic Pressure (mm Hg) | 95.33 ± 12.63 | 111.00 ± 8.10 * | 119.36 ± 12.63 * | 115.38 ± 11.92 * |
| Systolic Pressure percentile | 26.67 ± 29.61 | 62.30 ± 23.94 * | 79.55 ± 21.40 * | 71.33 ± 23.76 * |
| Systolic Pressure SDS | −0.83 ± 1.18 | 0.42 ± 0.80 * | 1.20 ± 1.04 * | 0.83 ± 1.00 * |
| Diastolic Pressure (mm Hg) | 66.67 ± 7.78 | 69.10 ± 7.62 | 61.91 ± 8.54 | 65.52 ± 8.81 |
| Diastolic Pressure percentile | 65.42 ± 18.30 | 69.10 ± 22.01 | 50.45 ± 23.67 | 59.33 ± 24.27 |
| Diastolic Pressure SDS | 0.47 ± 0.63 | 0.62 ± 0.74 | 0.00 ± 0.66 | 0.30 ± 0.75 |
| Glycaemia (mg/dL) | 78.25 ± 19.14 | 85.63 ± 8.48 * | 90.54 ± 8.20 * | 88.23 ± 8.45 * |
| Insulinemia (mU/mL) | 11.52 ± 4.32 | 12.24 ± 6.19 | 18.56 ± 12.15 | 16.13 ± 10.45 |
| HOMA-IR [(Glu *Ins)/405] | 2.24 ± 1.12 | 2.87 ± 1.51 | 3.70 ± 2.50 | 3.31 ± 2.07 |
| ALT (U/L) | 15.83 ± 8.65 | 20.88 ± 4.02 | 49.80 ± 15.75 * | 36.19 ± 18.78 * |
| AST (U/L) | 25.25 ± 2.96 | 25.36 ± 8.38 | 31.56 ± 14.52 | 28.46 ± 16.76 |
| Intestinal Permebility (L/M ratio) | 0.0158 ± 0.0121 | 0.0209 ± 0.0214 | 0.0630 ± 0.0328 * | 0.0420 ± 0.0392 * |
| SIBO (n/total) (% positive pts) | 0/14 (0%) | 5/10 (50.0%) | 7/12 (58.3%) | 12/22 (54.5%) * |
| KIDMED score | 7.88 + 1.69 | 5.00 + 2.23 * | 5.23 + 2.74 * | 5.12 + 2.52 * |
| Beta | Std.Err. | p-Value | ||
|---|---|---|---|---|
| WCE | Leucine | 0.883 | 0.054 | <0.001 |
| Glucose | 0.857 | 0.057 | <0.001 | |
| Xylitol | 0.174 | 0.046 | 0.003 | |
| Glycolic acid | 0.491 | 0.049 | <0.001 | |
| Methyloxovalerate | −0.669 | 0.068 | <0.001 | |
| Pseudouridin | 0.204 | 0.081 | 0.03 | |
| Methylmalonic acid | −0.263 | 0.050 | <0.001 | |
| Androstenedione | 0.355 | 0.060 | <0.001 | |
| Butanoic acid | −0.206 | 0.054 | <0.001 | |
| Phenylalanine | 0.154 | 0.069 | 0.04 | |
| Kynurenine | −0.089 | 0.052 | 0.01 | |
| Threose | 0.045 | 0.044 | 0.02 | |
| L/M | Glucose | 0.402 | 0.040 | <0.001 |
| Mannose | −1.083 | 0.046 | <0.001 | |
| Arachidate | −0.449 | 0.043 | <0.001 | |
| Palmitoleic acid | 0.202 | 0.044 | 0.001 | |
| Ascorbic acid | 0.672 | 0.041 | <0.001 | |
| Xylose | −0.720 | 0.065 | <0.001 | |
| Kynurenine | −0.207 | 0.037 | <0.001 | |
| Pseudouridine | −0.017 | 0.040 | 0.04 | |
| Valine | 0.317 | 0.053 | <0.001 | |
| Androstenedione | 0.357 | 0.061 | <0.001 | |
| Unknown | −0.213 | 0.044 | <0.001 | |
| KID-MED | Methyloxovalerate | −1.106 | 0.033 | <0.001 |
| Methylmalonic acid | −0.459 | 0.027 | <0.001 | |
| Butanoic acid | −0.347 | 0.035 | <0.001 | |
| Kynurenine | −0.145 | 0.027 | <0.001 | |
| p-cresylsulphate | −0.139 | 0.030 | <0.001 | |
| Threose | 0.082 | 0.032 | 0.024 | |
| Phenylalanine | 0.100 | 0.031 | 0.008 | |
| Xylitol | 0.117 | 0.036 | 0.007 | |
| Pseudouridin | 0.177 | 0.024 | <0.001 | |
| Androstenedione | 0.326 | 0.028 | <0.001 | |
| Glycolic acid | 0.438 | 0.029 | <0.001 | |
| Glucose | 1.015 | 0.046 | <0.001 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Troisi, J.; Pierri, L.; Landolfi, A.; Marciano, F.; Bisogno, A.; Belmonte, F.; Palladino, C.; Guercio Nuzio, S.; Campiglia, P.; Vajro, P. Urinary Metabolomics in Pediatric Obesity and NAFLD Identifies Metabolic Pathways/Metabolites Related to Dietary Habits and Gut-Liver Axis Perturbations. Nutrients 2017, 9, 485. https://doi.org/10.3390/nu9050485
Troisi J, Pierri L, Landolfi A, Marciano F, Bisogno A, Belmonte F, Palladino C, Guercio Nuzio S, Campiglia P, Vajro P. Urinary Metabolomics in Pediatric Obesity and NAFLD Identifies Metabolic Pathways/Metabolites Related to Dietary Habits and Gut-Liver Axis Perturbations. Nutrients. 2017; 9(5):485. https://doi.org/10.3390/nu9050485
Chicago/Turabian StyleTroisi, Jacopo, Luca Pierri, Annamaria Landolfi, Francesca Marciano, Antonella Bisogno, Federica Belmonte, Carmen Palladino, Salvatore Guercio Nuzio, Pietro Campiglia, and Pietro Vajro. 2017. "Urinary Metabolomics in Pediatric Obesity and NAFLD Identifies Metabolic Pathways/Metabolites Related to Dietary Habits and Gut-Liver Axis Perturbations" Nutrients 9, no. 5: 485. https://doi.org/10.3390/nu9050485







