Cosmetics Preservation: A Review on Present Strategies
Abstract
:1. Introduction
2. Overview of Cosmetics and Their Microbiological Safety
2.1. Definition and Classification of Cosmetics
2.2. Cosmetic Products with Antimicrobial Effect
2.3. Microbiological Safety of Cosmetic Products
2.4. Microbiological Specifications According to International Regulations
2.4.1. Legislation in the United States
2.4.2. Legislation in Japan
2.4.3. Legislation in the European Union
- Category 1—products specifically intended for children under three years, to be used in the eye area and on mucous membranes;
- Category 2—other products.
3. Preservation Strategies
3.1. Primary Preservation Strategy
3.2. Secondary Preservation Strategy
3.2.1. Physical Secondary Preservation
3.2.2. Physicochemical Secondary Preservation
Water Activity
Emulsion Form
pH Control
3.2.3. Chemical Secondary Preservation
Synthetic Chemical Preservatives
Natural Chemical Preservatives
Multifunctional Ingredients
3.3. Validation of Effective Preservation
3.3.1. Types of Primary Packaging
3.3.2. Microbiological Control of Raw Materials
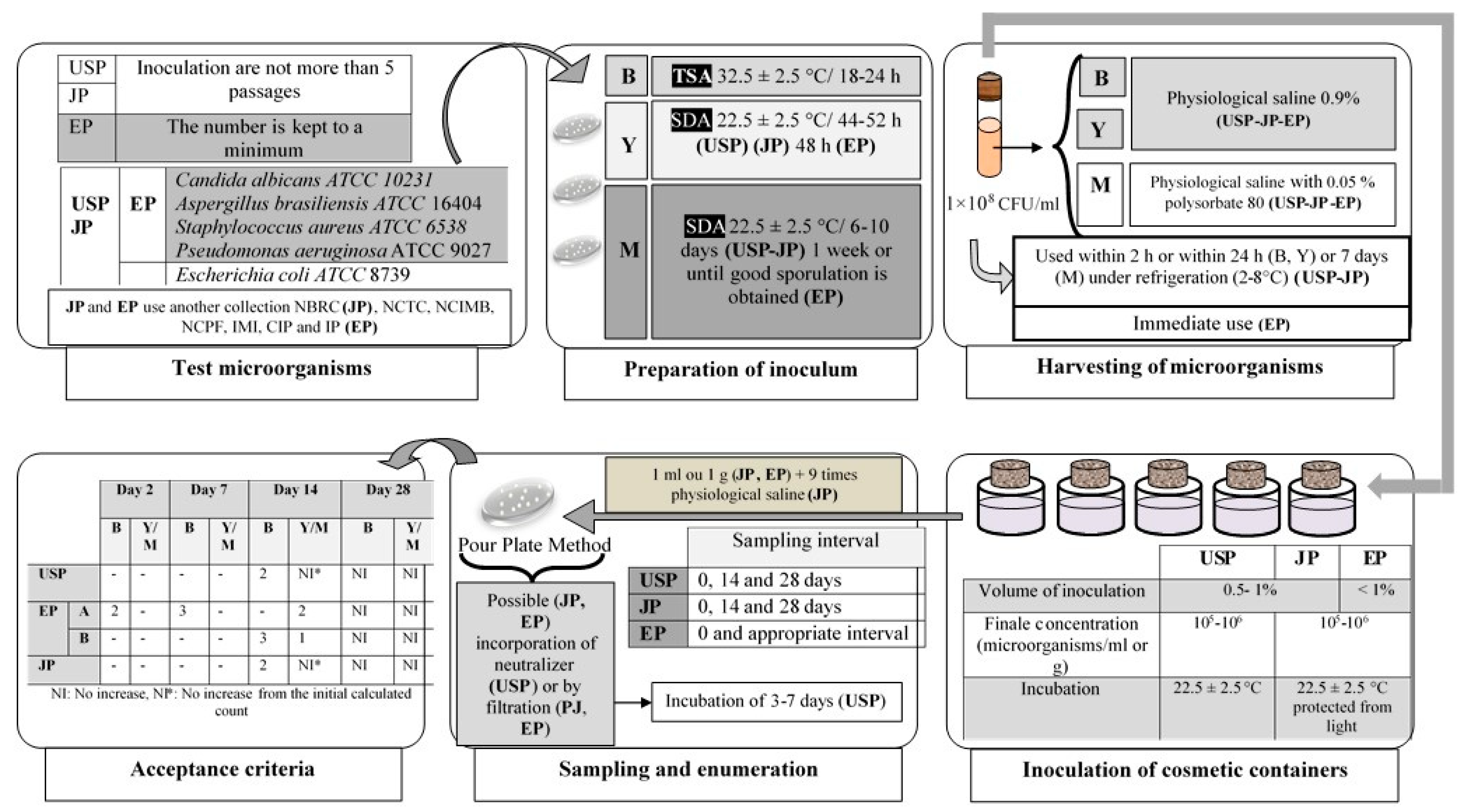
3.3.3. Antimicrobial Efficacy Test of the Preservation System
Challenge Test
Other Published Methods
Factors affecting preservation effectiveness tests
4. Synthetic Chemical Preservatives
4.1. Different Chemical Classes
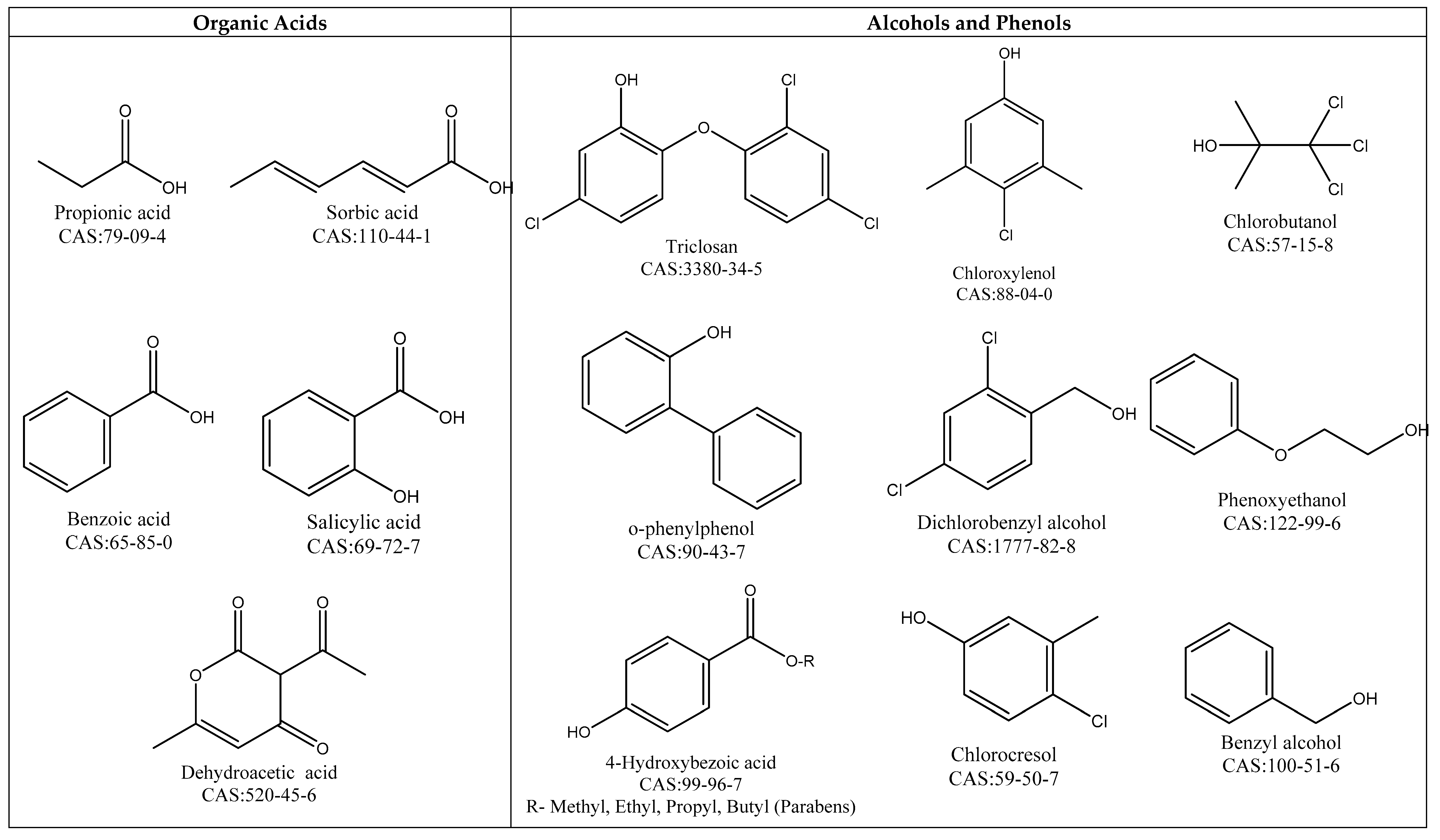
4.1.1. Organic Acids
4.1.2. Alcohols and Phenols
4.1.3. Aldehydes and Formaldehyde Releasers
4.1.4. Isothiazolinones
4.1.5. Biguanides
4.1.6. Quaternary Ammonium Compounds (QAC)
4.1.7. Nitrogen Compounds
4.1.8. Heavy Metal Derivatives
4.1.9. Inorganic Compounds
4.2. Analytical Methods Used to Determine Preservatives
4.3. Toxicity of Chemical Preservatives
4.4. Selection of Appropriate Preservatives
4.4.1. Stability
4.4.2. Compatibility
4.4.3. Safety
4.4.4. Compliance with Cosmetic Legislation
4.4.5. Cost
4.5. Preservative Mechanisms of Action
4.5.1. Organic Acids
4.5.2. Alcohols and Phenols
4.5.3. Aldehydes and Formaldehyde Releasers
4.5.4. Isothiazolinones
4.5.5. Biguanides
4.5.6. Quaternary Ammonium Compounds (QAC)
4.5.7. Nitrogen Compounds
4.5.8. Heavy Metal Derivatives
4.5.9. Inorganic Compounds
4.6. Microorganism’s Mechanisms of Resistance to Preservatives
4.6.1. Organic Acids
4.6.2. Alcohols and Phenols
4.6.3. Aldehydes and Formaldehyde Releasers
4.6.4. Biguanides
4.6.5. Quaternary Ammoniums Compounds (QAC)
4.6.6. Heavy Metal Derivatives
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wood, L. Global Cosmetics Market—By Product Type, Ingredient, Geography, and Vendors—Market Size, Demand Forecasts, Industry Trends and Updates, Supplier Market Shares 2014–2020. URL: Research and Markets. Available online: https://www.researchandmarkets.com/research/f2lvdg/global_cosmetics (accessed on 31 May 2018).
- Neza, E.; Centini, M. Microbiologically contaminated and over-preserved cosmetic products according rapex 2008–2014. Cosmetics 2016, 3, 3. [Google Scholar] [CrossRef]
- Martini, M.C. Conservateurs. In Cosmétologie et Dermatologie Esthétique; Elsevier Masson: Paris, France, 2006; Volume 1, pp. 50–120. [Google Scholar]
- The European Parliament and the Council of the European Union. Regulation (EC) No. 1223/2009 of the European parliament and of the council of 30 November 2009 on cosmetic products. Off. J. Eur. Union L 2009, 342, 59. [Google Scholar]
- Toler, J.C. Preservative stability and preservative systems. Int. J. Cosmet. Sci. 1985, 7, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Siquet, F. Antibacterial agents and preservatives. In Handbook of Cosmetic Science and Technology; Paye, M., Barel, A.O., Maibach, H.I., Eds.; Taylor & Francis Group, LLC: Boca Raton, FL, USA, 2009; pp. 223–231. [Google Scholar]
- Butler, H. Microbiological control of cosmetics. In Poucher’s Perfumes, Cosmetics and Soaps; Butler, H., Ed.; Springer: Dordrecht, The Netherlands, 1993; Volume 3, pp. 572–606. [Google Scholar]
- Siemer, E. Preparations for cleansing and caring for blemished skin. In Cosmetics and Toiletries—Development, Production and Use; Umbach, W., Ed.; Ellis Horwood: New York, NY, USA, 1991; pp. 124–128. [Google Scholar]
- Shai, A.; Baran, R.; Maibach, H.I. (Eds.) Cosmetics and Cosmetic Preparations: Basic Definitions; Informa UK Ltd.: London, UK, 2009; pp. 1–3. [Google Scholar]
- Mitsui, T. (Ed.) Preservation of cosmetics. In New Cosmetic Science; Elsevier: Amsterdam, The Netherlands, 1997; pp. 199–208. [Google Scholar]
- Barel, A.O.; Paye, M.; Maibach, H.I. Handbook of Cosmetic Science and Technology; Taylor & Francis Group: Boca Raton, FL, USA, 2006. [Google Scholar]
- Draelos, Z.D. Cosmetics, categories, and the future. Dermatol. Ther. 2012, 25, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Kabara, J.J.; Orth, D.S. Principles for product preservation. In Preservative-Free and Self-Preserving Cosmetics and Drugs: Principles and Practices; Kabara, J.J., Ed.; Marcel Dekker: New York, NY, USA, 1997; pp. 1–14. [Google Scholar]
- Huang, H.Y.; Lai, Y.C.; Chiu, C.W.; Yeh, J.M. Comparing micellar electrokinetic chromatography and microemulsion electrokinetic chromatography for the analysis of preservatives in pharmaceutical and cosmetic products. J. Chromatogr. A 2003, 993, 153–164. [Google Scholar] [CrossRef]
- Hellwege, K.D. The oral cavity. In Cosmetics and Toiletries: Development, Production and Use; Umbach, W., Ed.; Ellis Horwood: New York, NY, USA, 1991; pp. 31–37. [Google Scholar]
- Pitt, T.L.; McClure, J.; Parker, M.D.; Amezquita, A.; McClure, P.J. Bacillus cereus in personal care products: Risk to consumers. Int. J. Cosmet. Sci. 2015, 37, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.E.; Parker, M.D.; Amezquita, A.; Pitt, T.L. Microbiological risk assessment for personal care products. Int. J. Cosmet. Sci. 2016, 38, 634–645. [Google Scholar] [CrossRef] [PubMed]
- Scientific Committee on Consumer Safety. The SCCS Notes of Guidance for the Testing of Cosmetic Ingredients and Their Safety Evaluation, 9th ed.; SCCS, Ed.; European Union: Brussels, Belgium, 2016; Volume SCCS/1564/15. [Google Scholar]
- Smith, C.N.; Alexander, B.R. The relative cytotoxicity of personal care preservative systems in Balb/c 3T3 clone A31 embryonic mouse cells and the effect of selected preservative systems upon the toxicity of a standard rinse-off formulation. Toxicol. In Vitro 2005, 19, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Bremer, H.; Klein, W. Deodorants. In Cosmetics and Toiletries—Development, Production and Use, 1st ed.; Umbach, W., Ed.; Ellis Horwood: New York, NY, USA, 1991; pp. 115–121. [Google Scholar]
- U.S. Code. Regulations, Electronic Code of Federal Regulations (e-CFR). In Title 21: Food and Drugs; Legal Information Institute: Ithaca, NY, USA, 2016. [Google Scholar]
- Prabhamanju, M.; Shankar, S.G.; Babu, K.; Ranjith, M.S. Herbal vs. Chemical substances as antidandruff ingredients: Which are more effective in the management of dandruff? Egypt Dermatol. Online J. 2009, 5, 1–8. [Google Scholar]
- Dos Santos, R.M.; Dias-Souza, M.V. Effectiveness of five antidandruff cosmetic formulations against planktonic cells and biofilms of dermatophytes. Saudi J. Biol. Sci. 2017, 24, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Easley, J.; Gorman, W.; Mendoza, M. Approaches for adding antibacterial properties to cosmetic products. In Multifunctional Cosmetics; Schueller, R., Romanowski, P., Eds.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Chiller, K.; Selkin, B.A.; Murakawa, G.J. Skin microflora and bacterial infections of the skin. J. Investig. Dermatol. Symp. Proc. 2001, 6, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Settembrini, L.; Gultz, J.; Boylan, R.; Scherer, W. Antimicrobial activity produced by six dentifrices. Gen. Dent. 1998, 46, 286–288. [Google Scholar] [PubMed]
- Davies, R.; Scully, C.; Preston, A.J. Dentifrices—An update. Med. Oral Patol. Oral Cir. Bucal 2010, 16, 976–982. [Google Scholar] [CrossRef]
- Sutton, S.V.W. Antimicrobial preservative efficacy and microbial content testing. In Cosmetic Microbiology: A Practical Approach; Geis, P.A., Ed.; Taylor & Francis Group: New York, NY, USA, 2006; pp. 111–145. [Google Scholar]
- Kunicka-Styczynska, A.; Sikora, M.; Kalemba, D. Antimicrobial activity of lavender, tea tree and lemon oils in cosmetic preservative systems. J. Appl. Microbiol. 2009, 107, 1903–1911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geis, P.A. Cosmetic microbiology: A practical approach. In Cosmetic Microbiology: A Practical Approach; Geis, P.A., Ed.; Taylor & Francis: New York, NY, USA, 2006; pp. 163–180. [Google Scholar]
- Delarras, C. Microbiologie Pratique pour le Laboratoire d’Analyses ou de Contrôle Sanitaire; Tec & Doc Lavoisier: Paris, France, 2007. [Google Scholar]
- Sedlewicz, L.B. Cosmetic preservatives: Friend or foe? Skinmed 2005, 4, 98–100. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, L.; Dorato, S. General concepts and cosmetic legislation. In Analysis of Cosmetic Products; Amparo, S., Alberto, C., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2007; pp. 3–28. [Google Scholar]
- Milstein, S.R.; Halper, A.R.; Katz, L.M. Regulatory requirements for the marketing of cosmetics in the United States. In Handbook of Cosmetic Science and Technology; Barel, A.O., Paye, M., Maibach, H.I., Eds.; Taylor & Francis Group: New York, NY, USA, 2006; pp. 833–859. [Google Scholar]
- Benson, L.; Reczek, K. A Guide to United States Cosmetic Products Compliance Requirements; National Institute of Standards and Technology, Ed.; US Department of Commerce: Washington, DC, USA, 2017.
- U.S. Code. Subchapter VI—Cosmetics. In Title 21—Food and Drugs; Legal Information Institute: Ithaca, NY, USA, 2011; pp. 304–306. [Google Scholar]
- Huang, J.; Hitchins, A.D.; Tran, T.T.; McCarron, J.E. Microbiological methods for cosmetics. In Bacteriological Analytical Manual (BAM), 8th ed.; FDA: Silver Spring, MD, USA, 2017. [Google Scholar]
- Pharmacopeia, T.J. The Japanese Pharmacopeia, 64th ed.; Japan Ministry of Health, Labour and Welfare: Tokyo, Japan, 2016; pp. 2486–2489.
- International Organization for Standardization (ISO). Cosmétiques—Microbiologie—Limites Microbiologiques European Committee for Standardization; ISO 17516:2014; ISO: Geneva, Switzerland, 2014. [Google Scholar]
- Journal Officiel de la République Algérienne. Les conditions et les modalités de fabrication, de conditionnement, d’importation, et de commercialisation sur le marché national des produits cosmétiques et d’hygiène corporelle. In Décret exécutif n° 97-37; Imprimerie Officielle: Bir Mourad Raïs, Algeria, 1997; pp. 13–15. [Google Scholar]
- Journal Officiel de la République Algérienne. Décret exécutif n°10-114 du 18 Avril 2010 modifiant et complétant le décret exécutif n° 97-37 définissant les conditions et les modalités de fabrication, de conditionnement, d’importation et de de commercialisation, sur le marché national, des produits cosmétiques et d’hygiène corporelle; Imprimerie Officielle: Bir Mourad Raïs, Algeria, 2010. [Google Scholar]
- Varvaresou, A.; Papageorgiou, S.; Tsirivas, E.; Protopapa, E.; Kintziou, H.; Kefala, V.; Demetzos, C. Self-preserving cosmetics. Int. J. Cosmet. Sci. 2009, 31, 163–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghalleb, S.; De Vaugelade, S.; Sella, O.; Lavarde, M.; Mielcarek, C.; Pense-Lheritier, A.M.; Pirnay, S. Predictive microbiology for cosmetics based on physicals, chemicals and concentration parameters. Int. J. Cosmet. Sci. 2015, 37, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Kabara, J.J. Hurdle technology: Are biocides always necessary for product protection? J. Appl. Cosmetol. 1999, 17, 102–109. [Google Scholar]
- Leistner, L. Basic aspects of food preservation by hurdle technology. Int. J. Food Microbiol. 2000, 55, 181–186. [Google Scholar] [CrossRef]
- Stoffels, K.M. Modern and safe antimicrobial stabilization of cosmetic products. Househ. Pers. Care Today 2012, 7, 18–21. [Google Scholar]
- De Boer, E. Understanding and Implementing the Requirements of the ISO 22176 Good Manufacturing Practices (GMP) Certification Standard for Cosmetics Products. URL: SGS Offices & Labs. Available online: https://www.sgs.com/en/white-paper-library/cosmetics-gmp (accessed on 31 May 2018).
- Devlieghere, F.; De Loy-Hendrickx, A.; Rademaker, M.; Pipelers, P.; Crozier, A.; De Baets, B.; Joly, L.; Keromen, S. A new protocol for evaluating the efficacy of some dispensing systems of a packaging in the microbial protection of waterbased preservative-free cosmetic products. Int. J. Cosmet. Sci. 2015, 37, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Yablonski, J.I.; Mancuso, S.E. Microbial risks and eco-friendly packaging. In Formulating, Packaging, and Marketing of Natural Cosmetic Products; Dayan, N., Kromidas, L., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 179–211. [Google Scholar]
- Yablonski, J.I.; Mancuso, S.E. Personal care wipes: Manufacturing practices and microbiological control. Cosmet. Toilet. 2004, 119, 53–56. [Google Scholar]
- Lockhart, H.; Paine, F.A. Packaging of Pharmaceuticals and Healthcare Products; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Song, Y.S.; Al-Taher, F.; Sadler, G. Migration of volatile degradation products into ozonated water from plastic packaging materials. Food Addit. Contam. 2003, 20, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Zema, L.; Sangalli, M.E.; Maroni, A.; Foppoli, A.; Bettero, A.; Gazzaniga, A. Active packaging for topical cosmetic/drug products: A hot-melt extruded preservative delivery device. Eur. J. Pharm. Biopharm. 2010, 75, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Kabara, J.J. Preservative-Free and Self-Preserving Cosmetics and Drugs: Principles and Practices; CRC Press: Boca Raton, FL, USA, 1997; Volume 16. [Google Scholar]
- Kerdudo, A.; Fontaine-Vive, F.; Dingas, A.; Faure, C.; Fernandez, X. Optimization of cosmetic preservation: Water activity reduction. Int. J. Cosmet. Sci. 2015, 37, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Hiom, S.J. Preservation of medicines and cosmetics. In Russell, Hugo & Ayliffe’s: Principles and Practice of Disinfection, Preservation and Sterilization; Fraise, A.P., Maillard, J.-Y., Sattar, S.A., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2013; pp. 388–407. [Google Scholar]
- Berthele, H.; Sella, O.; Lavarde, M.; Mielcarek, C.; Pense-Lheritier, A.M.; Pirnay, S. Determination of the influence of factors (ethanol, pH and aw) on the preservation of cosmetics using experimental design. Int. J. Cosmet. Sci. 2014, 36, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Buranasuksombat, U.; Kwon, Y.J.; Turner, M.; Bhandari, B. Influence of emulsion droplet size on antimicrobial properties. Food Sci. Biotechnol. 2011, 20, 793–800. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Rojas-Graü, A.; Soliva-Fortuny, R.; Martín-Belloso, O. Physicochemical characterization and antimicrobial activity of food-grade emulsions and nanoemulsions incorporating essential oils. Food Hydrocoll. 2015, 43, 547–556. [Google Scholar] [CrossRef]
- Char, C.; Cisternas, L.; Pérez, F.; Guerrero, S. Effect of emulsification on the antimicrobial activity of carvacrol. CyTA J. Food 2016, 14, 186–192. [Google Scholar] [CrossRef]
- Dias, M.F.R.G. Hair cosmetics: An overview. Int. J. Trichol. 2015, 7, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Lukic, M.; Pantelic, I.; Savic, S. An overview of novel surfactants for formulation of cosmetics with certain emphasis on acidic active substances. Tenside Surfactants Deterg. 2016, 53, 7–19. [Google Scholar] [CrossRef]
- Truchliński, J.; Sembratowicz, I.; Gorzel, M.; Kiełtyka-Dadasiewicz, A. Allergenic potential of cosmetic ingredients. Arch Physiother. Glob. Res. 2015, 19, 7–15. [Google Scholar] [CrossRef]
- McCarthy, T.J. Formulated factors affecting the activity of preservatives. In Cosmetic and Drug Preservation, Principles and Practices, 1st ed.; Kabara, J.J., Ed.; Marcel Dekker: New York, NY, USA, 1984; pp. 359–387. [Google Scholar]
- Strilets, O.P.; Petrovska, L.S.; Baranova, I.I.; Bespala Yu, O. A study of antimicrobial activity of foam-washing agent specimens at acidic ph values. Anali Mečnikìvsʹkogo Institutu 2017, 23–26. [Google Scholar] [CrossRef]
- Kole, P.L.; Jadhav, H.R.; Thakurdesai, P.A.; Nagappa, A.N. Cosmetics: Potential of herbal extracts. Nat. Prod. Radiance 2005, 4, 315–321. [Google Scholar]
- Kerdudo, A.; Burger, P.; Merck, F.; Dingas, A.; Rolland, Y.; Michel, T.; Fernandez, X. Development of a natural ingredient—Natural preservative: A case study. C. R. Chim. 2016, 19, 1077–1089. [Google Scholar] [CrossRef]
- Popescu, C.; Popescu, C.; Popescu, B.; Daas, D.; Morgovan, C.; Olah, N.K. Antimicrobial efficacy of the organic greasy oils combination-sea buckthorn oil and maize germs oil. Farmacia 2014, 62, 743–752. [Google Scholar]
- Antignac, E.; Nohynek, G.J.; Re, T.; Clouzeau, J.; Toutain, H. Safety of botanical ingredients in personal care products/cosmetics. Food Chem. Toxicol. 2011, 49, 324–341. [Google Scholar] [CrossRef] [PubMed]
- Patrone, V.; Campana, R.; Vittoria, E.; Baffone, W. In vitro synergistic activities of essential oils and surfactants in combination with cosmetic preservatives against Pseudomonas aeruginosa and Staphylococcus aureus. Curr. Microbiol. 2010, 60, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Herman, A.; Herman, A.P.; Domagalska, B.W.; Mlynarczyk, A. Essential oils and herbal extracts as antimicrobial agents in cosmetic emulsion. Indian J. Microbiol. 2013, 53, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Kunicka-Styczynska, A.; Sikora, M.; Kalemba, D. Lavender, tea tree and lemon oils as antimicrobials in washing liquids and soft body balms. Int. J. Cosmet. Sci. 2011, 33, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Maccioni, A.M.; Anchisi, C.; Sanna, A.; Sardu, C.; Dessi, S. Preservative systems containing essential oils in cosmetic products. Int. J. Cosmet. Sci. 2002, 24, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Nostro, A.; Cannatelli, M.A.; Morelli, I.; Musolino, A.D.; Scuderi, F.; Pizzimenti, F.; Alonzo, V. Efficiency of Calamintha officinalis essential oil as preservative in two topical product types. J. Appl. Microbiol. 2004, 97, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Nostro, A.; Cannatelli, M.A.; Morelli, I.; Cioni, P.L.; Bader, A.; Marino, A.; Alonzo, V. Preservative properties of Calamintha officinalis essential oil with and without EDTA. Lett. Appl. Microbiol. 2002, 35, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Muyima, N.Y.O.; Zulu, G.; Bhengu, T.; Popplewell, D. The potential application of some novel essential oils as natural cosmetic preservatives in an aqueous cream formulation. Flavour Fragr. J. 2002, 17, 258–266. [Google Scholar] [CrossRef]
- Manou, I.; Bouillard, L.; Devleeschouwer, M.J.; Barel, A.O. Evaluation of the preservative properties of thymus vulgaris essential oil in topically applied formulations under a challenge test. J. Appl. Microbiol. 1998, 84, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.P.; Sati, N. Artificial preservatives and their harmful effects: Looking toward nature for safer alternatives. Int. J. Pharm. Sci. Res. 2013, 4, 2496–2501. [Google Scholar]
- Rodríguez, J.; Martín, M.J.; Ruiz, M.A.; Clares, B. Current encapsulation strategies for bioactive oils: From alimentary to pharmaceutical perspectives. Food Res. Int. 2016, 83, 41–59. [Google Scholar] [CrossRef]
- Hommoss, A. Preservative system development for argan oil-loaded nanostructured lipid carriers. Pharmazie 2011, 66, 187–191. [Google Scholar] [PubMed]
- Dreger, M.; Wielgus, K. Application of essential oils as natural cosmetic preservatives. Herba Pol. 2013, 59, 142–156. [Google Scholar] [CrossRef] [Green Version]
- Siegert, W. Boosting the antimicrobial efficiency of multifunctional additives by chelating agents. Int. J. Appl. Sci. 2014, 140, 1–6. [Google Scholar]
- Yoo, I.K.; Kim, J.I.; Kang, Y.K. Conformational preferences and antimicrobial activities of alkanediols. Int. J. Comput. Theor. Chem. 2015, 1064, 15–24. [Google Scholar] [CrossRef]
- Pillai, R.; Schmaus, G.; Pfeiffer, A.; Lange, S.; Trunet, A. 1,2-alkanediols for cosmetic preservation. Cosmet. Toiletries 2008, 123, 53–64. [Google Scholar]
- Laverty, G.; Gilmore, B.F.; Jones, D.S.; Coyle, L.; Folan, M.; Breathnach, R. Antimicrobial efficacy of an innovative emulsion of medium chain triglycerides against canine and feline periodontopathogens. J. Small Anim. Pract. 2015, 56, 253–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amaral, L.F.B.; Camilo, N.S.; Pereda, M.D.C.V.; Levy, C.E.; Moriel, P.; Mazzola, P.G. Evaluation of antimicrobial effectiveness of c-8 xylitol monoester as an alternative preservative for cosmetic products. Int. J. Cosmet. Sci. 2011, 33, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, M.; Sekar, P.; Pasupathi, M.; Mukhopadhyay, T. Self-preserving personal care products. Int. J. Cosmet. Sci. 2017, 39, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Siegert, W. Microbiological quality management for the production of cosmetics and detergents. Int. J. Appl. Sci. (SOFW) 2012, 138, 2–9. [Google Scholar]
- Brannan, D.K. The role of packaging in product preservation. In Preservation-Free and Self-Preserving Cosmetics and Drugs; Kabara, J.J., Orth, D.S., Eds.; Marcel Dekker: New York, NY, USA, 1997; pp. 227–249. [Google Scholar]
- Wallhäuser, K.H. Praxis der sterilisation, desinfektion, konservierung, keimidentifizierung, betriebshygiene, 3. Neubearb. U. Erweit. Auflage. In Pharmazie in unserer zeit; Scheer, R., Ed.; Wiley-VCH Verlag: Weinheim, Germany, 1985; Volume 14, p. 127. [Google Scholar]
- Katušin-Ražem, B.; Mihaljević, B.; Ražem, D. Microbial decontamination of cosmetic raw materials and personal care products by irradiation. Int. J. Radiat. Phys. Chem. 2003, 66, 309–316. [Google Scholar] [CrossRef]
- Devleeschouwer, M.; Siquet, F. Stability control: Microbiological tests. In Handbook of Cosmetic Science and Technology, 2nd ed.; Barel, A.O., Paye, M., Maibach, H.I., Eds.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Nagarnaik, M.; Sarjoshi, A.; Linge, P.; Bhore, S.; Pandya, G. A microbial study of some cosmetics and raw materials used in personal care products in urban area. Res. J. Top. Cosmet. Sci. 2015, 6, 48. [Google Scholar] [CrossRef]
- Todd, E.C.; Michaels, B.S.; Smith, D.; Greig, J.D.; Bartleson, C.A. Outbreaks where food workers have been implicated in the spread of foodborne disease. Part 9. Washing and drying of hands to reduce microbial contamination. J. Food Prot. 2010, 73, 1937–1955. [Google Scholar] [CrossRef] [PubMed]
- Lodhi, B.; Padamwar, P.; Patel, A. Cleaning validation for the pharmaceuticals, biopharmaceuticals, cosmetic and neutraceuticals industries. J. Innov. Pharm. Biol. Sci. 2014, 1, 27–38. [Google Scholar]
- Behravan, J.; Bazzaz, F.; Malaekeh, P. Survey of bacteriological contamination of cosmetic creams in Iran. Int. J. Dermatol. 2005, 44, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Cundell, A.M.; Chatellier, S.; Schumann, P.; Lilischkis, R. Equivalence of quality control strains of microorganisms used in the compendial microbiological tests: Are national culture collection strains identical? PDA J. Pharm. Sci. Technol. 2010, 64, 137–155. [Google Scholar] [PubMed]
- Sandle, T. Antibiotics and preservatives. In Pharmaceutical Microbiology: Essentials for Quality Assurance and Quality Control; Sandle, T., Ed.; Woodhead Publishing: Oxford, UK, 2016; pp. 171–183. [Google Scholar]
- Booth, C. Antimicrobial effectiveness testing validation strategies. J. GXP Compliance 2014, 18, 1–12. [Google Scholar]
- Scholtyssek, R. Protection of cosmetics and toiletries. In Directory of Microbicides for the Protection of Materials; Paulus, W., Ed.; Springer: Dordrecht, The Netherlands, 2004; pp. 263–266. [Google Scholar]
- Toiletry and Fragrance Association. CTFA Technical Guidelines; The Cosmetic Association: Brussels, Belgium, 1981. [Google Scholar]
- Office of Regulatory Affairs (Ed.) Pharmaceutical Microbiology Manual; Office of Regulatory Affairs: Silver Spring, MD, USA, 2015; Volume ORA.007. [Google Scholar]
- Siegert, W. Comparison of microbial challenge testing methods for cosmetics. Househ. Pers. Care Today 2013, 8, 32–39. [Google Scholar]
- Russell, A.D. Challenge testing: Principles and practice. Int. J. Cosmet. Sci. 2003, 25, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, S.F. Methods for assessing antimicrobial activity. In Mechanisms of Action of Chemical Biocides: Their Study and Exploitation; Denyer, S.P., Hugo, W.B., Eds.; Blackwell Scientific Publications: Oxford, UK, 1991; Volume 27, pp. 1–22. [Google Scholar]
- Sandle, T. Microbiological culture media. In Pharmaceutical Microbiology: Essentials for Quality Assurance and Quality Control; Woodhead Publishing: Oxford, UK, 2016; pp. 47–61. [Google Scholar]
- Orth, D.S.; Lutes, C.M.; Smith, D.K. Effect of culture conditions and method for inoculum preparation on the kinetics of bacterial death during preservative efficacy testing. J. Soc. Cosmet. Chem. 1989, 40, 193–204. [Google Scholar]
- United States Pharmacopeia Convention (Ed.) Antimicrobial preservatives: Effectiveness. In U.S. Pharmacopeia; United States Pharmacopeia Convention: Rockville, MD, USA, 2005. [Google Scholar]
- European Pharmacopeia (Ed.) Efficacy of antimicrobial preservation. In European Pharmacopeia, 7th ed.; Council of Europe: Strasbourg, France, 2011. [Google Scholar]
- Sutton, S.V.; Porter, D. Development of the antimicrobial effectiveness test as USP chapter 51. PDA J. Pharm. Sci. Technol. 2002, 56, 300–311. [Google Scholar] [PubMed]
- Bishop, J.R.; White, C.H.; Firstenberg-Eden, R. A rapid impedimetric method for determining the potential shelf-life of pasteurized whole milk. J. Food Prot. 1984, 47, 471–475. [Google Scholar] [CrossRef]
- Zhou, X.; King, V.M. An impedimetric method for rapid screening of cosmetic preservatives. J. Ind. Microbiol. 1995, 15, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Connolly, P.; Bloomfield, S.F.; Denyer, S.P. A study of the use of rapid methods for preservative efficacy testing of pharmaceuticals and cosmetics. J. Appl. Bacteriol. 1993, 75, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Connolly, P.; Bloomfield, S.F.; Denyer, S.P. The use of impedance for preservative efficacy testing of pharmaceuticals and cosmetic products. J. Appl. Bacteriol. 1994, 76, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.R.; Lourenco, F.R.; Ohara, M.T.; Bou-Chacra, N.A.; Pinto, T.J. An innovative challenge test for solid cosmetics using freeze-dried microorganisms and electrical methods. J. Microbiol. Methods 2014, 106, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Shintani, H.; Sakudo, A.; McDonnel, G.E. Methods of rapid microbiological assay and their application to pharmaceutical and medical device fabrication. Biocontrol. Sci. 2011, 16, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Chollet, R.; Ribault, S. Use of ATP bioluminescence for rapid detection and enumeration of contaminants: The milliflex rapid microbiology detection and enumeration system. In Bioluminescence: Recent Advances in Oceanic Measurements and Laboratory Applications; Lapota, D., Ed.; InTech: London, UK, 2012; pp. 99–118. [Google Scholar]
- Moser, C.L.; Meyer, B.K. Comparison of compendial antimicrobial effectiveness tests: A review. AAPS PharmSciTech 2011, 12, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Orth, D.S. Linear regression method for rapid determination of cosmetic preservative efficacy. J. Soc. Cosmet. Chem. 1979, 30, 321–332. [Google Scholar]
- Orth, D.S.; Enigl, D.C. Preservative efficacy testing by a rapid screening method for estimation of d-values. J. Soc. Cosmet. Chem. 1993, 44, 329–336. [Google Scholar]
- Hodges, N.A.; Denyer, S.P.; Hanlon, G.W.; Reynolds, J.P. Preservative efficacy tests in formulated nasal products: Reproducibility and factors affecting preservative activity. J. Pharm. Pharmacol. 1996, 48, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
- Drewry, D.T.; Galbraith, L.; Wilkinson, B.J.; Wilkinson, S.G. Staphylococcal slime: A cautionary tale. J. Clin. Microbiol. 1990, 28, 1292–1296. [Google Scholar] [PubMed]
- Gilbert, P.; Moore, L.E. Cationic antiseptics: Diversity of action under a common epithet. J. Appl. Microbiol. 2005, 99, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, P. The revival of micro-organisms sublethally injured by chemical inhibitors. Soc. Appl. Bacteriol. Symp. Ser. 1984, 175–197. [Google Scholar]
- Hedges, A.J. Estimating the precision of serial dilutions and viable bacterial counts. Int. J. Food Microbiol. 2002, 76, 207–214. [Google Scholar] [CrossRef]
- Ricke, S.C. Perspectives on the use of organic acids and short chain fatty acids as antimicrobials. Poult. Sci. 2003, 82, 632–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherrington, C.A.; Hinton, M.; Mead, G.C.; Chopra, I. Organic acids: Chemistry, antibacterial activity and practical applications. Adv. Microb. Physiol. 1990, 32, 87–108. [Google Scholar]
- Al-Adham, I.; Haddadin, R.; Collier, P. Types of microbicidal and microbistatic agents. In Russell, Hugo & Ayliffe’s: Principles and Practice of Disinfection, Preservation and Sterilization; Fraise, A.P., Maillard, J.-Y., Sattar, S.A., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2013; pp. 5–70. [Google Scholar]
- European Commission (Ed.) Commission Regulation (EU) No 866/2014 of 8 August 2014 Amending Annexes Iii, V and VI to Regulation (EC) No 1223/2009 of the European Parliament and the Council on Cosmetic Products; 866/2014; European Commission: Brussels, Belgium, 2014. [Google Scholar]
- Park, E.-S.; Moon, W.-S.; Song, M.-J.; Kim, M.-N.; Chung, K.-H.; Yoon, J.-S. Antimicrobial activity of phenol and benzoic acid derivatives. Int. Biodeterior. Biodegrad. 2001, 47, 209–214. [Google Scholar] [CrossRef]
- European Commission (Ed.) Commission Regulation (EU) No 344/2013 of 4 April 2013 Amending Annexes II, III, V and VI to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on Cosmetic Products; 344; European Commission: Brussels, Belgium, 2013; Volume 344. [Google Scholar]
- European Commission (Ed.) Commission Regulation (EU) No 358/2014 of 9 April 2014 Amending Annexes II and V to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on Cosmetic Products; 358/2014; European Commission: Brussels, Belgium, 2014. [Google Scholar]
- European Commission (Ed.) Commission Regulation (EU) No 1004/2014 of 18 September 2014 Amending Annex V to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on Cosmetic Products; 1004/2014; European Commission: Brussels, Belgium, 2014. [Google Scholar]
- Ministry of Health, Labour and Welfare. Standard for Cosmetics; Ed.; Ministry of Health, Labour and Welfare: Tokyo, Japan, 2000; Volume 331, pp. 1–8. [Google Scholar]
- De Groot, A.C.; Veenstra, M. Formaldehyde-releasers in cosmetics in the USA and in Europe. Contact Dermat. 2010, 62, 221–224. [Google Scholar] [CrossRef] [PubMed]
- De Groot, A.C.; White, I.R.; Flyvholm, M.A.; Lensen, G.; Coenraads, P.J. Formaldehyde- releasers in cosmetics: Relationship to formaldehyde contact allergy part 1. Characterization, frequency and relevance of sensitization, and frequency of use in cosmetics. Contact Dermat. 2010, 62, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Polati, S.; Gosetti, F.; Gennaro, M.C. Preservatives in cosmetics. Regulatory aspects and analytical methods In Analysis of Cosmetic Products; Chisvert, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 211–241. [Google Scholar]
- Lv, C.; Hou, J.; Xie, W.; Cheng, H. Investigation on formaldehyde release from preservatives in cosmetics. Int. J. Cosmet. Sci. 2015, 37, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Rowe, R.C.; Sheskey, P.J.; Quinn, M.E. Handbook of Pharmaceutical Excipients; Pharmaceutical Press: London, UK, 2009. [Google Scholar]
- De Groot, A.C.; Flyvholm, M.A.; Lensen, G.; Menné, T.; Coenraads, P.J. Formaldehyde-releasers: Relationship to formaldehyde contact allergy. Contact allergy to formaldehyde and inventory of formaldehyde releasers. Contact Dermat. 2009, 61, 63–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aerts, O.; Lambert, J.; Goossens, A. Isothiazolinone derivatives: Chemical structure and cross-reactivity patterns. Revue Française d’Allergologie 2017, 57, 178–180. [Google Scholar] [CrossRef]
- Xia, S.; Sun, W.; Yu, L.; Hua, Z. Qsar studies on the antibacterial activity of some substituted 3-lsothiazolinones. Acta Chim. Sin. Chin. Ed. 2007, 65, 2707–2714. [Google Scholar]
- Rezaee, S.; Khalaj, A.; Adibpour, N.; Saffary, M. Correlation between lipophilicity and antimicrobial activity of some 2-(4-substituted phenyl)-3 (2h)-isothiazolones. DARU J. Pharm. Sci. 2015, 82, 632–639. [Google Scholar]
- Rossmoore, H.W. Nitrogen compounds. In Disinfection, Sterilization, and Preservation; Block, S.S., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2001; pp. 349–395. [Google Scholar]
- European Commission (Ed.) Commission Regulation (EU) No 1003/2014 of 18 September 2014 Amending Annex V to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on Cosmetic Products; 1003/2014; European Commission: Brussels, Belgium, 2014. [Google Scholar]
- European Commission (Ed.) Commission Regulation (EU) 2016/1198 of 22 July 2016. Amending Annex V to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on Cosmetic Products; 2016/1198; European Commission: Brussels, Belgium, 2016. [Google Scholar]
- European Commission (Ed.) Commission Regulation (EU) 2017/1224 of 6 July 2017 Amending Annex V to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on Cosmetic Products; 2017/1224; European Commission: Brussels, Belgium, 2017. [Google Scholar]
- Baker, P.J.; Coburn, R.A.; Genco, R.J.; Evans, R.T. Structural determinants of activity of chlorhexidine and alkyl bisbiguanides against the human oral flora. J. Dent. Res 1987, 66, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, G.; Russell, A.D. Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [PubMed]
- Buffet-Bataillon, S.; Tattevin, P.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Emergence of resistance to antibacterial agents: The role of quaternary ammonium compounds—A critical review. Int. J. Antimicrob. Agents 2012, 39, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Kabara, J.J.; Conley, A.J.; Truant, J.P. Relationship of chemical structure and antimicrobial activity of alkyl amides and amines. Antimicrob. Agents Chemother. 1972, 2, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Doose, C.A.; Ranke, J.; Stock, F.; Bottin-Weber, U.; Jastorff, B. Structure–activity relationships of pyrithiones–IPC-81 toxicity tests with the antifouling biocide zinc pyrithione and structural analogs. Green Chem. 2004, 6, 259–266. [Google Scholar] [CrossRef]
- Khushal, M.K.; Kishor, M.K.; Parth, A.M.; Ranjan, C.K. Synthesis of nitrogen and oxygen based pyrazole derivatives and its antitubercular and antimicrobial activity. Anti-Infect. Agents 2015, 13, 129–138. [Google Scholar]
- European Commission (Ed.) Commission Regulation (EU) 2016/1121 of 11 July 2016 Amending Annex V to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on Cosmetic Products; 2016/1121; European Commission: Brussels, Belgium, 2016. [Google Scholar]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.H.; Park, J.E.; Osaka, T.; Park, S.G. The study of antimicrobial activity and preservative effects of nanosilver ingredient. Electrochim. Acta 2005, 51, 956–960. [Google Scholar] [CrossRef]
- Davidson, P.M.; Taylor, T.M. Chemical preservatives and natural antimicrobial compounds. In Food Microbiology: Fundamentals and Frontiers, 3rd ed.; Doyle, M.P., Buchanan, R.L., Eds.; American Society of Microbiology: Washington, DC, USA, 2007. [Google Scholar]
- Ough, C.S.; Were, L. Sulfur dioxide and sulfites. In Antimicrobials in Food, 3rd ed.; Davidson, P.M., Sofos, J.N., Branen, A.L., Eds.; CRC Press: Boca Raton, FL, USA, 2005; pp. 143–167. [Google Scholar]
- Lores, M.; Llompart, M.; Alvarez-Rivera, G.; Guerra, E.; Vila, M.; Celeiro, M.; Lamas, J.P.; Garcia-Jares, C. Positive lists of cosmetic ingredients: Analytical methodology for regulatory and safety controls—A review. Anal. Chim. Acta 2016, 915, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Diaz, I.; Zafra-Gomez, A.; Ballesteros, O.; Navalon, A. Analytical methods for the determination of personal care products in human samples: An overview. Talanta 2014, 129, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Yim, E.; Baquerizo Nole, K.L.; Tosti, A. Contact dermatitis caused by preservatives. Dermatitis 2014, 25, 215–231. [Google Scholar] [CrossRef] [PubMed]
- Harvey, P.W. Parabens, oestrogenicity, underarm cosmetics and breast cancer: A perspective on a hypothesis. J. Appl. Toxicol. 2003, 23, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.D.; J ohansen, J.D.; Menne, T.; Andersen, K.E. Methyldibromo glutaronitrile contact allergy: Effect of single versus repeated daily exposure. Contact Dermat. 2005, 52, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Crépy, M.N. Dermatite de contact d’origine professionnelle: Conduite à tenir. Réferences en Santé au Travail 2013, 133, 109–122. [Google Scholar]
- Uter, W.; Yazar, K.; Kratz, E.M.; Mildau, G.; Liden, C. Coupled exposure to ingredients of cosmetic products: II. Preservatives. Contact Dermat. 2014, 70, 219–226. [Google Scholar] [CrossRef] [PubMed]
- ANSM. Portant retrait et interdiction de la fabrication, de l’importation, de l’exportation, de la distribution en gros, de la mise sur le marché à titre gratuit ou onéreux, de la détention en vue de la vente ou de la distribution à titre gratuit et de l’utilisation de produits cosmétiques contenant la substance chloroacetamide. In DECISION du 14 juin 2012; ANSM: Saint-Denis, France, 2012. [Google Scholar]
- Scientific Committee on Consumer Safety. Opinion on Parabens. Updated Request for a Scientific Opinion on Propyl- and Butylparaben; COLIPA n° P82; European Union: Brussels, Belgium, 2013. [Google Scholar]
- Heid, S.E.; Kanti, A.; McNamee, P.M.; Apel, A.W. Consumer safety considerations of cosmetic preservation. In Cosmetic Microbiology: A Practical Approach, 2nd ed.; Geis, P.A., Ed.; Taylor & Francis: New York, NY, USA, 2006. [Google Scholar]
- Belsito, D.V. Contact dermatitis: Allergic and irritant. In Clinical and Basic Immunodermatology; Gaspari, A.A., Tyring, S.K., Eds.; Springer: London, UK, 2008; pp. 171–192. [Google Scholar]
- Guin, J.D. Contact dermatitis and other contact reactions. In Allergic Diseases: Diagnosis and Treatment; Lieberman, P., Anderson, J.A., Eds.; Humana Press: Totowa, NJ, USA, 2007; pp. 249–270. [Google Scholar]
- Goossens, A. Contact-allergic reactions to cosmetics. J. Allergy 2011, 2011, 467071. [Google Scholar] [CrossRef] [PubMed]
- Gökçe, K.; Benderli, Y. Antimicrobial action of various polyacrylic acids on Streptococcus mutans and Actinomyces viscosus. Oral Health Dent. Manag. 2003, 2, 42–46. [Google Scholar]
- Review, C.I. Cosmetic Ingredients Review. Available online: https://online.personalcarecouncil.org/jsp/IngredInfoSearchResultPage.jsp?searchLetter=P&CIRR=WO98JR3 (accessed on 7 March 2018).
- White, S. Consumer research and concept development for multifunctional products. In Multifunctional Cosmetics; Schueller, R., Romanowski, P., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 2003; pp. 209–228. [Google Scholar]
- Owh, C.; Chee, P.L.; Loh, X.J. A global analysis of the personal care market. In Polymers for Personal Care Products and Cosmetics; Loh, X.J., Ed.; Royal Society of Chemistry: Cambridge, UK, 2016; pp. 1–17. [Google Scholar]
- Yin, X.L.; Loh, X.J. Polymers for personal care–natural protein-based polymers. In Polymers for Personal Care Products and Cosmetics; Loh, X.J., Ed.; Royal Society of Chemistry: Cambridge, UK, 2016; pp. 18–36. [Google Scholar]
- Loh, X.J. Perspectives on the development of the personal care industry. In Polymers for Personal Care Products and Cosmetics; Loh, X.J., Ed.; Royal Society of Chemistry: Cambridge, UK, 2016; pp. 227–231. [Google Scholar]
- Zheng, Y.J.; Loh, X.J. Natural rheological modifiers for personal care. Polym. Adv. Technol. 2016, 27, 1664–1679. [Google Scholar] [CrossRef]
- Ellingson, K.; Haas, J.P.; Aiello, A.E.; Kusek, L.; Maragakis, L.L.; Olmsted, R.N.; Perencevich, E.; Polgreen, P.M.; Schweizer, M.L.; Trexler, P. Strategies to prevent healthcare-associated infections through hand hygiene. Infect. Control Hosp. Epidemiol. 2014, 35, 937–960. [Google Scholar] [CrossRef] [PubMed]
- Judah, G.; Aunger, R.; Schmidt, W.P.; Michie, S.; Granger, S.; Curtis, V. Experimental pretesting of hand-washing interventions in a natural setting. Am. J. Public Health 2009, 99 (Suppl. S2), S405–S411. [Google Scholar] [CrossRef] [PubMed]
- Williamson, D.A.; Carter, G.P.; Howden, B.P. Current and emerging topical antibacterials and antiseptics: Agents, action, and resistance patterns. Clin. Microbiol. Rev. 2017, 30, 827–860. [Google Scholar] [CrossRef] [PubMed]
- Ortega Morente, E.; Fernández-Fuentes, M.A.; Grande Burgos, M.J.; Abriouel, H.; Pérez Pulido, R.; Gálvez, A. Biocide tolerance in bacteria. Int. J. Food Microbiol. 2013, 162, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Stratford, M.; Eklund, T. Organic acids and esters. In Food Preservatives; Russell, N.J., Gould, G.W., Eds.; Springer: Boston, MA, USA, 2003; pp. 48–84. [Google Scholar]
- Alexandre, H.; Mathieu, B.; Charpentier, C. Alteration in membrane fluidity and composition in Saccharomyces cerevisiae caused by decanoic acid and modulation of atpase activity. Microbiology 1996, 142, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Haft, R.J.; Keating, D.H.; Schwaegler, T.; Schwalbach, M.S.; Vinokur, J.; Tremaine, M.; Peters, J.M.; Kotlajich, M.V.; Pohlmann, E.L.; Ong, I.M. Correcting direct effects of ethanol on translation and transcription machinery confers ethanol tolerance in bacteria. Proc. Natl. Acad. Sci. USA 2014, 111, E2576–E2585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, P.M.; Benndorf, D.; Sa-Correia, I. Insights into Pseudomonas putida kt2440 response to phenol-induced stress by quantitative proteomics. Proteomics 2004, 4, 2640–2652. [Google Scholar] [CrossRef] [PubMed]
- Lucchini, J.J.; Corre, J.; Cremieux, A. Antibacterial activity of phenolic compounds and aromatic alcohols. Res. Microbiol 1990, 141, 499–510. [Google Scholar] [CrossRef]
- Lambert, P.A. Mechanisms of action of microbicides. In Russell, Hugo and Ayliffe’s Principles and Practice of Disinfection, Preservation and Sterilization, 5th ed.; Fraise, A., Maillard, J.Y., Sattar, S., Eds.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2012; pp. 95–107. [Google Scholar]
- Jang, H.-J.; Nde, C.; Toghrol, F.; Bentley, W.E. Microarray analysis of toxicogenomic effects of ortho-phenylphenol in Staphylococcus aureus. BMC Genom. 2008, 9, 411. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.J.; Parikh, S.; Xiao, G.; Tonge, P.J.; Kisker, C. Structural basis and mechanism of enoyl reductase inhibition by triclosan. J. Mol. Biol. 1999, 290, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, H.P. Triclosan: A widely used biocide and its link to antibiotics. FEMS Mmicrobiol. Lett. 2001, 202, 1–7. [Google Scholar] [CrossRef]
- Yueh, M.-F.; Tukey, R.H. Triclosan: A widespread environmental toxicant with many biological effects. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 251–272. [Google Scholar] [CrossRef] [PubMed]
- Garner, N.; Siol, A.; Eilks, I. Parabens as preservatives in personal care products. Chem. Action 2014, 103, 36–43. [Google Scholar]
- Nes, I.F.; Eklund, T. The effect of parabens on DNA, RNA and protein synthesis in Escherichia coli and Bacillus subtilis. J. Appl. Bacteriol. 1983, 54, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Eklund, T. Inhibition of growth and uptake processes in bacteria by some chemical food preservatives. J. Appl. Microbiol 1980, 48, 423–432. [Google Scholar] [CrossRef]
- Nguyen, T.; Clare, B.; Guo, W.; Martinac, B. The effects of parabens on the mechanosensitive channels of E. coli. Eur. Biophys. J. 2005, 34, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Yazawa, S.; Nakagawa, Y.; Sasaki, Y.; Yajima, S. Effects of alkyl parabens on plant pathogenic fungi. Bioorg. Med. Chem. Lett. 2015, 25, 1774–1777. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.H.; Djoko, K.Y.; Veyrier, F.J.; McEwan, A.G. Formaldehyde stress responses in bacterial pathogens. Front. Microbiol. 2016, 7, 257. [Google Scholar] [CrossRef] [PubMed]
- Kireche, M.; Gimenez-Arnau, E.; Lepoittevin, J.P. Preservatives in cosmetics: Reactivity of allergenic formaldehyde-releasers towards amino acids through breakdown products other than formaldehyde. Contact Dermat. 2010, 63, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Kireche, M.; Peiffer, J.L.; Antonios, D.; Fabre, I.; Gimenez-Arnau, E.; Pallardy, M.; Lepoittevin, J.P.; Ourlin, J.C. Evidence for chemical and cellular reactivities of the formaldehyde releaser bronopol, independent of formaldehyde release. Chem. Res. Toxicol. 2011, 24, 2115–2128. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum, M.; Thomson, M.; Bowman, W.; Al-Khalil, S. Mode of action of the antimicrobial compound 5-bromo-5-nitro-1, 3-dioxane (bronidox). Folia Microbiol 1986, 31, 19–31. [Google Scholar] [CrossRef]
- Ioannou, C.J.; Hanlon, G.W.; Denyer, S.P. Action of disinfectant quaternary ammonium compounds against Staphylococcus aureus. Antimicrob. Agents Chemother. 2007, 51, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Ding, Y.; Gao, Y.; Zheng, S.; Chen, F. Fluorescence enhancement effect for the determination of DNA with calcein–cetyl trimethyl ammonium bromide system. Anal. Chim. Acta 2008, 625, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Ermolayeva, E.; Sanders, D. Mechanism of pyrithione-induced membrane depolarization in Neurospora crassa. Appl. Environ. Microbiol. 1995, 61, 3385–3390. [Google Scholar] [PubMed]
- Dinning, A.J.; Al-Adham, I.S.I.; Eastwood, I.M.; Austin, P.; Collier, P.J. Pyrithione biocides as inhibitors of bacterial ATP synthesis. J. Appl. Microbiol. 1998, 85, 141–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, S.E.; Maillard, J.Y.; Russell, A.D.; Catrenich, C.E.; Charbonneau, D.L.; Bartolo, R.G. Activity and mechanisms of action of selected biocidal agents on gram-positive and -negative bacteria. J. Appl. Microbiol. 2003, 94, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.D.; Chopra, I. Understanding Antibacterial Action and Resistance; Prentice Hall: Dunfermline, UK, 1990. [Google Scholar]
- Do Couto, F.M.M.; do Nascimento, S.C.; Júnior, S.F.P.; da Silva, V.K.A.; Leal, A.F.G.; Neves, R.P. Antifungal activity of the piroctone olamine in experimental intra-abdominal candidiasis. SpringerPlus 2016, 5, 468. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Alpmann, P.; Blaum-Feder, S.; Kramer, S.; Endo, T.; Lu, D.; Carson, D.; Schmidt-Wolf, I.G. Increased in vivo efficacy of lenalidomide by addition of piroctone olamine. In Vivo 2011, 25, 99–103. [Google Scholar] [PubMed]
- Holt, K.B.; Bard, A.J. Interaction of silver (I) ions with the respiratory chain of Escherichia coli: An electrochemical and scanning electrochemical microscopy study of the antimicrobial mechanism of micromolar Ag+. Biochemistry 2005, 44, 13214–13223. [Google Scholar] [CrossRef] [PubMed]
- Randall, C.P.; Oyama, L.B.; Bostock, J.M.; Chopra, I.; O’Neill, A.J. The silver cation (Ag+): Antistaphylococcal activity, mode of action and resistance studies. J. Antimicrob. Chemother. 2013, 68, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Kim, J.Y.; Kim, J.; Lee, J.H.; Hahn, J.S.; Gu, M.B.; Yoon, J. Silver-ion-mediated reactive oxygen species generation affecting bactericidal activity. Water Res. 2009, 43, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Hobman, J.L.; Crossman, L.C. Bacterial antimicrobial metal ion resistance. J. Med. Microbiol. 2015, 64, 471–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gould, G.W. The use of other chemical preservatives: Sulfite and nitrite. In Microbiological Safety and Quality of Food; Lund, B.M., Baird-Parker, T.C., Gould, G.W., Eds.; Springer: Gaithersburg, MD, USA, 2000; pp. 200–213. [Google Scholar]
- Maillard, J.-Y. Mechanisms of bacterial resistance to microbicides. In Russell, Hugo & Ayliffe’s: Principles and Practice of Disinfection, Preservation and Sterilization; Fraise, A.P., Maillard, J.-Y., Sattar, S.A., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2013; pp. 108–120. [Google Scholar]
- Hugo, W.B. The degradation of preservatives by microorganisms. Int. Biodeterior. 1991, 27, 185–194. [Google Scholar] [CrossRef]
- McMurry, L.M.; Oethinger, M.; Levy, S.B. Overexpression of marA, soxS, or acrAB produces resistance to triclosan in laboratory and clinical strains of Escherichia coli. FEMS Microbiol. Lett. 1998, 166, 305–309. [Google Scholar] [CrossRef] [PubMed]
- McMurry, L.M.; Oethinger, M.; Levy, S.B. Triclosan targets lipid synthesis. Nature 1998, 394, 531–532. [Google Scholar] [CrossRef] [PubMed]
- Karatzas, K.A.; Randall, L.P.; Webber, M.; Piddock, L.J.; Humphrey, T.J.; Woodward, M.J.; Coldham, N.G. Phenotypic and proteomic characterization of multiply antibiotic-resistant variants of Salmonella enterica serovar typhimurium selected following exposure to disinfectants. Appl. Environ. Microbiol. 2008, 74, 1508–1516. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Pi, B.; Zhou, H.; Yu, Y.; Li, L. Triclosan resistance in clinical isolates of Acinetobacter baumannii. J. Med. Microbiol. 2009, 58, 1086–1091. [Google Scholar] [CrossRef] [PubMed]
- Mavri, A.; Smole Mozina, S. Development of antimicrobial resistance in Campylobacter jejuni and Campylobacter coli adapted to biocides. Int. J. Food Microbiol. 2013, 160, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, P.; Moreno, E.; Martinez, J.L. The biocide triclosan selects Stenotrophomonas maltophilia mutants that overproduce the smedef multidrug efflux pump. Antimicrob. Agents Chemother. 2005, 49, 781–782. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.; Tremblay, J.; Deziel, E. Swarming motility: A multicellular behaviour conferring antimicrobial resistance. Environ. Microbiol. 2009, 11, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.; Chauhan, S.; Dare, M.; Bansal, A.K. Degradation of parabens by Pseudomonas beteli and Burkholderia latens. Eur. J. Pharm. Biopharm. 2010, 75, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Davin-Regli, A.; Chollet, R.; Bredin, J.; Chevalier, J.; Lepine, F.; Pagès, J.M. Enterobacter gergoviae and the prevalence of efflux in parabens resistance. J. Antimicrob. Chemother. 2006, 57, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.S. Characterizing bacterial resistance to preservatives and disinfectants. Int. Biodeterior. Biodegradation 1998, 41, 241–245. [Google Scholar] [CrossRef]
- Manzoor, S.E.; Lambert, P.A.; Griffiths, P.A.; Gill, M.J.; Fraise, A.P. Reduced glutaraldehyde susceptibility in Mycobacterium chelonae associated with altered cell wall polysaccharides. J. Antimicrob. Chemother. 1999, 43, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Kummerle, N.; Feucht, H.H.; Kaulfers, P.M. Plasmid-mediated formaldehyde resistance in Escherichia coli: Characterization of resistance gene. Antimicrob. Agents Chemother. 1996, 40, 2276–2279. [Google Scholar] [PubMed]
- Denyer, S.P.; Maillard, J.Y. Cellular impermeability and uptake of biocides and antibiotics in gram-negative bacteria. J. Appl. Microbiol. Symp. Suppl. 2002, 92, 35S–45S. [Google Scholar] [CrossRef]
- K, P. Efflux-mediated antimicrobial resistance. In Antibiotic Discovery and Development; Dougherty, T., Pucci, M., Eds.; Springer: Boston, MA, USA, 2012; pp. 349–395. [Google Scholar]
- Boeris, P.S.; Domenech, C.E.; Lucchesi, G.I. Modification of phospholipid composition in Pseudomonas putida A ATCC 12633 induced by contact with tetradecyltrimethylammonium. J. Appl. Microbiol. 2007, 103, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Jennings, M.C.; Minbiole, K.P.C.; Wuest, W.M. Quaternary ammonium compounds: An antimicrobial mainstay and platform for innovation to address bacterial resistance. ACS Infect. Dis. 2015, 1, 288–303. [Google Scholar] [CrossRef] [PubMed]
- Levy, S.B. Active efflux, a common mechanism for biocide and antibiotic resistance. J. Appl. Microbiol. 2002, 92 (Suppl. S1), 65s–71s. [Google Scholar] [CrossRef] [PubMed]
- Kiyono, M.; Sone, Y.; Nakamura, R.; Pan-Hou, H.; Sakabe, K. The MerE protein encoded by transposon Tn21 is a broad mercury transporter in Escherichia coli. FEBS Lett. 2009, 583, 1127–1131. [Google Scholar] [CrossRef] [PubMed]




| Class | Product | Application | Targeted Microorganism | Active Ingredient | References |
|---|---|---|---|---|---|
| Leave-on products | Deodorants | Inhibit the bacterial metabolism responsible for the degradation of sweat and subsequent production of unpleasant body odor | Staphylococci and diphtheroids of the Corynebacteriaceae family | Aluminum chlorohydrate, alcohol, triclosan, 3,4,4′-trichlorocarbanilide, chlorhexidine | [20,21] |
| Antiperspirants | Suppress the release of sweat and eliminates the bacteria responsible for the unpleasant body odor production | Aluminum chlorohydrate, aluminum salts, zirconium-aluminum tetrachlorohydrex glycine complex | |||
| Rinse-off hair products | Anti-dandruff shampoos | Reduces species of Malassezia (Pityrosporum); Inhibit yeast growth and eradicate dead cells adhering to the scalp | The genus Malassezia | Zinc pyrithione, salicylic acid, imidazole derivatives, glycolic acid, steroids, coal, tar and sulfur derivatives, piroctone olamine | [20,21,22,23] |
| Skin care products | Antibacterial soap bars | Cleaning and bacterial reduction | Staphylococci, Mocrococcus, Corynebacterium sp., Streptococcus | Triclocarban, triclosan | [6,21,24,25] |
| Disinfectants | Alcohol, triclosan, natural ingredients and glycerin | ||||
| Antibacterial wipes | Benzalkonium chloride | ||||
| Face care products | Acne products and antiseptic cuticle treatment | Skin care; Cleaning and anti-acne treatments | Staphylococcus aureus, Staphylococcus epidermis, Propionibacterium acnes | Benzalkonium chloride | [8,21,24] |
| Oral care products | Toothpaste | Prevention of bacterial growth and plaque formation | Firmicutes, Bacteroidetes The families: Proteobacteria, Actinobacteria, Spirochaetes, Fusobacteria and the yeast Candida albicans | Triclosan, chlorhexidine, natural extracts | [21,24,26,27] |
| Mouthwash | Alcohol+triclosan or alcohol+chlorhexidine | ||||
| Antibacterial toothbrushes | Inhibit bacteria growth | Microban®, triclosan |
| Component | Influence | Effects | Example | References | |
|---|---|---|---|---|---|
| Solvent | Water | Negative | Main source of contamination | - | [20] |
| Ethanol | Positive | Antimicrobial agent | Ethanol (more than 30%) | ||
| Thickener and emulsifiers based on lipids | - | - | Fats, oils, waxes | ||
| Surfactants | Cationic | Positive | Perturbation of cell membranes or increase in membrane porosity which also facilitates penetration of other antimicrobial substances | Alkylamines, quaternary ammonium compounds | [20] |
| Anionic | Sulfates, sulfonates and carboxylates | ||||
| Amphoteric | Alkylamidobetain and alkylamidoglycinate | ||||
| Non-ionic | Fatty acids monoethanolamides, ethoxylated fatty alcohols and alkyl polyglucosides | ||||
| Humectants | Positive | At concentrations of 5 to 10%, they can effectively reduce the amount of biologically available water. | Sugars (sorbitol), glycerol and gylcol | [20] | |
| Gelling agents | Positive | Antimicrobial agent and reduction of biologically available water | Polyacrylic acids and hydroxypropyl methylcellulose | [20,172] | |
| Emollients | Negative | Promote the growth of microorganisms | Silicon derivatives, proteins (milk proteins and albumin hydrolyzate) | [20] | |
| Plants extracts and mineral raw materials | Positive or negative | Positive: polyphenols can exert antibacterial effect; Negative: source of contamination especially for spores, mycotoxins and Clostridium | Melissa officinalis extract, rosmarinic acid and phenylethyl alcohol | [20,100] | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halla, N.; Fernandes, I.P.; Heleno, S.A.; Costa, P.; Boucherit-Otmani, Z.; Boucherit, K.; Rodrigues, A.E.; Ferreira, I.C.F.R.; Barreiro, M.F. Cosmetics Preservation: A Review on Present Strategies. Molecules 2018, 23, 1571. https://doi.org/10.3390/molecules23071571
Halla N, Fernandes IP, Heleno SA, Costa P, Boucherit-Otmani Z, Boucherit K, Rodrigues AE, Ferreira ICFR, Barreiro MF. Cosmetics Preservation: A Review on Present Strategies. Molecules. 2018; 23(7):1571. https://doi.org/10.3390/molecules23071571
Chicago/Turabian StyleHalla, Noureddine, Isabel P. Fernandes, Sandrina A. Heleno, Patrícia Costa, Zahia Boucherit-Otmani, Kebir Boucherit, Alírio E. Rodrigues, Isabel C. F. R. Ferreira, and Maria Filomena Barreiro. 2018. "Cosmetics Preservation: A Review on Present Strategies" Molecules 23, no. 7: 1571. https://doi.org/10.3390/molecules23071571









