Vermicompost Supplementation Improves the Stability of Bioactive Anthocyanin and Phenolic Compounds in Clinacanthus nutans Lindau
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Phytochemical Screening of Bioactive Compounds in C. nutans
2.2.1. Measurement of Total Anthocyanin Content
2.2.2. Measurement of Total Phenolic Content
2.2.3. Measurement of Total Flavonoid Content
2.3. DPPH (2,2-Diphenyl-1-picrylhydrazyl) Radical Scavenging Activity Assay
2.4. ABTS (2,2′-Azinobis(3-ethylbenzothiazoline-6-sulfonic acid)) Radical Scavenging Activity Assay
2.5. Extract Stability after Storage at Different Temperatures
2.6. Statistical Analysis
3. Results and Discussion
3.1. Phytochemical Screening
3.2. Determination of Pigments Content (Total Anthocyanin, Phenolic and Flavonoid)
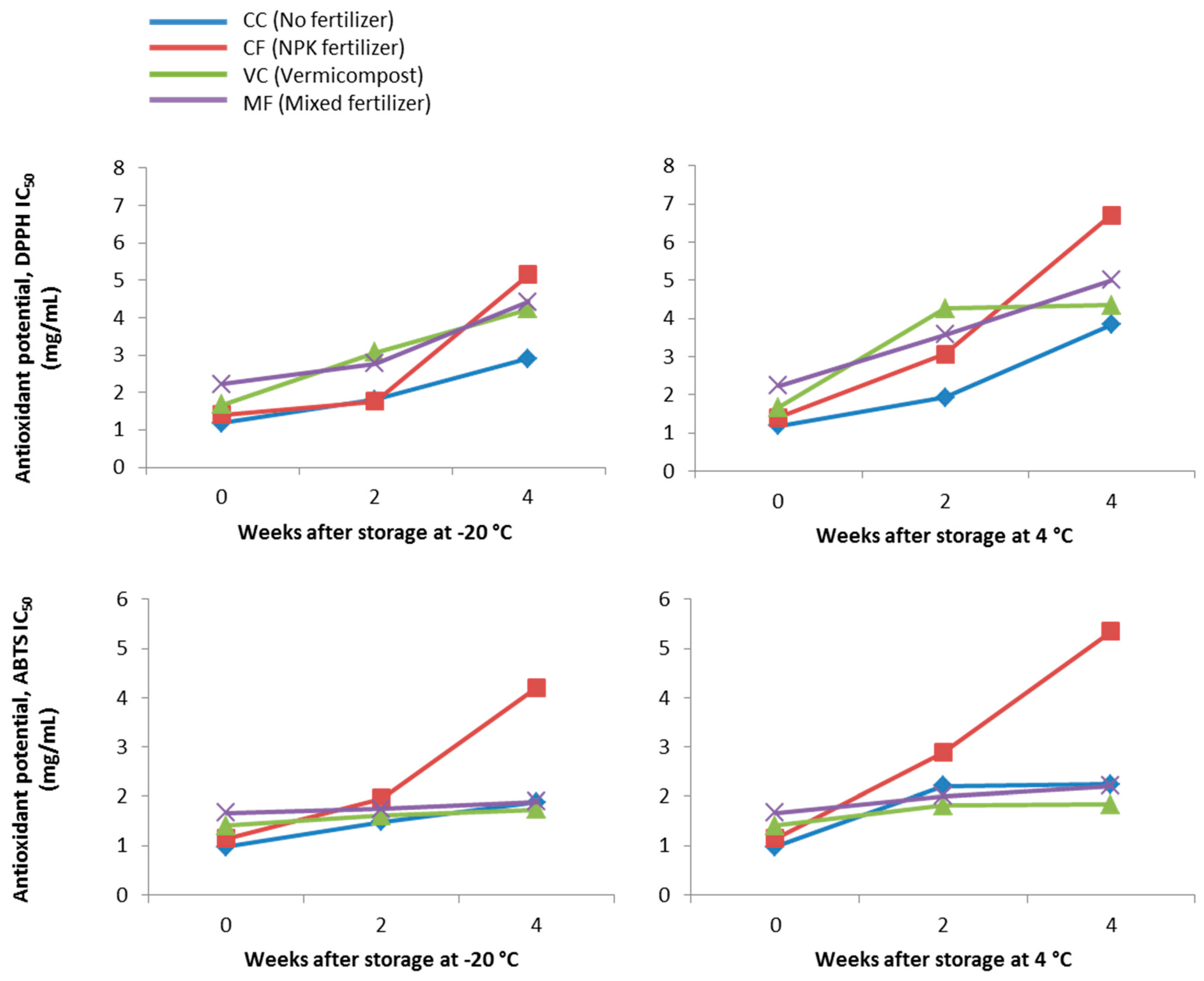
3.3. Antioxidant Potential of C. Nutans Methanolic Extracts against DPPH and ABTS Radicals
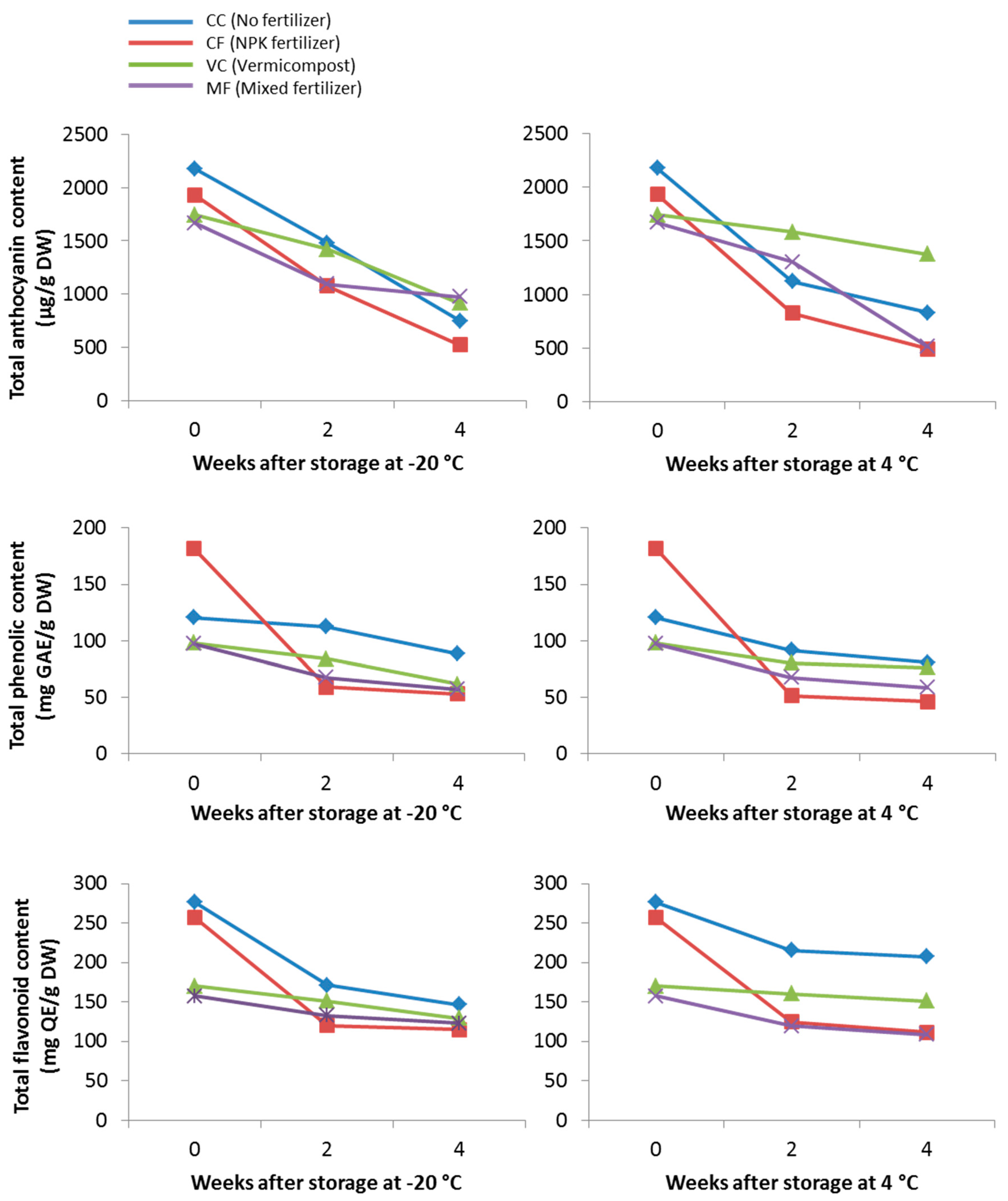
3.4. Effect of C. Nutans Extracts Storage (Duration and Temperature) on Stability of Pigments and Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zakaria, Z.A.; Rahim, M.H.A.; Mohtarrudin, N.; Kadir, A.A.; Cheema, M.S.; Ahmad, Z.; Mooi, C.S.; Tohid, S.F.M. Acute and sub-chronic oral toxicity studies of methanol extract of Clinacanthus nutans in mice. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 210–222. [Google Scholar] [CrossRef]
- Uawonggul, N.; Chaveerach, A.; Thammasirirak, S.; Arkaravichien, T.; Chuachan, C.; Daduang, S. Screening of plants acting against Heterometrus laoticus scorpion venom activity on fibroblast cell lysis. J. Ethnopharmacol. 2006, 103, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Kamarudin, M.N.A.; Sarker, M.M.R.; Kadir, H.A.; Ming, L.C. Ethnopharmacological uses, phytochemistry, biological activities, and therapeutic applications of Clinacanthus nutans (Burm. f.) Lindau: A comprehensive review. J. Ethnopharmacol. 2017, 206, 245–266. [Google Scholar] [CrossRef] [PubMed]
- Yong, Y.K.; Tan, J.J.; Teh, S.S.; Mah, S.H.; Ee, G.C.L.; Chiong, H.S.; Ahmad, Z. Clinacanthus nutans extracts are antioxidant with antiproliferative effect on cultured human cancer cell lines. Evid.-Based Complement. Altern. Med. 2013, 2013, 462751. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.; Ferdosh, S.; Ghafoor, K.; Hakim, A.; Juraimi, A.S.; Khatib, A.; Sarker, Z.I. Clinacanthus nutans: A review of the medicinal uses, pharmacology and phytochemistry. Asian Pac. J. Trop. Med. 2016, 9, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Arullappan, S.; Rajamanickam, P.; Thevar, N.; Kodimani, C.C. In vitro screening of cytotoxic, antimicrobial and antioxidant activities of Clinacanthus nutans (Acanthaceae) leaf extracts. Trop. J. Pharm. Res. 2014, 13, 1455–1461. [Google Scholar] [CrossRef]
- Wanikiat, P.; Panthong, A.; Sujayanon, P.; Yoosook, C.; Rossi, A.G.; Reutrakul, V. The anti-inflammatory effects and the inhibition of neutrophil responsiveness by Barleria lupulina and Clinacanthus nutans extracts. J. Ethnopharmacol. 2008, 116, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, M.; Hassali, M.A.; Shatar, A.K.A.; Farooqui, M.A.; Saleem, F.; ul Haq, N.; Othman, C.N. Use of complementary and alternative medicines among Malaysian cancer patients: A descriptive study. J. Tradit. Complement. Med. 2016, 6, 321–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jordan, B.R. Plant pigments and protection against UV-B radiation. In Plant Pigments Their Manipulation; Blackwell: Oxford, UK, 2004; pp. 275–292. [Google Scholar]
- Azmir, J.; Zaidul, I.; Rahman, M.; Sharif, K.; Mohamed, A.; Sahena, F.; Jahurul, M.; Ghafoor, K.; Norulaini, N.; Omar, A. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef] [Green Version]
- Gürses, A.; Açıkyıldız, M.; Güneş, K.; Gürses, M.S. Colorants in Health and Environmental Aspects. In Dyes and Pigments; Springer International Publishing: Cham, Switzerland, 2016; pp. 69–83. [Google Scholar]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed]
- Ananga, A.; Georgiev, V.; Ochieng, J.; Phills, B.; Tsolova, V. Production of anthocyanins in grape cell cultures: A potential source of raw material for pharmaceutical, food, and cosmetic industries. In The Mediterranean Genetic Code-Grapevine and Olive; In Tech: Vienna, Austria, 2013. [Google Scholar]
- Jacobo-Herrera, N.J.; Jacobo-Herrera, F.E.; Zentella-Dehesa, A.; Andrade-Cetto, A.; Heinrich, M.; Pérez-Plasencia, C. Medicinal plants used in Mexican traditional medicine for the treatment of colorectal cancer. J. Ethnopharmacol. 2016, 179, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Giusti, M.M.; Wallace, T.C. Flavonoids as Natural Pigments. In Handbook of Natural Colorants; John Wiley & Sons, Ltd.: Chichester, UK, 2009; pp. 255–275. [Google Scholar]
- Mateus, N.; de Freitas, V. Anthocyanins as Food Colorants. In Anthocyanins: Biosynthesis, Functions, and Applications; Winefield, C., Davies, K., Gould, K., Eds.; Springer: New York, NY, USA, 2009; pp. 284–304. [Google Scholar]
- Kim, S.H. Functional Dyes; Elsevier Science: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Ayalew, W.A.; Ayele, D.W. Dye-sensitized solar cells using natural dye as light-harvesting materials extracted from Acanthus sennii chiovenda flower and Euphorbia cotinifolia leaf. J. Sci. Adv. Mater. Devices 2016, 1, 488–494. [Google Scholar] [CrossRef]
- Mustroph, H.; Stollenwerk, M.; Bressau, V. Current Developments in Optical Data Storage with Organic Dyes. Angew. Chem. Int. Ed. 2006, 45, 2016–2035. [Google Scholar] [CrossRef] [PubMed]
- El-Shishtawy, R.M. Functional Dyes, and Some Hi-Tech Applications. Int. J. Photoenergy 2009, 2009, 434897. [Google Scholar] [CrossRef]
- Ramasamy, P.K.; Suresh, S.N. Effect of vermicompost on root numbers and length of sunflower plant (Helianthus annuus L.). J. Pure Appl. Microbiol. 2010, 4, 297–302. [Google Scholar]
- Chiluvuru, N.; Tartte, V.; Kalla, C.M.; Kommalapati, R. Plant bioassay for assessing the effects of vermicompost on growth and yield of Vigna radiata and Centella asiatica, two important medicinal plants. J. Dev. Sustain. Agric. 2009, 4, 160–164. [Google Scholar]
- Sundararasu, K.; Neelanarayanan, P. Effect of vermicompost and inorganic fertilizer on the growth and yield of tomato, Lycorpersium esculentum L. Int. J. Curr. Res. 2012, 4, 049–051. [Google Scholar]
- Kashem, M.A.; Sarker, A.; Hossain, I.; Islam, M.S. Comparison of the effect of vermicompost and inorganic fertilizers on vegetative growth and fruit production of tomato (Solanum lycopersicum L.). Open J. Soil Sci. 2015, 5, 53. [Google Scholar] [CrossRef]
- Yang, H.S.; Peng, T.W.; Madhavan, P.; Abdul, M.S.S.; Akowuah, G.A. Phytochemical Analysis and Antibacterial activity of Methanolic extract of Clinacanthus nutans Leaf. Int. J. Drug Dev. Res. 2013, 5, 349–355. [Google Scholar]
- Zucco, M.A.; Walters, S.A.; Chong, S.-K.; Klubek, B.P.; Masabni, J.G. Effect of soil type and vermicompost applications on tomato growth. Int. J. Recycl. Org. Waste Agric. 2015, 4, 135–141. [Google Scholar] [CrossRef]
- Cabanas-Echevarría, M.; Torres–García, A.; Díaz-Rodríguez, B.; Ardisana, E.F.H.; Creme-Ramos, Y. Influence of three bioproducts of organic origin on the production of two banana clones (Musa spp. AAB.) obtained by tissue cultures. Alimentaria 2005, 369, 111–116. [Google Scholar]
- Acevedo, I.C.; Pire, R. Effects of vermicompost as substrate amendment on the growth of papaya (Carica papaya L.). Interciencia 2004, 29, 274–279. [Google Scholar]
- Sardoei, A.S.; Roien, A.; Sadeghi, T.; Shahadadi, F.; Mokhtari, T.S. Effect of Vermicompost on the Growth and Flowering of African Marigold (Tagetes erecta). Am.-Euras. J. Agric. Environ. Sci. 2014, 14, 631–635. [Google Scholar]
- Chattopadhyay, A. Effect of Vermiwash and Vermicompost on an Ornamental Flower, Zinnia sp. J. Hortic. 2014, 1, 112. [Google Scholar] [CrossRef]
- Donald, D.G.M.; Visser, L.B. Vermicompost as a possible growth medium for the production of commercial forest nursery stock. Appl. Plant Sci. 1989, 3, 110–113. [Google Scholar]
- Lazcano, C.; Sampedro, L.; Zas, R.; Domínguez, J. Vermicompost enhances germination of the maritime pine (Pinus pinaster Ait.). New For. 2010, 39, 387–400. [Google Scholar] [CrossRef] [Green Version]
- Lazcano, C.; Sampedro, L.; Zas, R.; Domínguez, J. Assessment of plant growth promotion by vermicompost in different progenies of maritime pine (Pinus pinaster Ait.). Compost Sci. Util. 2010, 18, 111–118. [Google Scholar] [CrossRef]
- Solihah, M.; Wan Rosli, W.; Nurhanan, A. Phytochemicals screening and total phenolic content of Malaysian Zea mays hair extracts. Int. Food Res. J. 2012, 19, 1533–1538. [Google Scholar]
- Giusti, M.M.; Wrolstad, R.E. Characterization and measurement of anthocyanins by UV-visible spectroscopy. In Current Protocols in Food Analytical Chemistry; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2001. [Google Scholar]
- Sun, T.; Powers, J.R.; Tang, J. Effect of enzymatic macerate treatment on rutin content, antioxidant activity, yield, and physical properties of asparagus juice. J. Food Sci. 2007, 72, S267–S271. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-Y.; Tang, C.-Y. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem. 2007, 101, 140–147. [Google Scholar] [CrossRef]
- Sulaiman, S.F.; Ooi, K.L. Polyphenolic and vitamin C contents and antioxidant activities of aqueous extracts from mature-green and ripe fruit fleshes of Mangifera sp. J. Agric. Food Chem. 2012, 60, 11832–11838. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Chen, X.; Sun, P. Chemical characterization, antioxidant and antitumor activity of sulfated polysaccharide from Sargassum horneri. Carbohydr. Polym. 2014, 105, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Abdul Rahim, M.H.; Zakaria, Z.A.; Mohd Sani, M.H.; Omar, M.H.; Yakob, Y.; Cheema, M.S.; Ching, S.M.; Ahmad, Z.; Abdul Kadir, A. Methanolic extract of Clinacanthus nutans exerts antinociceptive activity via the opioid/nitric oxide-mediated, but cGMP-independent, pathways. Evid.-Based Complement. Altern. Med. 2016, 2016, 1494981. [Google Scholar] [CrossRef] [PubMed]
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Almeida, M.M.B.; de Sousa, P.H.M.; Arriaga, Â.M.C.; do Prado, G.M.; de Carvalho Magalhães, C.E.; Maia, G.A.; de Lemos, T.L.G. Bioactive compounds and antioxidant activity of fresh exotic fruits from northeastern Brazil. Food Res. Int. 2011, 44, 2155–2159. [Google Scholar] [CrossRef]
- Arnao, M.B. Some methodological problems in the determination of antioxidant activity using chromogen radicals: A practical case. Trends Food Sci. Technol. 2000, 11, 419–421. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and mechanisms of antioxidant activity using the DPPH. free radical method. LWT-Food Sci. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Kim, D.-O.; Lee, K.W.; Lee, H.J.; Lee, C.Y. Vitamin C equivalent antioxidant capacity (VCEAC) of phenolic phytochemicals. J. Agric. Food Chem. 2002, 50, 3713–3717. [Google Scholar] [CrossRef] [PubMed]
- Coria-Cayupán, Y.S.; Sánchez de Pinto, M.A.I.; Nazareno, M.N.A. Variations in bioactive substance contents and crop yields of lettuce (Lactuca sativa L.) cultivated in soils with different fertilization treatments. J. Agric. Food Chem. 2009, 57, 10122–10129. [Google Scholar] [CrossRef] [PubMed]
- Pant, A.; Radovich, T.J.K.; Hue, N.V.; Arancon, N.Q. Effects of vermicompost tea (aqueous extract) on pak choi yield, quality, and on soil biological properties. Compost Sci. Util. 2011, 19, 279–292. [Google Scholar] [CrossRef]
- Luján-Hidalgo, M.C.; Pérez-Gómez, L.E.; Abud-Archila, M.; Meza-Gordillo, R.; Ruiz-Valdiviezo, V.M.; Dendooven, L.; Gutiérrez-Miceli, F.A. Growth, phenolic content and antioxidant activity in Chincuya (Annona purpurea Moc & Sesse ex Dunal) cultivated with vermicompost and phosphate rock. Compost Sci. Util. 2015, 23, 276–283. [Google Scholar]
- Ksouri, R.; Megdiche, W.; Debez, A.; Falleh, H.; Grignon, C.; Abdelly, C. Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritima. Plant Physiol. Biochem. 2007, 45, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Downey, P.J.; Levine, L.H.; Musgrave, M.E.; McKeon-Bennett, M.; Moane, S. Effect of hypergravity and phytohormones on isoflavonoid accumulation in soybean (Glycine max L.) callus. Micrograv. Sci. Technol. 2013, 25, 9–15. [Google Scholar] [CrossRef]
- Adhikary, S. Vermicompost, the story of organic gold: A review. Agric. Sci. 2012, 3, 905. [Google Scholar] [CrossRef]
- Moldovan, B.; Popa, A.; David, L. Effects of storage temperature on the total phenolic content of Cornelian Cherry (Cornus mas L.) fruits extracts. J. Appl. Bot. Food Qual. 2016, 89. [Google Scholar] [CrossRef]
- Del-Toro-Sánchez, C.L.; Gutiérrez-Lomelí, M.; Lugo-Cervantes, E.; Zurita, F.; Robles-García, M.A.; Ruiz-Cruz, S.; Aguilar, J.A.; Rio, M.-D.; Alfredo, J.; Guerrero-Medina, P.J. Storage effect on phenols and on the antioxidant activity of extracts from Anemopsis californica and inhibition of elastase enzyme. J. Chem. 2015, 2015, 602136. [Google Scholar] [CrossRef]
- Ferrante, A.; Maggiore, T. Chlorophyll a fluorescence measurements to evaluate storage time and temperature of Valeriana leafy vegetables. Postharvest Biol. Technol. 2007, 45, 73–80. [Google Scholar] [CrossRef]
- Varela-Santos, E.; Ochoa-Martinez, A.; Tabilo-Munizaga, G.; Reyes, J.E.; Pérez-Won, M.; Briones-Labarca, V.; Morales-Castro, J. Effect of high hydrostatic pressure (HHP) processing on physicochemical properties, bioactive compounds and shelf-life of pomegranate juice. Innov. Food Sci. Emerg. Technol. 2012, 13, 13–22. [Google Scholar] [CrossRef]
- Serea, C.; Barna, O.; Manley, M.; Kidd, M. Effect of storage temperature on the ascorbic acid content, total phenolic content and antioxidant activity in lettuce (Lactuca sativa L.). J. Anim. Plant Sci. 2014, 24, 1173–1177. [Google Scholar]
- Lee, C.Y.; Kagan, V.; Jaworski, A.W.; Brown, S.K. Enzymic browning in relation to phenolic compounds and polyphenoloxidase activity among various peach cultivars. J. Agric. Food Chem. 1990, 38, 99–101. [Google Scholar] [CrossRef]
- Janovitz-Klapp, A.H.; Richard, F.C.; Goupy, P.M.; Nicolas, J.J. Kinetic studies on apple polyphenol oxidase. J. Agric. Food Chem. 1990, 38, 1437–1441. [Google Scholar] [CrossRef]
- Kim, D.O.; Padilla-Zakour, O.I. Jam processing effect on phenolics and antioxidant capacity in anthocyanin-rich fruits: Cherry, plum, and raspberry. J. Food Sci. 2004, 69, S395–S400. [Google Scholar] [CrossRef]
- He, J. Isolation of Anthocyanin Mixtures from Fruits and Vegetables and Evaluation of Their Stability, Availability and Biotransformation in the Gastrointestinal Tract. Ph.D. Thesis, The Ohio State University, Columbus, OH, USA, 2008. [Google Scholar]
- Shen, W.; Nada, K.; Tachibana, S. Involvement of polyamines in the chilling tolerance of cucumber cultivars. Plant Physiol. 2000, 124, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Holm, L.; Johansson, E. Soil and starter fertilizer and its effect on yield and protein composition of malting barley. J. Soil Sci. Plant Nutr. 2012, 12, 835–849. [Google Scholar] [CrossRef]
- Trombley, J.D.; Loegel, T.N.; Danielson, N.D.; Hagerman, A.E. Capillary electrophoresis methods for the determination of covalent polyphenol–protein complexes. Anal. Bioanal. Chem. 2011, 401, 1523–1529. [Google Scholar] [CrossRef] [PubMed]
- Le Bourvellec, C.; Renard, C. Interactions between polyphenols and macromolecules: Quantification methods and mechanisms. Crit. Rev. Food Sci. Nutr. 2012, 52, 213–248. [Google Scholar] [CrossRef] [PubMed]
- Ozdal, T.; Capanoglu, E.; Altay, F. A review on protein–phenolic interactions and associated changes. Food Res. Int. 2013, 51, 954–970. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| Phytochemical | Treatment | |||
|---|---|---|---|---|
| No Fertilizer (CC) | NPK Fertilizer (CF) | Vermicompost (VC) | Mixed Fertilizer (MF) | |
| Alkaloid | − | − | − | − |
| Tannin | − | − | − | − |
| Phenol | + | + | + | + |
| Flavonoid | + | + | + | + |
| Saponin | + | + | + | + |
| Treatment | Sample ID | Total Anthocyanin Content (µg/g DW) | Total Phenolic Content (mg GAE/g DW) | Total Flavonoid Content (mg QE/g DW) |
|---|---|---|---|---|
| No fertilizer | CC | 2180.14 ± 338.43 a | 120.48 ± 6.70 a | 276.25 ± 3.09 b |
| NPK fertilizer | CF | 1933.52 ± 66.06 a | 181.53 ± 35.58 b | 256.66 ± 45.43 b |
| Vermicompost | VC | 1742.86 ± 62.30 a | 98.06 ± 2.27 a | 170.42 ± 7.55 a |
| Mixed fertilizer | MF | 1669.91 ± 122.12 a | 97.47 ± 18.73 a | 157.30 ± 26.42 a |
| Treatment | Sample ID | DPPH IC50 (mg/mL) | ABTS IC50 (mg/mL) |
|---|---|---|---|
| No fertilizer | CC | 1.18 ± 0.05 a | 0.98 ± 0.05 a |
| NPK fertilizer | CF | 1.41 ± 0.02 b | 1.15 ± 0.16 a,b |
| Vermicompost | VC | 1.67 ± 0.04 c | 1.41 ± 0.01 b,c |
| Mixed fertilizer | MF | 2.23 ± 0.02 d | 1.67 ± 0.17 c |
| Variables | TFC | TPC | TAC | DPPH | ABTS |
|---|---|---|---|---|---|
| TFC | 1 | ||||
| TPC | 0.834 ** | 1 | |||
| TAC | 0.399 ** | 0.429 ** | 1 | ||
| DPPH | −0.465 ** | −0.464 ** | −0.518 ** | 1 | |
| ABTS | −0.376 ** | −0.401 ** | −0.427 ** | 0.545 ** | 1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yusof, Z.; Ramasamy, S.; Mahmood, N.Z.; Yaacob, J.S. Vermicompost Supplementation Improves the Stability of Bioactive Anthocyanin and Phenolic Compounds in Clinacanthus nutans Lindau. Molecules 2018, 23, 1345. https://doi.org/10.3390/molecules23061345
Yusof Z, Ramasamy S, Mahmood NZ, Yaacob JS. Vermicompost Supplementation Improves the Stability of Bioactive Anthocyanin and Phenolic Compounds in Clinacanthus nutans Lindau. Molecules. 2018; 23(6):1345. https://doi.org/10.3390/molecules23061345
Chicago/Turabian StyleYusof, Zuhaili, Sujatha Ramasamy, Noor Zalina Mahmood, and Jamilah Syafawati Yaacob. 2018. "Vermicompost Supplementation Improves the Stability of Bioactive Anthocyanin and Phenolic Compounds in Clinacanthus nutans Lindau" Molecules 23, no. 6: 1345. https://doi.org/10.3390/molecules23061345






