Design, Synthesis, and Antifungal Activity of Novel Aryl-1,2,3-Triazole-β-Carboline Hybrids
Abstract
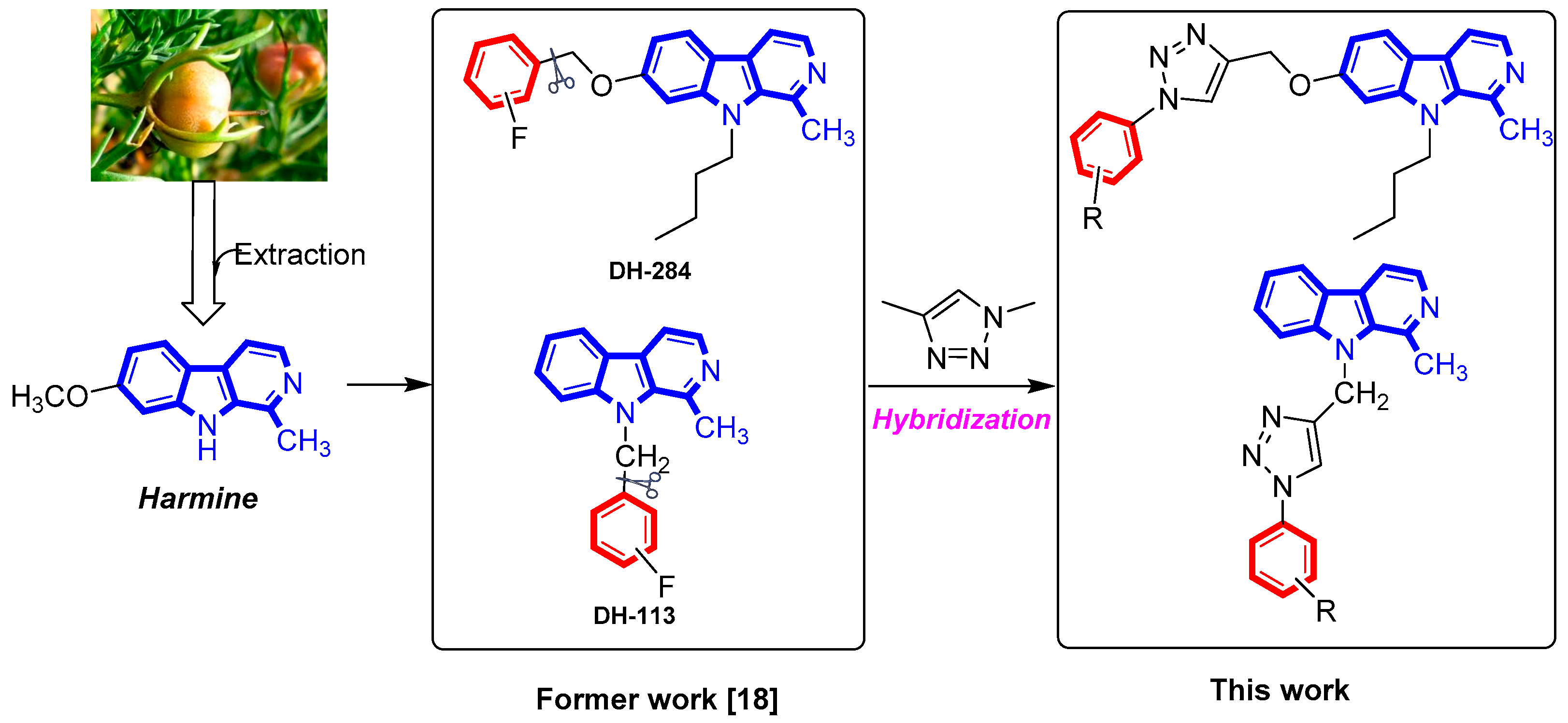
:1. Introduction
2. Results and Discussion
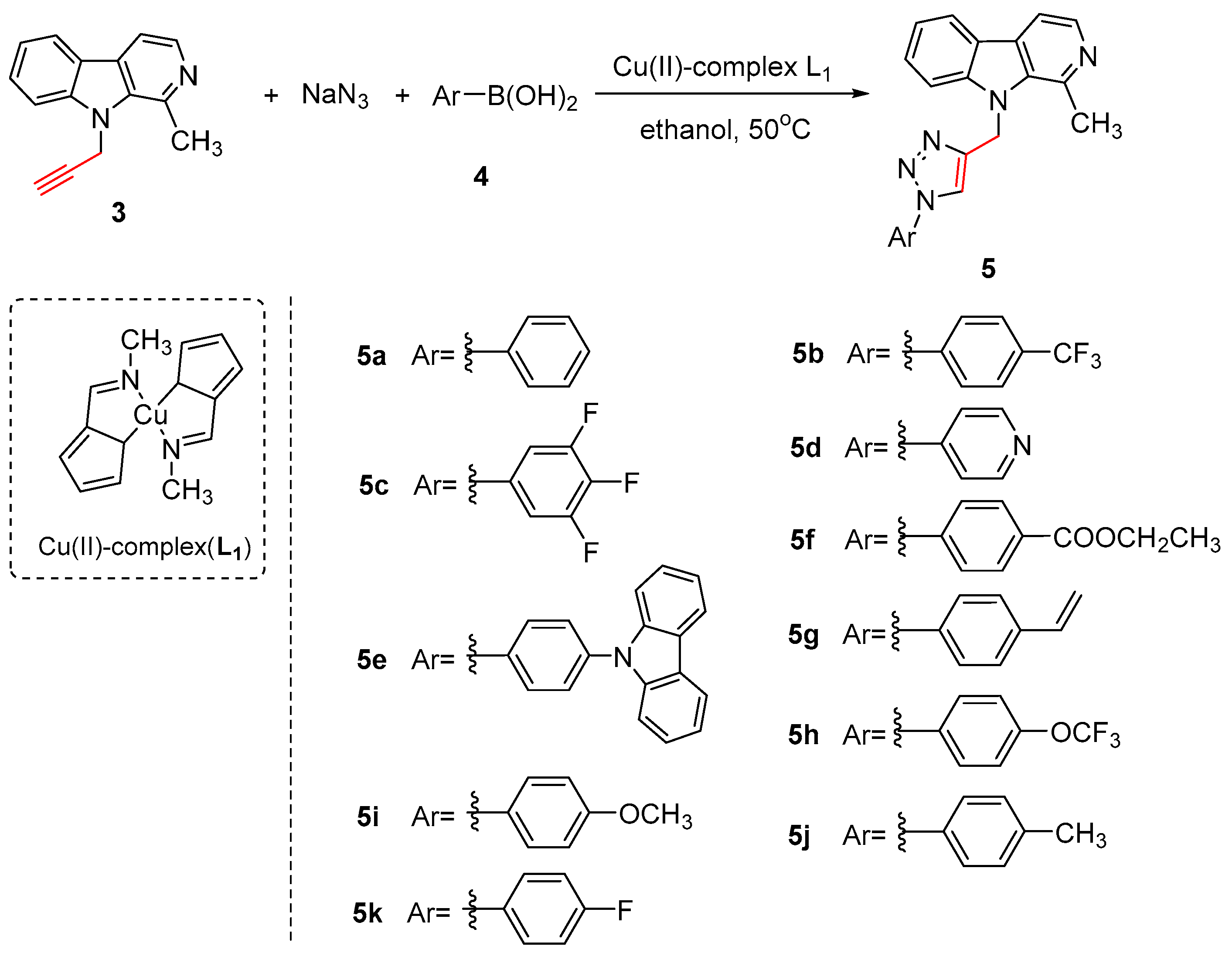
2.1. Chemistry
2.2. Fungicidal Activities
3. Materials and Methods
3.1. General Information
3.2. Synthesis of 1-Methyl-9-(prop-2-yn-1-yl)-β-carboline (3)
3.3. Synthesis of 9-Butyl-1-Methyl-7-(Prop-2-yn-1-yloxy)-β-Carboline (8)
3.4. General Procedure for the Synthesis of 1,2,3-Triazolyl-β-Carboline Hybrids (5 and 9)
3.5. Biological Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dayan, F.E.; Cantrell, C.L.; Duke, S.O. Natural products in crop protection. Bioorg. Med. Chem. 2009, 17, 4022–4034. [Google Scholar] [CrossRef] [PubMed]
- Rosell, G.; Quero, C.; Coll, J.; Guerrero, A. Biorational insecticides in pest management. J. Pestic. Sci. 2008, 33, 103–121. [Google Scholar] [CrossRef] [Green Version]
- Petroski, R.J.; Stanley, D.W. Natural compounds for pest and weed control. J. Agric. Food Chem. 2009, 57, 8171–8179. [Google Scholar] [CrossRef] [PubMed]
- Crombie, L. Natural product chemistry and its part in the defence against insects and fungi in agriculture. Pestic. Sci. 1999, 55, 761–774. [Google Scholar] [CrossRef]
- Meester, C.D. Genotoxic potential of β-carbolines: A review. Mutat. Res. 1995, 339, 139–153. [Google Scholar] [CrossRef]
- Cain, M.; Weber, R.W.; Guzman, F.; Cook, J.M.; Barker, S.A.; Rice, K.C.; Crawley, J.N.; Paul, S.M.; Skolnick, P. β-Carbolines: Synthesis and neurochemical and pharmacological actions on brain benzodiazepine receptors. J. Med. Chem. 1982, 25, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Bournine, L.; Bensalem, S.; Fatmi, S.; Bedjou, F.; Mathieu, V.; Iguer-Ouada, M.; Kiss, R.; Duez, P. Evaluation of the cytotoxic and cytostatic activities of alkaloid extracts from different parts of Peganum harmala L. (Zygophyllaceae). Eur. J. Integr. Med. 2017, 9, 91–96. [Google Scholar] [CrossRef]
- Asgarpanah, J.; Ramezanloo, F. Chemistry, pharmacology and medicinal properties of Peganum harmala L. Afr. J. Pharm. Pharmacol. 2012, 6, 1573–1580. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Agarwal, A.; Chauhan, P.M.S.; Agarwal, S.K.; Bhaduri, A.P.; Singh, S.N.; Fatima, N.; Chatterjee, R.K. Potent 1,3-disubstituted-9H-pyrido[3,4-b] indoles as new lead compounds in antifilarial chemotherapy. Bioorg. Med. Chem. 1999, 7, 1223–1236. [Google Scholar] [CrossRef]
- Wang, Y.H.; Tang, J.G.; Wang, R.R.; Yang, L.M.; Dong, Z.J.; Du, L.; Shen, X.; Liu, J.K.; Zheng, Y.T. Flazinamide, a novel β-carboline compound with anti-HIV actions. Biochem. Biophys. Res. Commun. 2007, 355, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.J.; Zhang, J.J.; Jiang, Z.Y.; Zhong, G.H. Design, Synthesis and Bioactivity Evaluation of Novel β-carboline 1,3,4-oxadiazole Derivatives. Molecules 2017, 22, 1811. [Google Scholar] [CrossRef] [PubMed]
- Nenaah, G. Toxicity and growth inhibitory activities of methanol extract and the beta-carboline alkaloids of Peganum harmala L. against two coleopteran stored-grain pests. J. Stored Prod. Res. 2011, 47, 255–261. [Google Scholar] [CrossRef]
- Abbasipour, H.; Mahmoudvand, M.; Rastegar, F.; Basij, M. Insecticidal activity of Peganum harmala seed extract against the diamondback moth, Plutella xylostella. Bull. Insectol. 2010, 63, 259–263. [Google Scholar]
- Shonouda, M.; Osman, S.; Salama, O.; Ayoub, A. Toxical effect of Peganum harmala L. leaves on the cotton leaf worm, Spodoptera littoralis boisd and its parasitoids Microplitis rufiventris Kok. Pak. J. Biol. Sci. 2008, 11, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Song, H.J.; Liu, Y.X.; Liu, Y.X.; Wang, Q.M. Synthesis and antiviral and fungicidal activity evaluation of β-carboline, dihydro-β-carboline, tetrahydro-β-carboline alkaloids, and their derivatives. J. Agric. Food Chem. 2014, 62, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Q.; Liu, Y.X.; Liu, Y.X.; Song, H.J.; Wang, Q.M. C ring may be dispensable for β-carboline: Design, synthesis, and bioactivities evaluation of tryptophan analog derivatives based on the biosynthesis of β-carboline alkaloids. Bioorg. Med. Chem. 2016, 24, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.B.; Chen, S.H.; Zhu, S.W.; Luo, J.J.; Zhang, Y.M.; Weng, Q.F. Synthesis and fungicidal activity of β-carboline alkaloids and their derivatives. Molecules 2015, 20, 13941–13957. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.Y.; Guo, L.; Wei, Y.T.; Zhang, J.; Han, X.Q. Synthesis and Fungicidal Activity of Novel β-Carboline Derivatives. Agrochemicals 2018, 57, 3–6. [Google Scholar] [CrossRef]
- Ruddarraju, R.R.; Murugulla, A.C.; Kotla, R.; Tirumalasetty, M.C.B.; Wudayagiri, R.; Donthabakthuni, S.; Maroju, R.; Baburao, K.; Parasa, L.S. Design, synthesis, anticancer, antimicrobial activities and molecular docking studies of theophylline containing acetylenes and theophylline containing 1,2,3-triazoles with variant nucleoside derivatives. Eur. J. Med. Chem. 2016, 123, 379–396. [Google Scholar] [CrossRef] [PubMed]
- Kant, R.; Kumar, D.; Agarwal, D.; Gupta, R.D.; Tilak, R.; Awasthi, S.K.; Agarwal, A. Synthesis of newer 1,2,3-triazole linked chalcone and flavone hybrid compounds and evaluation of their antimicrobial and cytotoxic activities. Eur. J. Med. Chem. 2016, 113, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.P.; Hu, H.W.; Zhang, M.; Hu, X.H.; Chen, M.; Chen, D.C.; Liu, J.W.; Xiao, G.F.; Wang, Y.; Wen, Z.L. A mini review of the synthesis of poly-1,2,3-triazole-based functional materials. RSC Adv. 2017, 7, 2281–2287. [Google Scholar] [CrossRef] [Green Version]
- Floros, M.C.; Bortolatto, J.F.; Oliveira, J.O.B.; Salvador, S.L.; Narine, S.S. Antimicrobial activity of amphiphilic triazole-linked polymers derived from renewable sources. ACS Biomater. Sci. Eng. 2016, 2, 336–343. [Google Scholar] [CrossRef]
- Vatmurge, N.S.; Hazra, B.G.; Pore, V.S.; Shirazi, F.; Chavan, P.S.; Dehpande, M.V. Synthesis and antimicrobial activity of β-lactam-bile acid conjugates linked via triazole. Bioorg. Med. Chem. Lett. 2008, 18, 2043–2047. [Google Scholar] [CrossRef] [PubMed]
- Jagasia, R.; Holub, J.M.; Bollinger, M.; Kirshenbaum, K.; Finn, M.G. Peptide Cyclization and Cyclodimerization by CuI-Mediated Azide−Alkyne Cycloaddition. J. Org. Chem. 2009, 74, 2964–2974. [Google Scholar] [CrossRef] [PubMed]
- Huber, D.; Hübner, H.; Gmeiner, P. 1,1′-Disubstituted Ferrocenes as Molecular Hinges in Mono- and Bivalent Dopamine Receptor Ligands. J. Med. Chem. 2009, 52, 6860–6870. [Google Scholar] [CrossRef] [PubMed]
- Dubovis, M.V.; Rudakov, G.F.; Kulagin, A.S.; Tsarkova, K.V.; Popkov, S.V.; Goloveshkin, A.S.; Cherkaev, G.V. A new method of synthesis of substituted 1-(1H-imidazole-4-yl)-1H-1,2,3-triazoles and their fungicidal activity. Tetrahedron 2018, 74, 672–683. [Google Scholar] [CrossRef]
- Zheng, X.C.; Wan, Y.J.; Ling, F.; Ma, C. Copper-Catalyzed Tandem Reaction of Terminal Alkynes and Sulfonyl Azides for the Assembly of Substituted Aminotriazoles. Org. Lett. 2017, 19, 3859–3862. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.B.; Zhou, C.J.; Xie, J.W.; Zhang, J.; Liu, P.; Dai, B. An Efficient Copper-Catalyzed One-Pot Synthesis of 1-Aryl-1,2,3-triazoles from Arylboronic Acids in Water under Mild Conditions. Chin. J. Chem. 2015, 33, 1317–1320. [Google Scholar] [CrossRef]
- Zhou, C.J.; Zhang, J.; Liu, P.; Xie, J.W.; Dai, B. 2-Pyrrolecarbaldiminato–Cu(II) complex catalyzed three-component 1,3-dipolar cycloaddition for 1,4-disubstituted 1,2,3-triazoles synthesis in water at room temperature. RSC Adv. 2015, 5, 6661–6665. [Google Scholar] [CrossRef]
- Cao, R.H.; Chen, Q.; Hou, X.R.; Chen, H.S.; Guan, H.J.; Ma, Y.; Peng, W.L.; Xu, A.L. Synthesis, acute toxicities and antitumor effects of novel 9-substituted β-carboline derivatives. Bioorg. Med. Chem. 2004, 12, 4613–4623. [Google Scholar] [CrossRef] [PubMed]
- Xinjiang Huashidan Pharmaceutical Research Co., Ltd. Harmine Derivatives, Intermediates Used in Their Preparation, Preparation Processes and Use Thereof. EP 1634881 A1, 15 March 2006. [Google Scholar]
- Cao, R.H.; Guan, X.D.; Shi, B.X.; Chen, Z.Y.; Ren, Z.H.; Peng, W.L.; Song, H.C. Design, synthesis and 3D-QSAR of β-carboline derivatives as potent antitumor agents. Eur. J. Med. Chem. 2010, 45, 2503–2515. [Google Scholar] [CrossRef] [PubMed]
- Du, S.J.; Lu, H.Z.; Yang, D.Y.; Li, H.; Gu, X.L.; Wan, C.; Jia, C.Q.; Wang, M.; Li, X.Y.; Qin, Z.H. Synthesis, Antifungal Activity and QSAR of Some Novel Carboxylic Acid Amides. Molecules 2015, 20, 4071–4087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of the compounds 5a–k, 9a–f are available from the authors. |


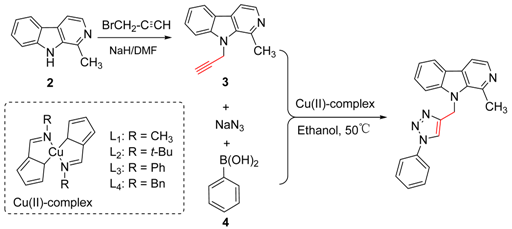 | |||
|---|---|---|---|
| Entry | Catalyst (mol %) | t/h | Yield/% |
| 1 | L1(1) | 8 + 2 | 84 |
| 2 | L2(1) | 8 + 2 | 80 |
| 3 | L3(1) | 8 + 2 | 69 |
| 4 | L4(1) | 8 + 2 | 71 |
| 5 | - | 8 + 2 | 0 |
| 6 | L1(0.5) | 8 + 2 | 67 |
| Componds | Inhibition Ratio (%) b | ClogP c | ||||
|---|---|---|---|---|---|---|
| RS | FO | BCP | SCR | RSR | ||
| 5a | 35.57 | −2.60 | 51.14 | 85.04 | 22.22 | 4.251 |
| 5b | 30.68 | −0.28 | 38.64 | 86.93 | 0.00 | 5.435 |
| 5c | 33.52 | 0.65 | 34.85 | 85.98 | 53.26 | 4.788 |
| 5d | 25.23 | −1.67 | 36.36 | 58.14 | 0.00 | 3.468 |
| 5e | 28.07 | −2.37 | 15.53 | 71.78 | 17.05 | 8.437 |
| 5f | 27.84 | 1.11 | 14.02 | 73.48 | 35.44 | 5.063 |
| 5g | 33.52 | −0.28 | 15.91 | 67.99 | 26.05 | 4.976 |
| 5h | 35.80 | −2.60 | 19.70 | 44.70 | 55.36 | 5.588 |
| 5i | 30.45 | 1.81 | 16.29 | 65.34 | 19.54 | 4.479 |
| 5j | 34.09 | −3.76 | 7.39 | 67.80 | 35.25 | 4.750 |
| 5k | 34.09 | 0.19 | 5.11 | 70.08 | 0.00 | 4.566 |
| 9a | 36.59 | −0.97 | 12.69 | 40.53 | 10.76 | 7.306 |
| 9b | 58.30 | 18.52 | 63.07 | 84.47 | 81.23 | 6.659 |
| 9c | 39.20 | 7.85 | 16.86 | 79.31 | 52.11 | 7.459 |
| 9d | 28.75 | 2.27 | 63.45 | 76.70 | 47.31 | 6.350 |
| 9e | 47.72 | −7.24 | 28.03 | 44.51 | 28.91 | 6.621 |
| 9f | 35.34 | 0.65 | 24.43 | 75.57 | 19.28 | 6.437 |
| carbendazim | 81.82 | 70.98 | 88.07 | 89.77 | 100 | |
| azoxystrobin | 54.55 | 51.25 | 83.71 | 88.07 | 88.51 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huo, X.-Y.; Guo, L.; Chen, X.-F.; Zhou, Y.-T.; Zhang, J.; Han, X.-Q.; Dai, B. Design, Synthesis, and Antifungal Activity of Novel Aryl-1,2,3-Triazole-β-Carboline Hybrids. Molecules 2018, 23, 1344. https://doi.org/10.3390/molecules23061344
Huo X-Y, Guo L, Chen X-F, Zhou Y-T, Zhang J, Han X-Q, Dai B. Design, Synthesis, and Antifungal Activity of Novel Aryl-1,2,3-Triazole-β-Carboline Hybrids. Molecules. 2018; 23(6):1344. https://doi.org/10.3390/molecules23061344
Chicago/Turabian StyleHuo, Xin-Yu, Liang Guo, Xiao-Fei Chen, Yue-Ting Zhou, Jie Zhang, Xiao-Qiang Han, and Bin Dai. 2018. "Design, Synthesis, and Antifungal Activity of Novel Aryl-1,2,3-Triazole-β-Carboline Hybrids" Molecules 23, no. 6: 1344. https://doi.org/10.3390/molecules23061344
APA StyleHuo, X.-Y., Guo, L., Chen, X.-F., Zhou, Y.-T., Zhang, J., Han, X.-Q., & Dai, B. (2018). Design, Synthesis, and Antifungal Activity of Novel Aryl-1,2,3-Triazole-β-Carboline Hybrids. Molecules, 23(6), 1344. https://doi.org/10.3390/molecules23061344





