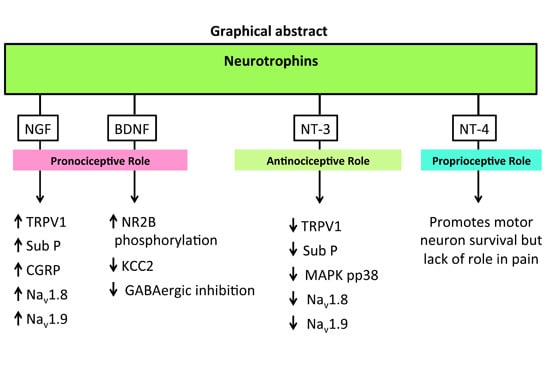
Neurotrophins and Neuropathic Pain: Role in Pathobiology
Abstract
:1. Introduction
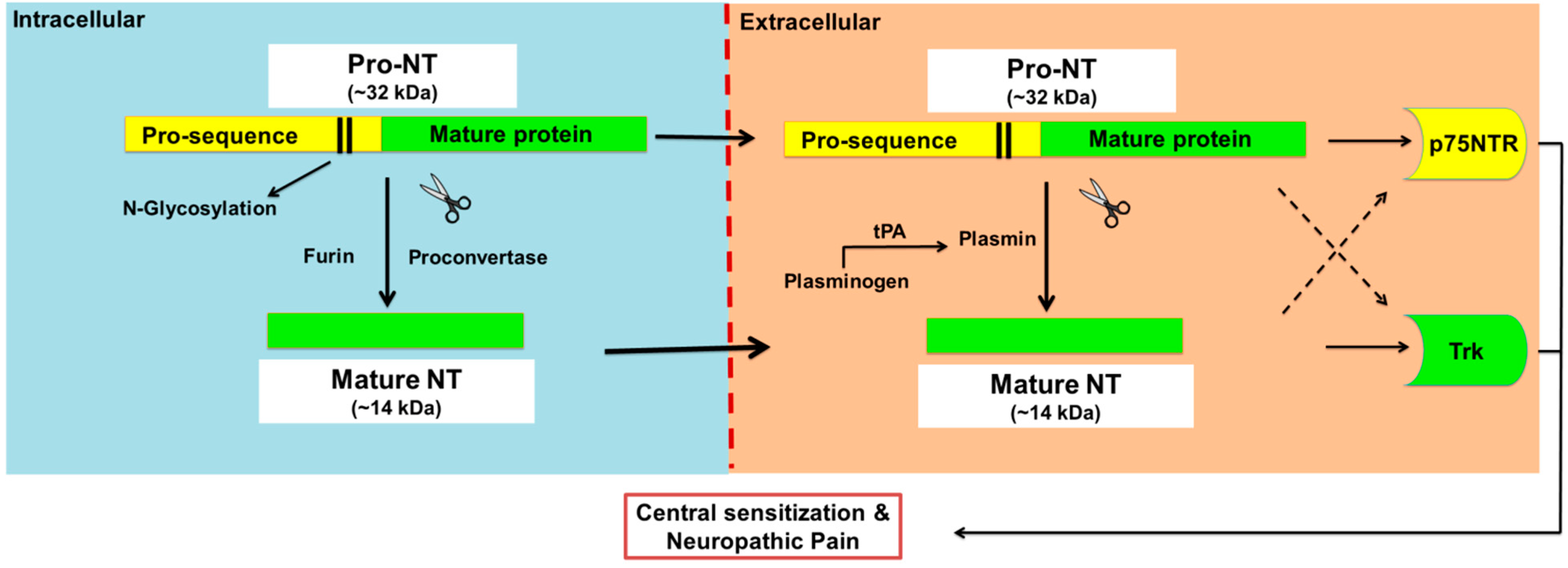
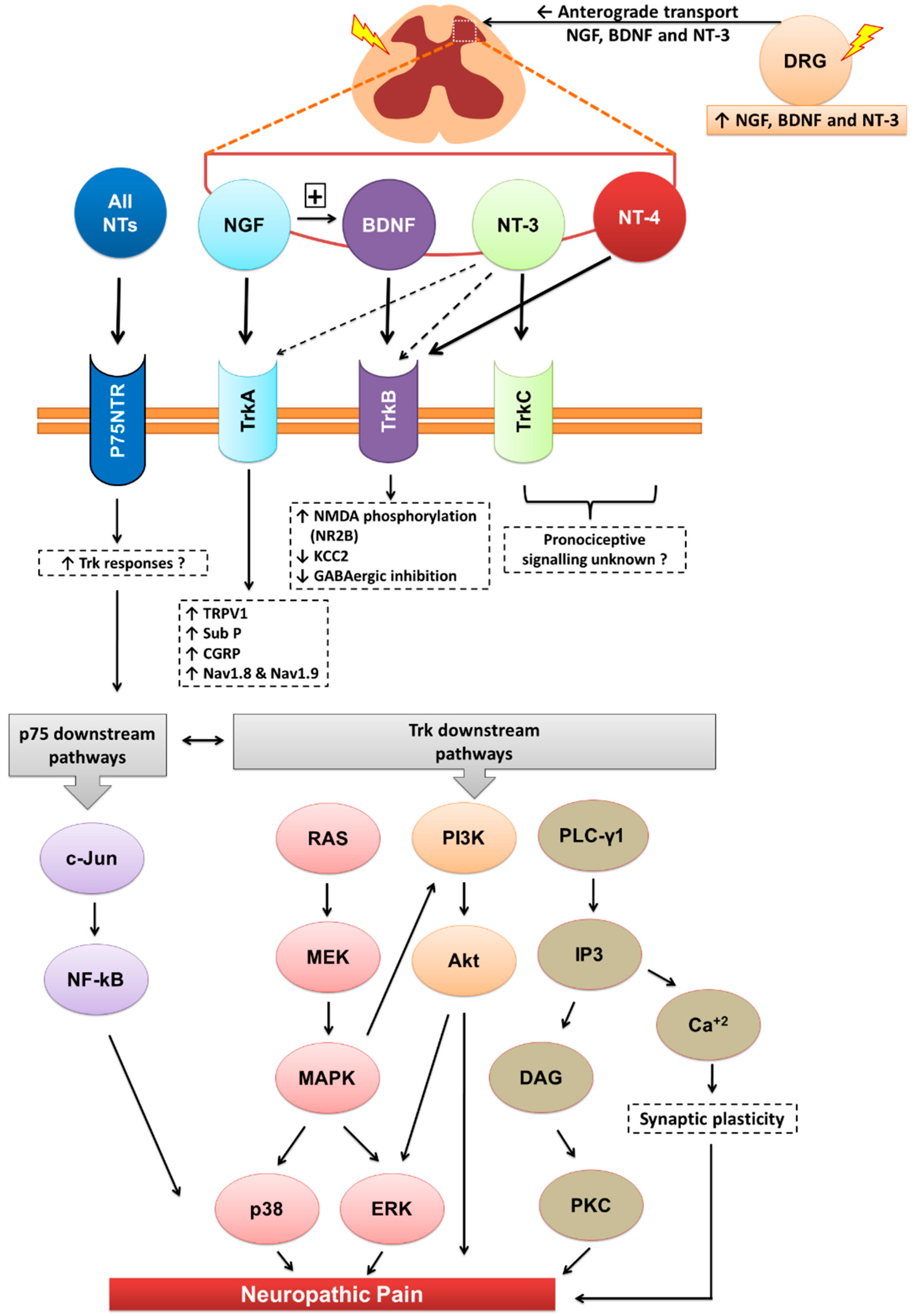
2. Neuropathic Pain
3. Neurotrophins and Neuropathic Pain
4. Nerve Growth Factor (NGF)
| NTs | Rodent Models of Neuropathic Pain | |||||
|---|---|---|---|---|---|---|
| Tissue | Nerve Ligation/Axotomy(e.g., CCI, SNL) | CIPN | DPN | EAE (MS) | SCI | |
| NGF | DRG | ↑ [22,23] | ↑ [24] or ↓ [25] | ↑ [26] or ↓ [27] | NS | NS |
| SC | ↑ [23] | ↑ [24,28] | ↓ [27] | ↓ [29,30] (Correlation with pain not investigated) | Not changed [31] or ↑ [32] (Indirect evidence) | |
| Pronociception | ||||||
| ● Micro-injected NGF (50 µg) via a catheter into an L5 DRG of un-injured rodents, induced ipsilateral persistent mechanical allodynia [33] | ||||||
| ● Intrathecal (i.t.) infusion of NGF (12 µg/day) for 9 days induced thermal hyperalgesia in rats [34] | ||||||
| ● Intraplantar (i.pl.) injection of NGF (0.3–5 µg) into a hindpaw produced dose-dependent mechanical allodynia and thermal hyperalgesia that persisted for ≥1 week and 24–48 h respectively [35]. Both mechanical allodynia and thermal hyperalgesia were partially attenuated by a TRPV1 antagonist [35] suggesting that NGF-mediated hyperalgesia is driven at least in part by increased TRPV1 expression [35,36] | ||||||
| ● NGF (1.0 mg/kg s.c.) significantly increased Sub-P and CGRP expression levels in the dorsal horn of the spinal cord [37] thereby producing neuropathic pain [38] | ||||||
| ● Overexpression of NGF may induce dysregulation of DRG Na+ channel expression, particularly Nav 1.8 (SNS/PN3), Nav 1.9 (NaN), Nav 1.6, Nav 2.1 (NaG) as well as various Na+ channel subunits including α-I, α-II, β-I and β-II in sensory neurons leading to neuropathic pain symptoms [39,40,41,42 ] | ||||||
| Antinociception | ||||||
| ● I.t. infusion of NGF (125 ng/µL/h) for 7 days reversed mechanical allodynia and thermal hyperalgesia in the hindpaws of CCI-rats. The analgesic effects of NGF were correlated with neuroprotection and decreased astrocytosis [43] | ||||||
| BDNF | DRG | ↑ [44] | NS | ↑ [45] | ↑ [46] (Not investigated in relation with pain) | NS |
| SC | ↑ [44] | ↑ [28] | NS | ↑ [47] | ↑ [48] | |
| Pronociception | ||||||
| ● Microinjection of BDNF (27–270 pg) into the midbrain facilitated nociception dependent on phosphorylation of NMDA receptors [49] | ||||||
| ● Micro-injection of BDNF (50 μg) into an L5 DRG of control (non-injured) rodents induced persistent mechanical allodynia in the hindpaws [33] | ||||||
| ● I.pl. injection of BDNF (200 ng) into rodent hindpaws produced transient thermal hyperalgesia and was significantly less potent (p < 0.05) than a similar dose of NGF [36] | ||||||
| ● In rodent models of peripheral neuropathic pain, upregulated BDNF induced phosphorylation of the NR2B subunit of the NMDA receptor [50] that was accompanied by downregulation of Kv channels [45] as well as expression levels of KCC2 in lamina-I of the spinal cord, thereby disrupting GABAergic inhibition [51] | ||||||
| ● Migration of inflammatory cells into the spinal cord may contribute to upregulation of BDNF in rodent models of peripheral neuropathic pain (e.g., DPN) [52] or CNP (e.g., MS-neuropathic pain) [47] | ||||||
| Antinociception | ||||||
| ● BDNF-infusion (12 µg/day) into the midbrain for 1–11 days evoked antinociception in the tail flick test in rats [18]. The proposed mechanism was via activation of descending opiodergic and serotonergic inhibitory signalling [18] | ||||||
| NT-4 | DRG | NS | NS | |||
| SC | ↓ [53] (Short-term) or Unchanged [54] | |||||
| Lack of a role in neuropathic pain | ||||||
| ● NT-4 appears to have no effect on activity-dependent synaptic plasticity or neuropathic pain [55,56]. | ||||||
| ● In one study, transient thermal hyperalgesia was observed in rats followed by i.pl. injection of NT-4 (200 ng) however it was worn off by 24 h [36] | ||||||
| ● Although there was a significant decrease in NT-4 expression levels in rodent models of neuropathic pain, e.g., DPN (at sciatic nerve) [57] and EAE (at brain) [58], it’s possible role in the pathogenesis of neuropathic pain remains to be investigated. | ||||||
| NT-3 | DRG | ↑ [22] | ↓ [59] (Indirect evidence) | ↓ [60] | NS | NS |
| SC | ↑ [61] | NS | NS | ↑ [62] (Not investigated in relation with pain) | ↑ [63] or ↓ [31] (Not investigated in relation with pain) | |
| Pronociception | ||||||
| ● Micro-injection of NT-3 (50 µg) into the L5 DRGs of control non-injured rodents produced transient mechanical allodynia in the hindpaws [33] | ||||||
| ● I.t infusion of NT-3 at 200 ng/day for 20-days produced pronounced but delayed mechanical allodynia in the hindpaws of non-injured rats at days 10–20 after dose initiation [64] | ||||||
| Antinociception | ||||||
| ● NT-3 infusion (12 µg/day) into the midbrain for 1–11 days showed delayed but stable antinociception in the tail-flick test in rats [18] | ||||||
| ● I.t. administration of NT-3 (600 ng/μL/h) for 7 days suppressed the over-expression of TRPV1 channels, p38 MAPK and Na+ channels (Nav 1.8 and Nav 1.9) in the ipsilateral DRGs of CCI-rats [65,66] | ||||||
| ● Down-regulation of Kv channel gene expression in DRG neurons following sciatic nerve transection was reversed by ex vivo incubation of DRGs collected from nerve-injured rats, with NT-3 (100 ng/mL) [67] | ||||||
| ● Acute i.p. injection of NT-3 (10–20 mg/kg) evoked transient mechanical but not thermal hypoalgesia in the hindpaws of rats that appeared to be underpinned by inhibition of SP release in the spinal cord [68] | ||||||
| ● NT-3 (200 ng) injected locally into rodent hindpaws did not produce hyperalgesia in contrast to that evoked by either NGF or BDNF [36] | ||||||
5. Pronociceptive Effects of NGF in Rodent Models
6. Antinociceptive Effects of NGF in Rodent Models
7. NGF: Role in Peripheral Neuropathic Pain
7.1. NGF in Rodent Models of Peripheral Nerve Ligation
7.2. Rodent Models of PDN and CIPN: Controversial Reports of NGF Expression
8. NGF: Role in Central Neuropathic Pain (CNP)
8.1. NGF in Spinal Cord Injury Induced Neuropathic Pain Rodent Model
8.2. NGF in Multiple Sclerosis-Associated Neuropathic Pain Mouse Model
9. BDNF
10. Pronociceptive Effects of BDNF in Rodent Models
11. Antinociceptive Effects of BDNF in Rodent Models
12. BDNF: Role in Peripheral Neuropathic Pain
12.1. BDNF in Rodent Models of Peripheral Nerve Ligation
12.2. BDNF in Rodent Models of PDN
12.3. BDNF in Rodent Models of CIPN
13. BDNF: Role in Central Neuropathic Pain
13.1. BDNF in Spinal Cord Injury Induced Neuropathic Pain Rodent Model
13.2. BDNF in Multiple Sclerosis-Associated Neuropathic Pain Mouse Model
14. NT-3
15. Pronociceptive Effects of NT-3 in Rodent Models
16. Antinociceptive Effects of NT-3 in Rodent Models
17. NT-3 in Peripheral Neuropathic Pain
17.1. NT-3 in Rodent Models of Peripheral Nerve Ligation
17.2. NT-3 in Rodent Models of PDN
17.3. NT-3 in Rodent Model of CIPN
18. NT-3: Role in Central Neuropathic Pain
18.1. NT-3 in Spinal Cord Injury Induced Neuropathic Pain Rodent Models
18.2. NT-3 in Multiple Sclerosis-Associated Neuropathic Pain Mouse Model
19. NT-4 (NT-4/5)
20. NT-4: Lack of a Role in Neuropathic Pain
21. Proneurotrophins, p75NTR and Sortilin: Potential Modulatory Role in Pain
21.1. Proneurotrophins
21.2. P75NTR
21.3. Sortilin
22. Neurotrophin Induced Activation of Trk or p75 Receptors: Downstream Signalling
23. Clinical Studies Using rNTs or Anti-NTs for Pain Relief
24. Conclusions
Acknowledgments
Conflicts of Interest
References
- Ceni, C.; Unsain, N.; Zeinieh, M.P.; Barker, P.A. Neurotrophins in the regulation of cellular survival and death. Handb. Exp. Pharmacol. 2014, 220, 193–221. [Google Scholar] [PubMed]
- Skaper, S.D. The neurotrophin family of neurotrophic factors: An overview. Methods Mol. Biol. 2012, 846, 1–12. [Google Scholar] [PubMed]
- Davies, A.M. Neurotrophins giveth and they taketh away. Nat. Neurosci. 2008, 11, 627–628. [Google Scholar] [CrossRef] [PubMed]
- Teng, K.K.; Felice, S.; Kim, T.; Hempstead, B.L. Understanding proneurotrophin actions: Recent advances and challenges. Dev. Neurobiol. 2010, 70, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Patapoutian, A.; Reichardt, L.F. Trk receptors: Mediators of neurotrophin action. Curr. Opin. Neurobiol. 2001, 11, 272–280. [Google Scholar] [CrossRef]
- Davies, A.M.; Minichiello, L.; Klein, R. Developmental changes in NT3 signalling via TrkA and TrkB in embryonic neurons. EMBO J. 1995, 14, 4482–4489. [Google Scholar] [PubMed]
- Lewin, G.R.; Nykjaer, A. Pro-neurotrophins, sortilin, and nociception. Eur. J. Neurosci. 2014, 39, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Nykjaer, A.; Willnow, T.E. Sortilin: A receptor to regulate neuronal viability and function. Trends Neurosci. 2012, 35, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Masoudi, R.; Ioannou, M.S.; Coughlin, M.D.; Pagadala, P.; Neet, K.E.; Clewes, O.; Allen, S.J.; Dawbarn, D.; Fahnestock, M. Biological activity of nerve growth factor precursor is dependent upon relative levels of its receptors. J. Biol. Chem. 2009, 284, 18424–18433. [Google Scholar] [CrossRef] [PubMed]
- Fayard, B.; Loeffler, S.; Weis, J.; Vögelin, E.; Krüttgen, A. The secreted brain-derived neurotrophic factor precursor pro-BDNF binds to TrkB and p75NTR but not to TrkA or TrkC. J. Neurosci. Res. 2005, 80, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Lane, N.E.; Schnitzer, T.J.; Birbara, C.A.; Mokhtarani, M.; Shelton, D.L.; Smith, M.D.; Brown, M.T. Tanezumab for the treatment of pain from osteoarthritis of the knee. N. Engl. J. Med. 2010, 363, 1521–1531. [Google Scholar] [CrossRef] [PubMed]
- Treede, R.D.; Jensen, T.S.; Campbell, J.N.; Cruccu, G.; Dostrovsky, J.O.; Griffin, J.W.; Hansson, P.; Hughes, R.; Nurmikko, T.; Serra, J. Neuropathic pain: Redefinition and a grading system for clinical and research purposes. Neurology 2008, 70, 1630–1635. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, R.H.; Levy, R.M.; Mackey, S.C.; Mayer, J.; Miaskowski, C.; Raja, S.N.; Rice, A.S.C.; Schmader, K.E.; Stacey, B.; Stanos, S.; et al. Recommendations for the pharmacological management of neuropathic pain: An overview and literature update. Mayo Clin. Proc. 2010, 85, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, R.H.; O’Connor, A.B.; Kent, J.; Mackey, S.C.; Raja, S.N.; Stacey, B.R.; Levy, R.M.; Backonja, M.; Baron, R.; Harke, H.; et al. Interventional management of neuropathic pain: NeuPSIG recommendations. Pain 2013, 154, 2249–2261. [Google Scholar] [CrossRef] [PubMed]
- Nijs, J.; Meeus, M.; Versijpt, J.; Moens, M.; Bos, I.; Knaepen, K.; Meeusen, R. Brain-derived neurotrophic factor as a driving force behind neuroplasticity in neuropathic and central sensitization pain: A new therapeutic target? Expert Opin. Ther. Targets 2015, 19, 565–576. [Google Scholar] [CrossRef] [PubMed]
- McMahon, S.B.; Jones, N.G. Plasticity of pain signaling: Role of neurotrophic factors exemplified by acid-induced pain. J. Neurobiol. 2004, 61, 72–87. [Google Scholar] [CrossRef] [PubMed]
- Pezet, S.; McMahon, S.B. Neurotrophins: Mediators and modulators of pain. Annu. Rev. Neurosci. 2006, 29, 507–538. [Google Scholar] [CrossRef] [PubMed]
- Siuciak, J.A.; Altar, C.A.; Wiegand, S.J.; Lindsay, R.M. Antinociceptive effect of brain-derived neurotrophic factor and neurotrophin-3. Brain Res. 1994, 633, 326–330. [Google Scholar] [CrossRef]
- Siuciak, J.A.; Wong, V.; Pearsall, D.; Wiegand, S.J.; Lindsay, R.M. Bdnf produces analgesia in the formalin test and modifies neuropeptide levels in rat brain and spinal cord areas associated with nociception. Eur. J. Neurosci. 1995, 7, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Aloe, L.; Rocco, M.; Bianchi, P.; Manni, L. Nerve growth factor: From the early discoveries to the potential clinical use. J. Transl. Med. 2012, 10, 239. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.S.; Nishimura, M.C.; Armanini, M.P.; Crowley, C.; Spencer, S.D.; Phillips, H.S. Disruption of a single allele of the nerve growth factor gene results in atrophy of basal forebrain cholinergic neurons and memory deficits. J. Neurosci. 1997, 17, 7288–7296. [Google Scholar] [PubMed]
- Zhou, X.F.; Deng, Y.S.; Chie, E.; Xue, Q.; Zhong, J.H.; McLachlan, E.M.; Rush, R.A.; Xian, C.J. Satellite-cell-derived nerve growth factor and neurotrophin-3 are involved in noradrenergic sprouting in the dorsal root ganglia following peripheral nerve injury in the rat. Eur. J. Neurosci. 1999, 11, 1711–1722. [Google Scholar] [CrossRef] [PubMed]
- Vivoli, E.; di Cesare Mannelli, L.; Salvicchi, A.; Bartolini, A.; Koverech, A.; Nicolai, R.; Benatti, P.; Ghelardini, C. Acetyl-l-carnitine increases artemin level and prevents neurotrophic factor alterations during neuropathy. Neuroscience 2010, 167, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Nakahashi, Y.; Kamiya, Y.; Funakoshi, K.; Miyazaki, T.; Uchimoto, K.; Tojo, K.; Ogawa, K.; Fukuoka, T.; Goto, T. Role of nerve growth factor-tyrosine kinase receptor a signaling in paclitaxel-induced peripheral neuropathy in rats. Biochem. Biophys. Res. Commun. 2014, 444, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Apfel, S.C.; Arezzo, J.C.; Lipson, L.; Kessler, J.A. Nerve growth factor prevents experimental cisplatin neuropathy. Ann. Neurol. 1992, 31, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.T.; Dauch, J.R.; Hayes, J.M.; Hong, Y.; Feldman, E.L. Nerve growth factor mediates mechanical allodynia in a mouse model of type 2 diabetes. J. Neuropathol. Exp. Neurol. 2009, 68, 1229–1243. [Google Scholar] [CrossRef] [PubMed]
- Unger, J.W.; Klitzsch, T.; Pera, S.; Reiter, R. Nerve growth factor (NGF) and diabetic neuropathy in the rat: Morphological investigations of the sural nerve, dorsal root ganglion, and spinal cord. Exp. Neurol. 1998, 153, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Aloe, L.; Manni, L.; Properzi, F.; de Santis, S.; Fiore, M. Evidence that nerve growth factor promotes the recovery of peripheral neuropathy induced in mice by cisplatin: Behavioral, structural and biochemical analysis. Auton. Neurosci. 2000, 86, 84–93. [Google Scholar] [CrossRef]
- Calza, L.; Fernandez, M.; Giuliani, A.; Aloe, L.; Giardino, L. Thyroid hormone activates oligodendrocyte precursors and increases a myelin-forming protein and NGF content in the spinal cord during experimental allergic encephalomyelitis. Proc. Natl. Acad. Sci. USA 2002, 99, 3258–3263. [Google Scholar] [CrossRef] [PubMed]
- Calza, L.; Giardino, L.; Pozza, M.; Micera, A.; Aloe, L. Time-course changes of nerve growth factor, corticotropin-releasing hormone, and nitric oxide synthase isoforms and their possible role in the development of inflammatory response in experimental allergic encephalomyelitis. Proc. Natl. Acad. Sci. USA 1997, 94, 3368–3373. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.Y.; Kim, D.Y.; Yune, T.Y.; Shin, D.H.; Baek, S.B.; Kim, C.J. Treadmill exercise reduces spinal cord injury-induced apoptosis by activating the PI3K/Akt pathway in rats. Exp. Ther. Med. 2014, 7, 587–593. [Google Scholar] [PubMed]
- Christensen, M.D.; Hulsebosch, C.E. Spinal cord injury and anti-NGF treatment results in changes in cgrp density and distribution in the dorsal horn in the rat. Exp. Neurol. 1997, 147, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.F.; Deng, Y.S.; Xian, C.J.; Zhong, J.H. Neurotrophins from dorsal root ganglia trigger allodynia after spinal nerve injury in rats. Eur J. Neurosci. 2000, 12, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Malcangio, M.; Ramer, M.S.; Boucher, T.J.; McMahon, S.B. Intrathecally injected neurotrophins and the release of substance P from the rat isolated spinal cord. Eur. J. Neurosci. 2000, 12, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D.; Nguyen, T.; Tanga, F.Y.; Zhong, C.; Gauvin, D.M.; Mikusa, J.; Gomez, E.J.; Salyers, A.K.; Bannon, A.W. Characterization of nerve growth factor-induced mechanical and thermal hypersensitivity in rats. Eur. J. Pain 2013, 17, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Mendell, L.M. Nerve growth factor acutely sensitizes the response of adult rat sensory neurons to capsaicin. Neurosci. Lett. 1999, 274, 159–162. [Google Scholar] [CrossRef]
- Malcangio, M.; Garrett, N.E.; Tomlinson, D.R. Nerve growth factor treatment increases stimulus-evoked release of sensory neuropeptides in the rat spinal cord. Eur. J. Neurosci. 1997, 9, 1101–1104. [Google Scholar] [CrossRef] [PubMed]
- Woolf, C.J. Phenotypic modification of primary sensory neurons: The role of nerve growth factor in the production of persistent pain. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1996, 351, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Gu, J.; Li, Y.Q.; Tao, Y.X. Are voltage-gated sodium channels on the dorsal root ganglion involved in the development of neuropathic pain? Mol. Pain 2011, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Waxman, S.G. The molecular pathophysiology of pain: Abnormal expression of sodium channel genes and its contributions to hyperexcitability of primary sensory neurons. Pain 1999, 81, S133–S140. [Google Scholar] [CrossRef]
- Fjell, J.; Cummins, T.R.; Fried, K.; Black, J.A.; Waxman, S.G. In vivo NGF deprivation reduces SNS expression and TTX-R sodium currents in IB4-negative DRG neurons. J. Neurophysiol. 1999, 81, 803–810. [Google Scholar] [PubMed]
- Fjell, J.; Cummins, T.R.; Dib-Hajj, S.D.; Fried, K.; Black, J.A.; Waxman, S.G. Differential role of GDNF and NGF in the maintenance of two TTX-resistant sodium channels in adult drg neurons. Mol. Brain Res. 1999, 67, 267–282. [Google Scholar] [CrossRef]
- Cirillo, G.; Cavaliere, C.; Bianco, M.R.; de Simone, A.; Colangelo, A.M.; Sellitti, S.; Alberghina, L.; Papa, M. Intrathecal NGF administration reduces reactive astrocytosis and changes neurotrophin receptors expression pattern in a rat model of neuropathic pain. Cell. Mol. Neurobiol. 2010, 30, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.O.; Kim, J.K.; Hong, H.S.; Kim, D.S.; Cho, H.J. Expression of brain-derived neurotrophic factor in rat dorsal root ganglia, spinal cord and gracile nuclei in experimental models of neuropathic pain. Neuroscience 2001, 107, 301–309. [Google Scholar] [CrossRef]
- Cao, X.H.; Byun, H.S.; Chen, S.R.; Cai, Y.Q.; Pan, H.L. Reduction in voltage-gated K(+) channel activity in primary sensory neurons in painful diabetic neuropathy: Role of brain-derived neurotrophic factor. J. Neurochem. 2010, 114, 1460–1475. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Frost, E.E.; Begum, F.; Vora, P.; Au, K.; Gong, Y.; MacNeil, B.; Pillai, P.; Namaka, M. The role of dorsal root ganglia activation and brain-derived neurotrophic factor in multiple sclerosis. J. Cell Mol. Med. 2012, 16, 1856–1865. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Gordon, R.; Woodruff, T.M.; Smith, M.T. Antiallodynic effects of alpha lipoic acid in an optimized RR-EAE mouse model of MS-neuropathic pain are accompanied by attenuation of upregulated BDNF-TrkB-ERK signaling in the dorsal horn of the spinal cord. Pharm. Res. Perpect. 2015, 3, e00137. [Google Scholar]
- Wu, J.; Renn, C.L.; Faden, A.I.; Dorsey, S.G. TrkB.T1 contributes to neuropathic pain after spinal cord injury through regulation of cell cycle pathways. J. Neurosci. 2013, 33, 12447–12463. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Robbins, M.T.; Wei, F.; Zou, S.; Dubner, R.; Ren, K. Supraspinal brain-derived neurotrophic factor signaling: A novel mechanism for descending pain facilitation. J. Neurosci. 2006, 26, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.J.; Liao, F.F.; Dang, W.H.; Ding, X.; Liu, X.D.; Cai, J.; Han, J.S.; Wan, Y.; Xing, G.G. Contribution of the spinal cord BDNF to the development of neuropathic pain by activation of the NR2B-containing nmda receptors in rats with spinal nerve ligation. Exp. Neurol. 2010, 222, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Coull, J.A.; Boudreau, D.; Bachand, K.; Prescott, S.A.; Nault, F.; Sik, A.; De Koninck, P.; De Koninck, Y. Trans-synaptic shift in anion gradient in spinal lamina-I neurons as a mechanism of neuropathic pain. Nature 2003, 424, 938–942. [Google Scholar] [CrossRef] [PubMed]
- Sobue, G.; Yamamoto, M.; Doyu, M.; Li, M.; Yasuda, T.; Mitsuma, T. Expression of mRNAs for neurotrophins (NGF, BDNF and NT-3) and their receptors (p75NGFR, Trk, TrkB, and TrkC) in human peripheral neuropathies. Neurochem. Res. 1998, 23, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi, H.; Frisen, J.; Barbany, G.; Timmusk, T.; Zachrisson, O.; Verge, V.M.; Persson, H. Differential expression of mrnas for neurotrophins and their receptors after axotomy of the sciatic nerve. J. Cell Biol. 1993, 123, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Ramer, L.M.; McPhail, L.T.; Borisoff, J.F.; Soril, L.J.; Kaan, T.K.; Lee, J.H.; Saunders, J.W.; Hwi, L.P.; Ramer, M.S. Endogenous TrkB ligands suppress functional mechanosensory plasticity in the deafferented spinal cord. J. Neurosci. 2007, 27, 5812–5822. [Google Scholar] [CrossRef] [PubMed]
- Yajima, Y.; Narita, M.; Narita, M.; Matsumoto, N.; Suzuki, T. Involvement of a spinal brain-derived neurotrophic factor/full-length TrkB pathway in the development of nerve injury-induced thermal hyperalgesia in mice. Brain Res. 2002, 958, 338–346. [Google Scholar] [CrossRef]
- Heppenstall, P.A.; Lewin, G.R. BDNF but not NT-4 is required for normal flexion reflex plasticity and function. Proc. Natl. Acad. Sci. USA 2001, 98, 8107–8112. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Pena, A.; Botana, M.; Gonzalez, M.; Requejo, F. Expression of neurotrophins and their receptors in sciatic nerve of experimentally diabetic rats. Neurosci. Lett. 1995, 200, 37–40. [Google Scholar] [CrossRef]
- Aharoni, R.; Eilam, R.; Domev, H.; Labunskay, G.; Sela, M.; Arnon, R. The immunomodulator glatiramer acetate augments the expression of neurotrophic factors in brains of experimental autoimmune encephalomyelitis mice. Proc. Natl. Acad. Sci. USA 2005, 102, 19045–19050. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.Q.; Dybdal, N.; Shinsky, N.; Murnane, A.; Schmelzer, C.; Siegel, M.; Keller, G.; Hefti, F.; Phillips, H.S.; Winslow, J.W. Neurotrophin-3 reverses experimental cisplatin-induced peripheral sensory neuropathy. Ann. Neurol. 1995, 38, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Fernyhough, P.; Diemel, L.T.; Tomlinson, D.R. Target tissue production and axonal transport of neurotrophin-3 are reduced in streptozotocin-diabetic rats. Diabetologia 1998, 41, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.H.; Meng, Q.S.; Qi, J.G.; Zhang, W.M.; Chen, J.; Wu, L.F. NT-3 expression in spared DRG and the associated spinal laminae as well as its anterograde transport in sensory neurons following removal of adjacent DRG in cats. Neurochem. Res. 2008, 33, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Muhallab, S.; Lundberg, C.; Gielen, A.W.; Lidman, O.; Svenningsson, A.; Piehl, F.; Olsson, T. Differential expression of neurotrophic factors and inflammatory cytokines by myelin basic protein-specific and other recruited T cells infiltrating the central nervous system during experimental autoimmune encephalomyelitis. Scand. J. Immunol. 2002, 55, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Shine, H.D. Neurotrophic factors expressed in both cortex and spinal cord induce axonal plasticity after spinal cord injury. J. Neurosci. Res. 2003, 74, 221–226. [Google Scholar] [CrossRef] [PubMed]
- White, D.M. Contribution of neurotrophin-3 to the neuropeptide Y-induced increase in neurite outgrowth of rat dorsal root ganglion cells. Neuroscience 1998, 86, 257–263. [Google Scholar] [CrossRef]
- Wilson-Gerwing, T.D.; Dmyterko, M.V.; Zochodne, D.W.; Johnston, J.M.; Verge, V.M. Neurotrophin-3 suppresses thermal hyperalgesia associated with neuropathic pain and attenuates transient receptor potential vanilloid receptor-1 expression in adult sensory neurons. J. Neurosci. 2005, 25, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Wilson-Gerwing, T.D.; Stucky, C.L.; McComb, G.W.; Verge, V.M. Neurotrophin-3 significantly reduces sodium channel expression linked to neuropathic pain states. Exp. Neurol. 2008, 213, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Choi, J.Y.; Kim, R.U.; Lee, Y.S.; Cho, H.J.; Kim, D.S. Downregulation of voltage-gated potassium channel alpha gene expression by axotomy and neurotrophins in rat dorsal root ganglia. Mol. Cells 2003, 16, 256–259. [Google Scholar] [PubMed]
- Malcangio, M.; Garrett, N.E.; Cruwys, S.; Tomlinson, D.R. Nerve growth factor- and neurotrophin-3-induced changes in nociceptive threshold and the release of substance P from the rat isolated spinal cord. J. Neurosci. 1997, 17, 8459–8467. [Google Scholar] [PubMed]
- Lewin, G.R.; Ritter, A.M.; Mendell, L.M. Nerve growth factor-induced hyperalgesia in the neonatal and adult rat. J. Neurosci. 1993, 13, 2136–2148. [Google Scholar] [PubMed]
- Rukwied, R.; Mayer, A.; Kluschina, O.; Obreja, O.; Schley, M.; Schmelz, M. NGF induces non-inflammatory localized and lasting mechanical and thermal hypersensitivity in human skin. Pain 2010, 148, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.W.; Dray, A.; McCarson, K.E.; Krause, J.E.; Urban, L. Nerve growth factor induces mechanical allodynia associated with novel a fibre-evoked spinal reflex activity and enhanced neurokinin-1 receptor activation in the rat. Pain 1995, 62, 219–231. [Google Scholar] [CrossRef]
- Lewin, G.R.; Rueff, A.; Mendell, L.M. Peripheral and central mechanisms of NGF-induced hyperalgesia. Eur. J. Neurosci. 1994, 6, 1903–1912. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.G.; Munson, J.B.; Thompson, S.W. A role for nerve growth factor in sympathetic sprouting in rat dorsal root ganglia. Pain 1999, 79, 21–29. [Google Scholar] [CrossRef]
- Deng, Y.S.; Zhong, J.H.; Zhou, X.F. Effects of endogenous neurotrophins on sympathetic sprouting in the dorsal root ganglia and allodynia following spinal nerve injury. Exp. Neurol. 2000, 164, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Mearow, K.M. The effects of NGF and sensory nerve stimulation on collateral sprouting and gene expression in adult sensory neurons. Exp. Neurol. 1998, 151, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Yen, L.D.; Bennett, G.J.; Ribeiro-da-Silva, A. Sympathetic sprouting and changes in nociceptive sensory innervation in the glabrous skin of the rat hind paw following partial peripheral nerve injury. J. Comp. Neurol. 2006, 495, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Otten, U.; Schwab, M.; Gagnon, C.; Thoenen, H. Selective induction of tyrosine hydroxylase and dopamine beta-hydroxylase by nerve growth factor: Comparison between adrenal medulla and sympathetic ganglia of adult and newborn rats. Brain Res. 1977, 133, 291–303. [Google Scholar] [CrossRef]
- Shadiack, A.M.; Sun, Y.; Zigmond, R.E. Nerve growth factor antiserum induces axotomy-like changes in neuropeptide expression in intact sympathetic and sensory neurons. J. Neurosci. 2001, 21, 363–371. [Google Scholar] [PubMed]
- Zhang, X.; Huang, J.; McNaughton, P.A. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005, 24, 4211–4223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Nicol, G.D. NGF-mediated sensitization of the excitability of rat sensory neurons is prevented by a blocking antibody to the p75 neurotrophin receptor. Neurosci. Lett. 2004, 366, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Khodorova, A.; Nicol, G.D.; Strichartz, G. The p75NTR signaling cascade mediates mechanical hyperalgesia induced by nerve growth factor injected into the rat hind paw. Neuroscience 2013, 254, 312–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahluwalia, J.; Urban, L.; Bevan, S.; Capogna, M.; Nagy, I. Cannabinoid 1 receptors are expressed by nerve growth factor- and glial cell-derived neurotrophic factor-responsive primary sensory neurones. Neuroscience 2002, 110, 747–753. [Google Scholar] [CrossRef]
- Agarwal, N.; Pacher, P.; Tegeder, I.; Amaya, F.; Constantin, C.E.; Brenner, G.J.; Rubino, T.; Michalski, C.W.; Marsicano, G.; Monory, K.; et al. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat. Neurosci. 2007, 10, 870–879. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Otto, W.R.; Sanchez-Herrera, D.; Facer, P.; Yiangou, Y.; Korchev, Y.; Birch, R.; Benham, C.; Bountra, C.; Chessell, I.P.; et al. Cannabinoid receptor CB2 localisation and agonist-mediated inhibition of capsaicin responses in human sensory neurons. Pain 2008, 138, 667–680. [Google Scholar] [CrossRef] [PubMed]
- McDowell, T.S.; Wang, Z.Y.; Singh, R.; Bjorling, D. CB1 cannabinoid receptor agonist prevents NGF-induced sensitization of TRPV1 in sensory neurons. Neurosci. Lett. 2013, 551, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, M.D.; Richardson, D.; Chapman, V. Endocannabinoid metabolism and uptake: Novel targets for neuropathic and inflammatory pain. Br. J. Pharmacol. 2007, 152, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Luongo, L.; Maione, S.; di Marzo, V. Endocannabinoids and neuropathic pain: Focus on neuron-glia and endocannabinoid-neurotrophin interactions. Eur. J. Neurosci. 2014, 39, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Petrosino, S.; Palazzo, E.; de Novellis, V.; Bisogno, T.; Rossi, F.; Maione, S.; di Marzo, V. Changes in spinal and supraspinal endocannabinoid levels in neuropathic rats. Neuropharmacology 2007, 52, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Guindon, J.; Lai, Y.; Takacs, S.M.; Bradshaw, H.B.; Hohmann, A.G. Alterations in endocannabinoid tone following chemotherapy-induced peripheral neuropathy: Effects of endocannabinoid deactivation inhibitors targeting fatty-acid amide hydrolase and monoacylglycerol lipase in comparison to reference analgesics following cisplatin treatment. Pharmacol. Res. 2013, 67, 94–109. [Google Scholar] [PubMed]
- Owolabi, J.B.; Rizkalla, G.; Tehim, A.; Ross, G.M.; Riopelle, R.J.; Kamboj, R.; Ossipov, M.; Bian, D.; Wegert, S.; Porreca, F.; et al. Characterization of antiallodynic actions of ALE-0540, a novel nerve growth factor receptor antagonist, in the rat. J. Pharmacol. Exp. Ther. 1999, 289, 1271–1276. [Google Scholar] [PubMed]
- Ro, L.S.; Chen, S.T.; Tang, L.M.; Jacobs, J.M. Effect of NGF and anti-NGF on neuropathic pain in rats following chronic constriction injury of the sciatic nerve. Pain 1999, 79, 265–274. [Google Scholar] [CrossRef]
- Wild, K.D.; Bian, D.; Zhu, D.; Davis, J.; Bannon, A.W.; Zhang, T.J.; Louis, J.C. Antibodies to nerve growth factor reverse established tactile allodynia in rodent models of neuropathic pain without tolerance. J. Pharmacol. Exp. Ther. 2007, 322, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Ugolini, G.; Marinelli, S.; Covaceuszach, S.; Cattaneo, A.; Pavone, F. The function neutralizing anti-TrkA antibody MNAC13 reduces inflammatory and neuropathic pain. Proc. Natl. Acad. Sci. USA 2007, 104, 2985–2990. [Google Scholar] [CrossRef] [PubMed]
- Verge, V.M.; Riopelle, R.J.; Richardson, P.M. Nerve growth factor receptors on normal and injured sensory neurons. J. Neurosci. 1989, 9, 914–922. [Google Scholar] [PubMed]
- Verge, V.M.; Richardson, P.M.; Wiesenfeld-Hallin, Z.; Hokfelt, T. Differential influence of nerve growth factor on neuropeptide expression in vivo: A novel role in peptide suppression in adult sensory neurons. J. Neurosci. 1995, 15, 2081–2096. [Google Scholar] [PubMed]
- Schmidt, Y.; Unger, J.W.; Bartke, I.; Reiter, R. Effect of nerve growth factor on peptide neurons in dorsal root ganglia after taxol or cisplatin treatment and in diabetic (db/db) mice. Exp. Neurol. 1995, 132, 16–23. [Google Scholar] [CrossRef]
- Hellweg, R.; Hartung, H.D. Endogenous levels of nerve growth factor (NGF) are altered in experimental diabetes mellitus: A possible role for NGF in the pathogenesis of diabetic neuropathy. J. Neurosci. Res. 1990, 26, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Konings, P.N.; Makkink, W.K.; van Delft, A.M.; Ruigt, G.S. Reversal by NGF of cytostatic drug-induced reduction of neurite outgrowth in rat dorsal root ganglia in vitro. Brain Res. 1994, 640, 195–204. [Google Scholar] [CrossRef]
- Cavaletti, G.; Bogliun, G.; Marzorati, L.; Zincone, A.; Piatti, M.; Colombo, N.; Franchi, D.; la Presa, M.T.; Lissoni, A.; Buda, A.; et al. Early predictors of peripheral neurotoxicity in cisplatin and paclitaxel combination chemotherapy. Ann. Oncol. 2004, 15, 1439–1442. [Google Scholar] [CrossRef] [PubMed]
- Apfel, S.C.; Arezzo, J.C.; Brownlee, M.; Federoff, H.; Kessler, J.A. Nerve growth factor administration protects against experimental diabetic sensory neuropathy. Brain Res. 1994, 634, 7–12. [Google Scholar] [CrossRef]
- Watson, J.J.; Allen, S.J.; Dawbarn, D. Targeting nerve growth factor in pain: What is the therapeutic potential? BioDrugs 2008, 22, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Mantyh, P.W.; Koltzenburg, M.; Mendell, L.M.; Tive, L.; Shelton, D.L. Antagonism of nerve growth factor-TrkA signaling and the relief of pain. Anesthesiology 2011, 115, 189–204. [Google Scholar] [CrossRef] [PubMed]
- McKelvey, L.; Shorten, G.D.; O’Keeffe, G.W. Nerve growth factor-mediated regulation of pain signalling and proposed new intervention strategies in clinical pain management. J. Neurochem. 2013, 124, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.I.; Rangappa, N.; Li, L.; Lightfoot, E.; Garry, M.G.; Smith, G.M. Extensive sprouting of sensory afferents and hyperalgesia induced by conditional expression of nerve growth factor in the adult spinal cord. J. Neurosci. 2000, 20, 4435–4445. [Google Scholar] [PubMed]
- Taniuchi, M.; Clark, H.B.; Johnson, E.M., Jr. Induction of nerve growth factor receptor in schwann cells after axotomy. Proc. Natl. Acad. Sci. USA 1986, 83, 4094–4098. [Google Scholar] [CrossRef] [PubMed]
- Taniuchi, M.; Clark, H.B.; Schweitzer, J.B.; Johnson, E.M., Jr. Expression of nerve growth factor receptors by schwann cells of axotomized peripheral nerves: Ultrastructural location, suppression by axonal contact, and binding properties. J. Neurosci. 1988, 8, 664–681. [Google Scholar] [PubMed]
- Lindholm, D.; Heumann, R.; Meyer, M.; Thoenen, H. Interleukin-1 regulates synthesis of nerve growth factor in non-neuronal cells of rat sciatic nerve. Nature 1987, 330, 658–659. [Google Scholar] [CrossRef] [PubMed]
- Heumann, R.; Lindholm, D.; Bandtlow, C.; Meyer, M.; Radeke, M.J.; Misko, T.P.; Shooter, E.; Thoenen, H. Differential regulation of mrna encoding nerve growth factor and its receptor in rat sciatic nerve during development, degeneration, and regeneration: Role of macrophages. Proc. Natl. Acad. Sci. USA 1987, 84, 8735–8739. [Google Scholar] [CrossRef] [PubMed]
- Ramer, M.S.; Kawaja, M.D.; Henderson, J.T.; Roder, J.C.; Bisby, M.A. Glial overexpression of NGF enhances neuropathic pain and adrenergic sprouting into drg following chronic sciatic constriction in mice. Neurosci. Lett. 1998, 251, 53–56. [Google Scholar] [CrossRef]
- Woolf, C.J.; Safieh-Garabedian, B.; Ma, Q.P.; Crilly, P.; Winter, J. Nerve growth factor contributes to the generation of inflammatory sensory hypersensitivity. Neuroscience 1994, 62, 327–331. [Google Scholar] [CrossRef]
- Donnerer, J.; Schuligoi, R.; Stein, C. Increased content and transport of substance P and calcitonin gene-related peptide in sensory nerves innervating inflamed tissue: Evidence for a regulatory function of nerve growth factor in vivo. Neuroscience 1992, 49, 693–698. [Google Scholar] [CrossRef]
- Michael, G.J.; Averill, S.; Nitkunan, A.; Rattray, M.; Bennett, D.L.; Yan, Q.; Priestley, J.V. Nerve growth factor treatment increases brain-derived neurotrophic factor selectively in TrkA-expressing dorsal root ganglion cells and in their central terminations within the spinal cord. J. Neurosci. 1997, 17, 8476–8490. [Google Scholar] [PubMed]
- Ondarza, A.B.; Ye, Z.; Hulsebosch, C.E. Direct evidence of primary afferent sprouting in distant segments following spinal cord injury in the rat: Colocalization of GAP-43 and CGRP. Exp. Neurol. 2003, 184, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Gwak, Y.S.; Nam, T.S.; Paik, K.S.; Hulsebosch, C.E.; Leem, J.W. Attenuation of mechanical hyperalgesia following spinal cord injury by administration of antibodies to nerve growth factor in the rat. Neurosci. Lett. 2003, 336, 117–120. [Google Scholar] [CrossRef]
- Khan, N.; Smith, M.T. Multiple sclerosis-induced neuropathic pain: Pharmacological management and pathophysiological insights from rodent EAE models. Inflammopharmacology 2014, 22, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Althaus, H.H. Remyelination in multiple sclerosis: A new role for neurotrophins? Prog. Brain Res. 2004, 146, 415–432. [Google Scholar] [PubMed]
- Colafrancesco, V.; Villoslada, P. Targeting NGF pathway for developing neuroprotective therapies for multiple sclerosis and other neurological diseases. Arch. Ital. Biol. 2011, 149, 183–192. [Google Scholar] [PubMed]
- Laudiero, L.B.; Aloe, L.; Levi-Montalcini, R.; Buttinelli, C.; Schilter, D.; Gillessen, S.; Otten, U. Multiple sclerosis patients express increased levels of beta-nerve growth factor in cerebrospinal fluid. Neurosci. Lett. 1992, 147, 9–12. [Google Scholar] [CrossRef]
- Olechowski, C.J.; Truong, J.J.; Kerr, B.J. Neuropathic pain behaviours in a chronic-relapsing model of experimental autoimmune encephalomyelitis (EAE). Pain 2009, 141, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Hui, J.; Zhang, Z.J.; Zhang, X.; Shen, Y.; Gao, Y.J. Repetitive hyperbaric oxygen treatment attenuates complete Freund’s adjuvant-induced pain and reduces glia-mediated neuroinflammation in the spinal cord. J. Pain 2013, 14, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, V.; Tanga, F.Y.; DeLeo, J.A. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the cns. Eur. J. Neurosci. 2004, 20, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Seybold, V.S.; Galeazza, M.T.; Garry, M.G.; Hargreaves, K.M. Plasticity of calcitonin gene related peptide neurotransmission in the spinal cord during peripheral inflammation. Can. J. Physiol. Pharmacol. 1995, 73, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Woodruff, T.M.; Smith, M.T. Establishment and characterization of an optimized mouse model of multiple sclerosis-induced neuropathic pain using behavioral, pharmacologic, histologic and immunohistochemical methods. Pharmacol. Biochem. Behav. 2014, 126, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Obata, K.; Noguchi, K. Bdnf in sensory neurons and chronic pain. Neurosci. Res. 2006, 55, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Groth, R.; Aanonsen, L. Spinal brain-derived neurotrophic factor (BDNF) produces hyperalgesia in normal mice while antisense directed against either BDNF or TrkB, prevent inflammation-induced hyperalgesia. Pain 2002, 100, 171–181. [Google Scholar] [CrossRef]
- Wang, X.; Ratnam, J.; Zou, B.; England, P.M.; Basbaum, A.I. TrkB signaling is required for both the induction and maintenance of tissue and nerve injury-induced persistent pain. J. Neurosci. 2009, 29, 5508–5515. [Google Scholar] [CrossRef] [PubMed]
- Pezet, S.; Malcangio, M.; McMahon, S.B. Bdnf: A neuromodulator in nociceptive pathways? Brain Res. Rev. 2002, 40, 240–249. [Google Scholar] [CrossRef]
- Geranton, S.M.; Tochiki, K.K.; Chiu, W.W.; Stuart, S.A.; Hunt, S.P. Injury induced activation of extracellular signal-regulated kinase (ERK) in the rat rostral ventromedial medulla (RVM) is age dependant and requires the lamina-I projection pathway. Mol. Pain 2010, 6, 54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Chi, X.X.; Nicol, G.D. Brain-derived neurotrophic factor enhances the excitability of rat sensory neurons through activation of the p75 neurotrophin receptor and the sphingomyelin pathway. J. Physiol. 2008, 586, 3113–3127. [Google Scholar] [CrossRef] [PubMed]
- Kerr, B.J.; Bradbury, E.J.; Bennett, D.L.; Trivedi, P.M.; Dassan, P.; French, J.; Shelton, D.B.; McMahon, S.B.; Thompson, S.W. Brain-derived neurotrophic factor modulates nociceptive sensory inputs and nmda-evoked responses in the rat spinal cord. J. Neurosci. 1999, 19, 5138–5148. [Google Scholar] [PubMed]
- Ossipov, M.H.; Dussor, G.O.; Porreca, F. Central modulation of pain. J. Clin. Investig. 2010, 120, 3779–3787. [Google Scholar] [CrossRef] [PubMed]
- Balkowiec, A.; Katz, D.M. Cellular mechanisms regulating activity-dependent release of native brain-derived neurotrophic factor from hippocampal neurons. J. Neurosci. 2002, 22, 10399–10407. [Google Scholar] [PubMed]
- Sarhan, M.; Pawlowski, S.A.; Barthas, F.; Yalcin, I.; Kaufling, J.; Dardente, H.; Zachariou, V.; DiLeone, R.J.; Barrot, M.; Veinante, P. BDNF parabrachio-amygdaloid pathway in morphine-induced analgesia. Int. J. Neuropsychopharmacol. 2013, 16, 1649–1660. [Google Scholar] [CrossRef] [PubMed]
- Eaton, M.J.; Blits, B.; Ruitenberg, M.J.; Verhaagen, J.; Oudega, M. Amelioration of chronic neuropathic pain after partial nerve injury by adeno-associated viral (AAV) vector-mediated over-expression of BDNF in the rat spinal cord. Gene Ther. 2002, 9, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Cejas, P.J.; Martinez, M.; Karmally, S.; McKillop, M.; McKillop, J.; Plunkett, J.A.; Oudega, M.; Eaton, M.J. Lumbar transplant of neurons genetically modified to secrete brain-derived neurotrophic factor attenuates allodynia and hyperalgesia after sciatic nerve constriction. Pain 2000, 86, 195–210. [Google Scholar] [CrossRef]
- Zhang, R.; Lao, L.; Ren, K.; Berman, B.M. Mechanisms of acupuncture-electroacupuncture on persistent pain. Anesthesiology 2014, 120, 482–503. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Xie, L.; Li, X.; Cai, J.; Gu, Z.; Wang, K. Analgesic mechanism of electroacupuncture in a rat L5 spinal nerve ligation model. Exp. Ther. Med. 2015, 9, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Wang, W.; Xi, H.; He, R.; Gao, L.; Jiang, S. Regulation of neurotrophin-3 and interleukin-1 and inhibition of spinal glial activation contribute to the analgesic effect of electroacupuncture in chronic neuropathic pain states of rats. Evid. Based Complement. Alternat. Med. 2014. [Google Scholar] [CrossRef]
- Kim, S.K.; Park, J.H.; Bae, S.J.; Kim, J.H.; Hwang, B.G.; Min, B.I.; Park, D.S.; Na, H.S. Effects of electroacupuncture on cold allodynia in a rat model of neuropathic pain: Mediation by spinal adrenergic and serotonergic receptors. Exp. Neurol. 2005, 195, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Gim, G.T.; Lee, J.H.; Park, E.; Sung, Y.H.; Kim, C.J.; Hwang, W.W.; Chu, J.P.; Min, B.I. Electroacupuncture attenuates mechanical and warm allodynia through suppression of spinal glial activation in a rat model of neuropathic pain. Brain Res. Bull. 2011, 86, 403–411. [Google Scholar] [CrossRef] [PubMed]
- McKelvey, R.; Berta, T.; Old, E.; Ji, R.R.; Fitzgerald, M. Neuropathic pain is constitutively suppressed in early life by anti-inflammatory neuroimmune regulation. J. Neurosci. 2015, 35, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Foster, E.; Robertson, B.; Fried, K. TrkB-like immunoreactivity in rat dorsal root ganglia following sciatic nerve injury. Brain Res. 1994, 659, 267–271. [Google Scholar] [CrossRef]
- Narita, M.; Yajima, Y.; Aoki, T.; Ozaki, S.; Mizoguchi, H.; Tseng, L.F.; Suzuki, T. Up-regulation of the TrkB receptor in mice injured by the partial ligation of the sciatic nerve. Eur. J. Pharmacol. 2000, 401, 187–190. [Google Scholar] [CrossRef]
- Deng, Y.S.; Zhong, J.H.; Zhou, X.F. BDNF is involved in sympathetic sprouting in the dorsal root ganglia following peripheral nerve injury in rats. Neurotox Res. 1999, 1, 311–322. [Google Scholar] [CrossRef]
- Hayashida, K.; Clayton, B.A.; Johnson, J.E.; Eisenach, J.C. Brain derived nerve growth factor induces spinal noradrenergic fiber sprouting and enhances clonidine analgesia following nerve injury in rats. Pain 2008, 136, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Yajima, Y.; Narita, M.; Usui, A.; Kaneko, C.; Miyatake, M.; Narita, M.; Yamaguchi, T.; Tamaki, H.; Wachi, H.; Seyama, Y.; et al. Direct evidence for the involvement of brain-derived neurotrophic factor in the development of a neuropathic pain-like state in mice. J. Neurochem. 2005, 93, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Apfel, S.C.; Kessler, J.A. Neurotrophic factors in the treatment of peripheral neuropathy. Ciba Found. Symp. 1996, 196, 98–108, discussion 108–112. [Google Scholar] [PubMed]
- Zhou, X.F.; Rush, R.A. Endogenous brain-derived neurotrophic factor is anterogradely transported in primary sensory neurons. Neuroscience 1996, 74, 945–953. [Google Scholar] [CrossRef]
- Matsuoka, Y.; Yang, J. Selective inhibition of ERK1/2 blocks NGF to BDNF signaling and suppresses the development of and reverses already established pain behavior in rats. Neuroscience 2012, 206, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Verge, V.M.K.; Andreassen, C.S.; Arnason, T.G.; Andersen, H. Mechanisms of Disease: Role of Neurotrophins in Diabetes and Diabetic Neuropathy. In Handbook of Clinical Neurology; Douglas, W.Z., Rayaz, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 126, pp. 443–460. [Google Scholar]
- Fernyhough, P.; Diemel, L.T.; Brewster, W.J.; Tomlinson, D.R. Altered neurotrophin mrna levels in peripheral nerve and skeletal muscle of experimentally diabetic rats. J. Neurochem. 1995, 64, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Liebl, D.J.; Huang, W.; Young, W.; Parada, L.F. Regulation of Trk receptors following contusion of the rat spinal cord. Exp. Neurol. 2001, 167, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Stadelmann, C.; Kerschensteiner, M.; Misgeld, T.; Bruck, W.; Hohlfeld, R.; Lassmann, H. BDNF and gp145TrkB in multiple sclerosis brain lesions: Neuroprotective interactions between immune and neuronal cells? Brain 2002, 125, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Gielen, A.; Khademi, M.; Muhallab, S.; Olsson, T.; Piehl, F. Increased brain-derived neurotrophic factor expression in white blood cells of relapsing-remitting multiple sclerosis patients. Scand. J. Immunol. 2003, 57, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, E.J.; Khemani, S.; Von, R.; King; Priestley, J.V.; McMahon, S.B. NT-3 promotes growth of lesioned adult rat sensory axons ascending in the dorsal columns of the spinal cord. Eur. J. Neurosci. 1999, 11, 3873–3883. [Google Scholar] [CrossRef] [PubMed]
- Francis, N.; Farinas, I.; Brennan, C.; Rivas-Plata, K.; Backus, C.; Reichardt, L.; Landis, S. NT-3, like NGF, is required for survival of sympathetic neurons, but not their precursors. Dev. Biol. 1999, 210, 411–427. [Google Scholar] [CrossRef] [PubMed]
- Ivanisevic, L.; Zheng, W.; Woo, S.B.; Neet, K.E.; Saragovi, H.U. TrkA receptor “hot spots” for binding of NT-3 as a heterologous ligand. J. Biol. Chem. 2007, 282, 16754–16763. [Google Scholar] [CrossRef] [PubMed]
- Gratto, K.A.; Verge, V.M.K. Neurotrophin-3 down-regulates TrkA mRNA, NGF high-affinity binding sites, and associated phenotype in adult DRG neurons. Eur J. Neurosci. 2003, 18, 1535–1548. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.E.; Zhou, L.; Kucera, J.; Snider, W.D. Introduction of a neurotrophin-3 transgene into muscle selectively rescues proprioceptive neurons in mice lacking endogenous neurotrophin-3. Neuron 1997, 19, 503–517. [Google Scholar] [CrossRef]
- Apfel, S.C.; Wright, D.E.; Wiideman, A.M.; Dormia, C.; Snider, W.D.; Kessler, J.A. Nerve growth factor regulates the expression of brain-derived neurotrophic factor mRNA in the peripheral nervous system. Mol. Cell. Neurosci. 1996, 7, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Pradat, P.F.; Kennel, P.; Naimi-Sadaoui, S.; Finiels, F.; Orsini, C.; Revah, F.; Delaere, P.; Mallet, J. Continuous delivery of neurotrophin 3 by gene therapy has a neuroprotective effect in experimental models of diabetic and acrylamide neuropathies. Hum. Gene Ther. 2001, 12, 2237–2249. [Google Scholar] [CrossRef] [PubMed]
- Mizisin, A.P.; Calcutt, N.A.; Tomlinson, D.R.; Gallagher, A.; Fernyhough, P. Neurotrophin-3 reverses nerve conduction velocity deficits in streptozotocin-diabetic rats. J. Peripher. Nerv. Syst. 1999, 4, 211–221. [Google Scholar] [PubMed]
- Boyce, V.S.; Park, J.; Gage, F.H.; Mendell, L.M. Differential effects of brain-derived neurotrophic factor and neurotrophin-3 on hindlimb function in paraplegic rats. Eur. J. Neurosci. 2012, 35, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Kalinowska-Lyszczarz, A.; Pawlak, M.A.; Michalak, S.; Paprzycki, W.; Losy, J. Immune cell NT-3 expression is associated with brain atrophy in multiple sclerosis patients. J. NeuroImmunol. 2011, 240–241, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Hallbook, F.; Ibanez, C.F.; Persson, H. Evolutionary studies of the nerve growth factor family reveal a novel member abundantly expressed in xenopus ovary. Neuron 1991, 6, 845–858. [Google Scholar] [CrossRef]
- Ip, N.Y.; Ibáñez, C.F.; Nye, S.H.; McClain, J.; Jones, P.F.; Gies, D.R.; Belluscio, L.; le Beau, M.M.; Espinosa, R.; Squinto, S.P. Mammalian neurotrophin-4: Structure, chromosomal localization, tissue distribution, and receptor specificity. Proc. Natl. Acad. Sci. USA 1992, 89, 3060–3064. [Google Scholar] [CrossRef] [PubMed]
- Berkemeier, L.R.; Winslow, J.W.; Kaplan, D.R.; Nikolics, K.; Goeddel, D.V.; Rosenthal, A. Neurotrophin-5: A novel neurotrophic factor that activates Trk and TrkB. Neuron 1991, 7, 857–866. [Google Scholar] [CrossRef]
- Hibbert, A.P.; Morris, S.J.; Seidah, N.G.; Murphy, R.A. Neurotrophin-4, alone or heterodimerized with brain-derived neurotrophic factor, is sorted to the constitutive secretory pathway. J. Biol. Chem. 2003, 278, 48129–48136. [Google Scholar] [CrossRef] [PubMed]
- Buck, C.R.; Seburn, K.L.; Cope, T.C. Neurotrophin expression by spinal motoneurons in adult and developing rats. J. Comp. Neurol. 2000, 416, 309–318. [Google Scholar] [CrossRef]
- Stephens, H.E.; Belliveau, A.C.; Gupta, J.S.; Mirkovic, S.; Kablar, B. The role of neurotrophins in the maintenance of the spinal cord motor neurons and the dorsal root ganglia proprioceptive sensory neurons. Int. J. Dev. Neurosci. 2005, 23, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Lucas, G.; Hendolin, P.; Harkany, T.; Agerman, K.; Paratcha, G.; Holmgren, C.; Zilberter, Y.; Sairanen, M.; Minichiello, L.; Castren, E.; et al. Neurotrophin-4 mediated TRKB activation reinforces morphine-induced analgesia. Nat. Neurosci. 2003, 6, 221–222. [Google Scholar] [CrossRef] [PubMed]
- Sakuragi, S.; Tominaga-Yoshino, K.; Ogura, A. Involvement of TrkB- and p75NTR-signaling pathways in two contrasting forms of long-lasting synaptic plasticity. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Boutilier, J.; Ceni, C.; Pagdala, P.C.; Forgie, A.; Neet, K.E.; Barker, P.A. Proneurotrophins require endocytosis and intracellular proteolysis to induce TrkA activation. J. Biol. Chem. 2008, 283, 12709–12716. [Google Scholar] [CrossRef] [PubMed]
- Peleshok, J.C.; Ribeiro-da-Silva, A. Neurotrophic factor changes in the rat thick skin following chronic constriction injury of the sciatic nerve. Mol. Pain 2012, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.J.; Ji, R.R. c-FOS and pERK, which is a better marker for neuronal activation and central sensitization after noxious stimulation and tissue injury? Open Pain J. 2009, 2, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, C.F.; Simi, A. P75 neurotrophin receptor signaling in nervous system injury and degeneration: Paradox and opportunity. Trends Neurosci. 2012, 35, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Tomita, K.; Kubo, T.; Matsuda, K.; Fujiwara, T.; Yano, K.; Winograd, J.M.; Tohyama, M.; Hosokawa, K. The neurotrophin receptor p75NTR in schwann cells is implicated in remyelination and motor recovery after peripheral nerve injury. Glia 2007, 55, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Karchewski, L.A.; Kim, F.A.; Johnston, J.; McKnight, R.M.; Verge, V.M. Anatomical evidence supporting the potential for modulation by multiple neurotrophins in the majority of adult lumbar sensory neurons. J. Comp. Neurol. 1999, 413, 327–341. [Google Scholar] [CrossRef]
- Obata, K.; Katsura, H.; Sakurai, J.; Kobayashi, K.; Yamanaka, H.; Dai, Y.; Fukuoka, T.; Noguchi, K. Suppression of the p75 neurotrophin receptor in uninjured sensory neurons reduces neuropathic pain after nerve injury. J. Neurosci. 2006, 26, 11974–11986. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.F.; Li, E.; Huber, L.J.; Landis, S.C.; Sharpe, A.H.; Chao, M.V.; Jaenisch, R. Targeted mutation of the gene encoding the low affinity NGF receptor p75 leads to deficits in the peripheral sensory nervous system. Cell 1992, 69, 737–749. [Google Scholar] [CrossRef]
- Vaegter, C.B.; Jansen, P.; Fjorback, A.W.; Glerup, S.; Skeldal, S.; Richner, M.; Erdmann, B.; Nyengaard, J.R.; Tessarollo, L.; Lewin, G.R.; et al. Sortilin associates with trk receptors to enhance anterograde transport and signaling by neurotrophins. Nat. Neurosci. 2011, 14, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, D.R.; Miller, F.D. Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol. 2000, 10, 381–391. [Google Scholar] [CrossRef]
- Ji, R.R.; Kawasaki, Y.; Zhuang, Z.Y.; Wen, Y.R.; Zhang, Y.Q. Protein kinases as potential targets for the treatment of pathological pain. Handb. Exp. Pharmacol. 2007, 359–389. [Google Scholar]
- Zhuang, Z.Y.; Gerner, P.; Woolf, C.J.; Ji, R.R. Erk is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain 2005, 114, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.T.; Tu, H.Y.; Xin, W.J.; Liu, X.G.; Zhang, G.H.; Zhai, C.H. Activation of phosphatidylinositol 3-kinase and protein kinase B/Akt in dorsal root ganglia and spinal cord contributes to the neuropathic pain induced by spinal nerve ligation in rats. Exp. Neurol. 2007, 206, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.J.; Reichardt, L.F. Trk receptors: Roles in neuronal signal transduction. Annu. Rev. Biochem. 2003, 72, 609–642. [Google Scholar] [CrossRef] [PubMed]
- Canossa, M.; Griesbeck, O.; Berninger, B.; Campana, G.; Kolbeck, R.; Thoenen, H. Neurotrophin release by neurotrophins: Implications for activity-dependent neuronal plasticity. Proc. Natl. Acad. Sci. USA 1997, 94, 13279–13286. [Google Scholar] [CrossRef] [PubMed]
- Coderre, T.J. Contribution of protein kinase c to central sensitization and persistent pain following tissue injury. Neurosci. Lett. 1992, 140, 181–184. [Google Scholar] [CrossRef]
- Reichardt, L.F. Neurotrophin-regulated signalling pathways. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 1545–1564. [Google Scholar] [CrossRef] [PubMed]
- Apfel, S.C.; Schwartz, S.; Adornato, B.T.; Freeman, R.; Biton, V.; Rendell, M.; Vinik, A.; Giuliani, M.; Stevens, J.C.; Barbano, R.; et al. Efficacy and safety of recombinant human nerve growth factor in patients with diabetic polyneuropathy: A randomized controlled trial. rhNGF clinical investigator group. JAMA 2000, 284, 2215–2221. [Google Scholar] [CrossRef] [PubMed]
- Cavaletti, G.; Marmiroli, P. The role of growth factors in the prevention and treatment of chemotherapy-induced peripheral neurotoxicity. Curr. Drug Saf. 2006, 1, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.T.; Murphy, F.T.; Radin, D.M.; Davignon, I.; Smith, M.D.; West, C.R. Tanezumab reduces osteoarthritic knee pain: Results of a randomized, double-blind, placebo-controlled phase-III trial. J. Pain 2012, 13, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Bramson, C.; Herrmann, D.N.; Carey, W.; Keller, D.; Brown, M.T.; West, C.R.; Verburg, K.M.; Dyck, P.J. Exploring the role of tanezumab as a novel treatment for the relief of neuropathic pain. Pain Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Katz, N.; Borenstein, D.G.; Birbara, C.; Bramson, C.; Nemeth, M.A.; Smith, M.D.; Brown, M.T. Efficacy and safety of tanezumab in the treatment of chronic low back pain. Pain 2011, 152, 2248–2258. [Google Scholar] [CrossRef] [PubMed]
- Schnitzer, T.J.; Marks, J.A. A systematic review of the efficacy and general safety of antibodies to ngf in the treatment of oa of the hip or knee. Osteoarthr. Cartil. 2015, 23, S8–S17. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.T.; Herrmann, D.N.; Goldstein, M.; Burr, A.M.; Smith, M.D.; West, C.R.; Verburg, K.M.; Dyck, P.J. Nerve safety of tanezumab, a nerve growth factor inhibitor for pain treatment. J. Neurol. Sci. 2014, 345, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Danehy, S. Pfizer and Lilly Preparing to Resume Phase-3 Chronic Pain Program for Tanezumab. Available online: http://www.pfizer.com/news/press-release/press-release-detail/pfizer_and_lilly_preparing_to_resume_phase_3_chronic_pain_program_for_tanezumab (accessed on 20 April 2015).
- Lange, J. Glenmark’s Novel Monoclonal Antibody gbr 900 for Treatment of Chronic Pain Entering Human Trials. Available online: http://www.prnewswire.com/news-releases/glenmarks-novel-monoclonal-antibody-gbr-900-for-treatment-of-chronic-pain-entering-human-trials-257130311.html (accessed on 20 April 2015).
- Chaudhry, V.; Giuliani, M.; Petty, B.G.; Lee, D.; Seyedsadr, M.; Hilt, D.; Cornblath, D.R. Tolerability of recombinant-methionyl human neurotrophin-3 (r-metHuNT3) in healthy subjects. Muscle Nerve 2000, 23, 189–192. [Google Scholar] [CrossRef]
- Leinninger, G.M.; Vincent, A.M.; Feldman, E.L. The role of growth factors in diabetic peripheral neuropathy. J. Peripher. Nerv. Syst. 2004, 9, 26–53. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, N.; Smith, M.T. Neurotrophins and Neuropathic Pain: Role in Pathobiology. Molecules 2015, 20, 10657-10688. https://doi.org/10.3390/molecules200610657
Khan N, Smith MT. Neurotrophins and Neuropathic Pain: Role in Pathobiology. Molecules. 2015; 20(6):10657-10688. https://doi.org/10.3390/molecules200610657
Chicago/Turabian StyleKhan, Nemat, and Maree T. Smith. 2015. "Neurotrophins and Neuropathic Pain: Role in Pathobiology" Molecules 20, no. 6: 10657-10688. https://doi.org/10.3390/molecules200610657