Integrated LC-MS/MS Analytical Systems and Physical Inspection for the Analysis of a Botanical Herbal Preparation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Method Development
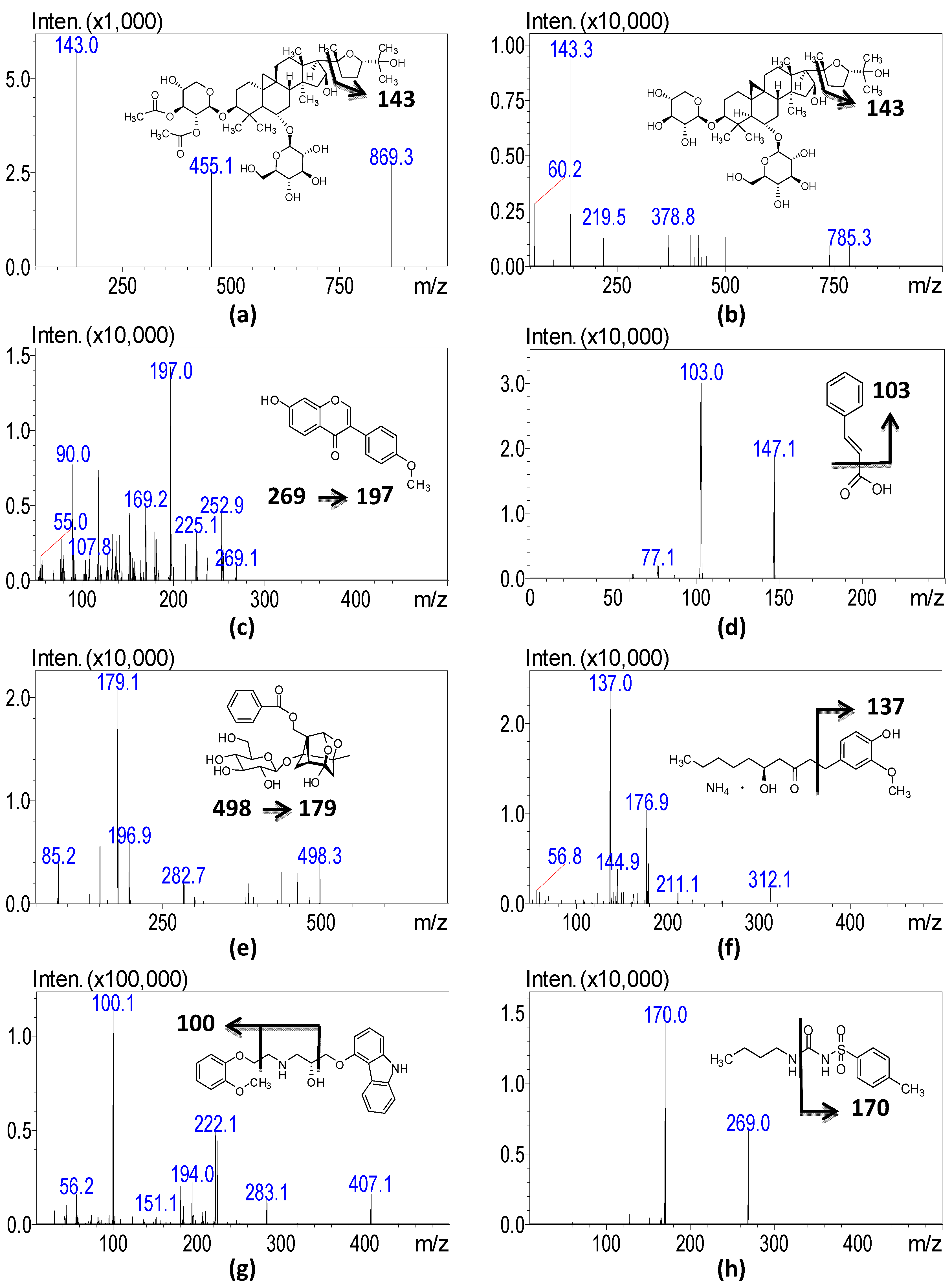
| MW a | Ion Mode | RT b | CE c | Q1 Mass d | Q3 Mass d | |
|---|---|---|---|---|---|---|
| Astragalus membranaceus | ||||||
| Astragaloside I | 868.4 | + | 5.8 | −19 | 869.3 [M + H]+ | 143.0 |
| Astragaloside IV | 784.9 | + | 6.4 | −21 | 785.3 [M + H]+ | 143.3 |
| Formononetin | 268.3 | + | 5.2 | −42 | 269.1 [M + H]+ | 197.0 |
| Paeonia lactiflora | ||||||
| Paeoniflorin | 480.2 | + | 3.6 | −26 | 498.3 [M + NH4]+ | 179.1 |
| Zingiber officinale | ||||||
| Gingerol | 294.3 | + | 5.2 | −25 | 312.0 [M + NH4]+ | 137.2 |
| Cinnamomum cassia | ||||||
| Cinnamic acid | 148.1 | − | 3.4 | 15 | 147.1 [M − H]− | 103.0 |
| Carvedilol (IS) | 406.4 | + | 4.9 | −35 | 407.1 [M + H]+ | 100.1 |
| Tolbutamide (IS) | 270.3 | − | 4.4 | 20 | 269.0 [M − H]− | 170.0 |
2.2. Method Validation
| Nominal Concentration (ng/mL) | Intra-Day | Inter-Day | ||||
|---|---|---|---|---|---|---|
| Observed Concentration (ng/mL) | Accuracy (%) | Precision (%) | Observed Concentration (ng/mL) | Accuracy (%) | Precision (%) | |
| Astragaloside I | ||||||
| 25 | 25.21 ± 1.49 | 0.86 | 5.90 | 23.83 ± 0.85 | −4.68 | 3.55 |
| 50 | 47.68 ± 3.93 | −4.64 | 8.24 | 48.78 ± 1.73 | −2.44 | 3.56 |
| 250 | 259.48 ± 5.70 | 3.79 | 2.20 | 237.78 ± 5.93 | −4.89 | 2.49 |
| 500 | 488.38 ±15.04 | −2.32 | 3.08 | 512.51 ± 13.76 | 2.50 | 2.69 |
| 1000 | 987.19 ± 9.88 | −1.28 | 1.00 | 983.27 ± 22.24 | −1.67 | 2.26 |
| Astragaloside IV | ||||||
| 10 | 9.23 ± 0.46 | −7.72 | 4.98 | 9.82 ± 1.34 | −1.84 | 13.61 |
| 50 | 23.64 ± 1.44 | 5.43 | 6.08 | 49.21 ± 3.29 | −1.58 | 6.68 |
| 250 | 50.21 ± 1.14 | 0.41 | 2.26 | 250.03 ± 7.69 | 0.01 | 3.08 |
| 500 | 246.48 ± 8.80 | −1.41 | 3.57 | 515.21 ± 5.45 | 3.04 | 1.06 |
| 1000 | 517.22 ± 6.27 | 3.44 | 1.21 | 997.18 ± 0.68 | −0.28 | 0.07 |
| Formononetin | ||||||
| 25 | 22.81 ± 1.33 | −8.75 | 5.82 | 22.08 ± 0.47 | −11.67 | 2.12 |
| 50 | 46.01 ± 1.93 | −7.98 | 4.19 | 46.77 ± 2.34 | −6.46 | 5.01 |
| 250 | 253.44 ± 10.43 | 1.38 | 4.11 | 254.42 ± 5.85 | 1.77 | 2.30 |
| 500 | 506.04 ± 10.04 | 1.21 | 1.98 | 509.04 ± 15.79 | 1.81 | 3.10 |
| 1000 | 995.55 ± 2.29 | −0.44 | 0.23 | 994.74 ± 6.45 | −0.53 | 0.65 |
| Cinnamic acid | ||||||
| 25 | 27.69 ± 1.95 | 10.74 | 7.05 | 26.32 ± 1.53 | 5.29 | 5.83 |
| 50 | 53.33 ± 1.62 | 6.65 | 3.04 | 49.83 ± 3.40 | −0.33 | 6.82 |
| 250 | 243.41 ± 5.31 | −2.64 | 2.18 | 236.01 ± 11.10 | −5.60 | 4.70 |
| 500 | 497.55 ± 10.83 | −0.49 | 2.18 | 487.97 ± 23.74 | −2.41 | 4.87 |
| 1000 | 1001.63 ± 5.97 | 0.16 | 0.60 | 997.47 ± 8.11 | −0.25 | 0.81 |
| Paeoniflorin | ||||||
| 25 | 24.99 ± 1.55 | −0.04 | 6.21 | 24.50 ± 3.31 | −2.01 | 13.50 |
| 50 | 49.44 ± 3.38 | −1.13 | 6.83 | 51.09 ± 4.00 | 2.17 | 7.84 |
| 250 | 257.68 ± 16.41 | 3.07 | 6.37 | 245.60 ± 10.78 | −1.76 | 4.39 |
| 500 | 501.05 ± 7.91 | 0.21 | 1.58 | 505.76 ± 1.82 | 1.15 | 0.36 |
| 1000 | 993.47 ± 9.92 | −0.65 | 1.00 | 998.49 ± 0.90 | −0.15 | 0.09 |
| Gingerol | ||||||
| 10 | 9.58 ± 0.84 | −4.25 | 8.82 | 9.26 ± 0.47 | 7.38 | 5.10 |
| 50 | 49.13 ± 1.79 | −1.74 | 3.65 | 50.31 ± 2.26 | 0.61 | 4.49 |
| 250 | 251.93 ± 2.66 | 0.77 | 1.05 | 251.84 ± 7.15 | 0.73 | 2.84 |
| 500 | 500.08 ± 7.34 | 0.02 | 1.47 | 497.17 ± 8.01 | −0.57 | 1.61 |
| 1000 | 999.65 ± 3.05 | −0.04 | 0.31 | 1000.92 ± 2.27 | 0.09 | 0.23 |
2.3. Application to the HGW Samples
| Components | EX | A | B | C | D | E |
|---|---|---|---|---|---|---|
| Astragaloside I | 0.46 ± 0.024 | 0.31 ± 0.007 | 0.52 ± 0.005 | 0.81 ± 0.004 | 0.39 ± 0.006 | 0.36 ± 0.001 |
| Astragaloside IV | 0.09 ± 0.002 | 0.12± 0.005 | 0.16 ± 0.003 | 0.10 ± 0.003 | 0.08± 0.002 | 0.07 ± 0.001 |
| Formononetin | 0.83 ± 0.021 | 0.31 ± 0.004 | 0.68 ± 0.010 | 0.46 ± 0.006 | 0.54 ± 0.006 | 0.34 ± 0.002 |
| Cinnamic acid | 0.24 ± 0.010 | 0.32 ± 0.009 | 0.34 ± 0.005 | 0.19 ± 0.006 | 0.31 ± 0.007 | 0.17 ± 0.004 |
| Paeoniflorin | 4.15 ± 0.044 | 2.26 ± 0.013 | 3.72 ± 0.003 | 2.09 ± 0.047 | 2.97 ± 0.002 | 1.67 ± 0.016 |
| Gingerol | 0.08 ± 0.003 | 0.91 ± 0.006 | 0.25 ± 0.007 | 0.36 ± 0.010 | 0.30 ± 0.007 | 0.80 ± 0.001 |
2.4. Physical Examination of the Pharmaceutical Additives
2.4.1. Scanning Electron Microscopy
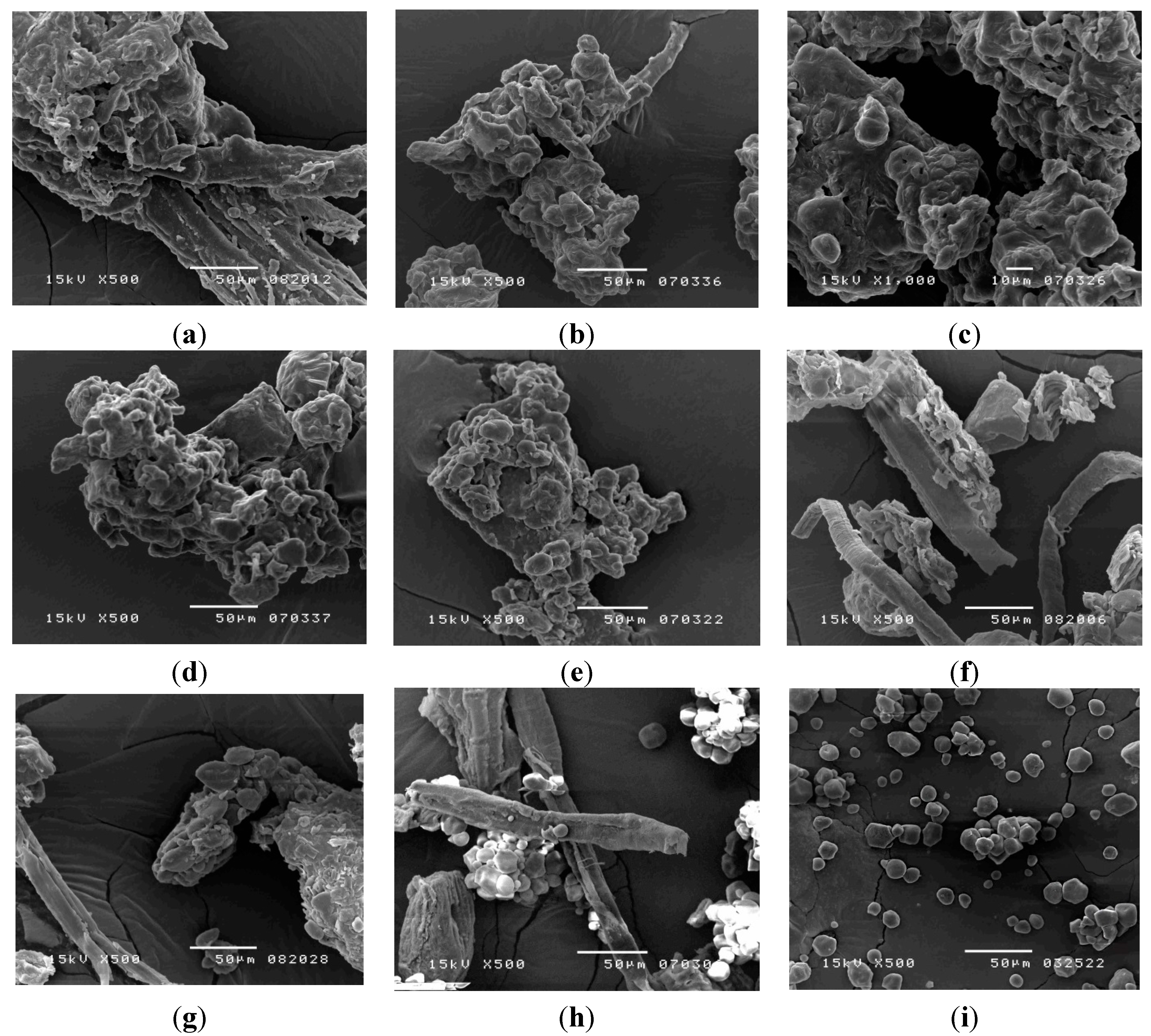
2.4.2. Light Microscopy Images of Congo red and Iodine-KI Stained Samples
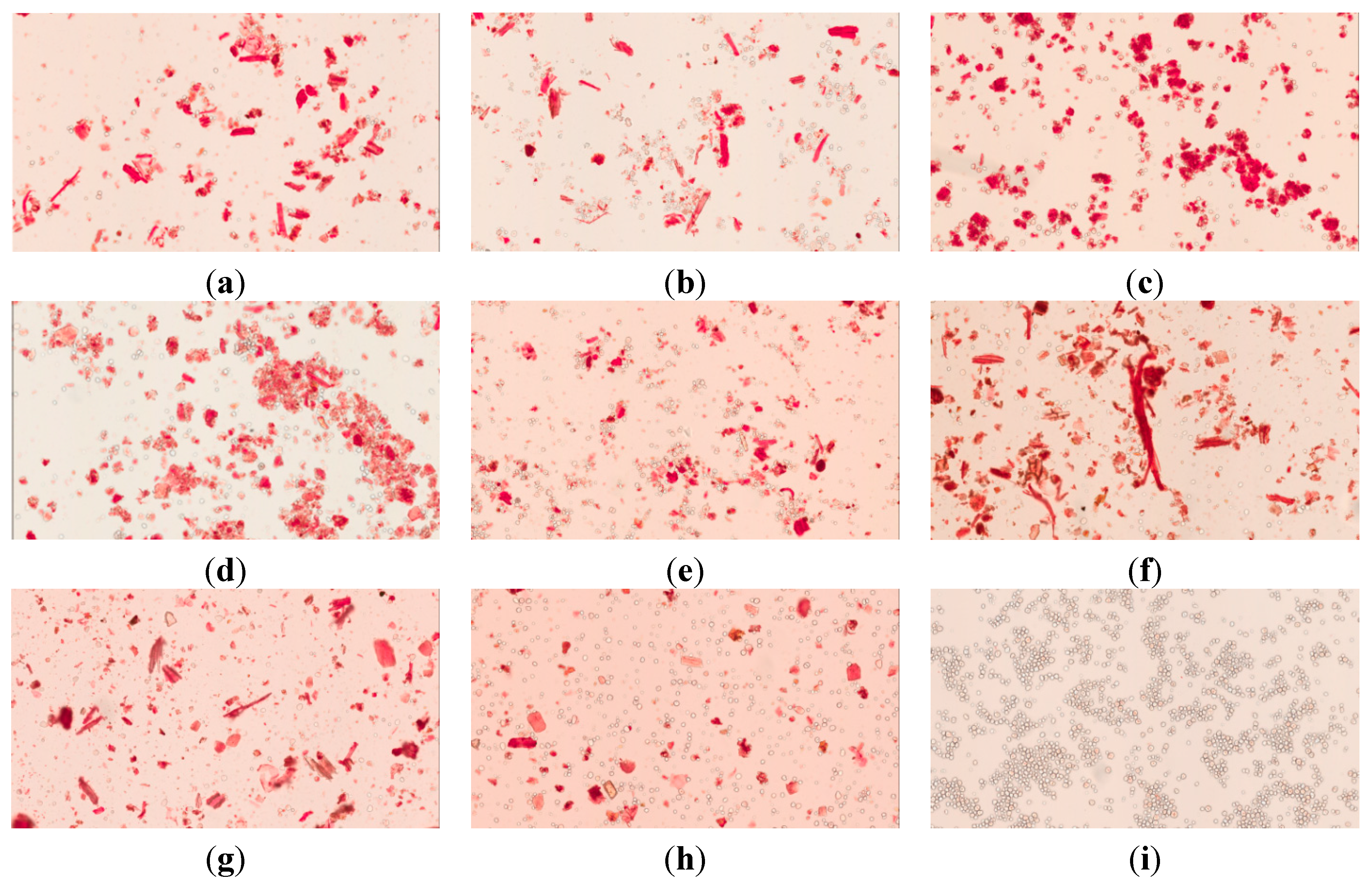

2.4.3. Solubility and Swelling Experiment
| Sample Brand | 55 °C | 65 °C | 75 °C | 85 °C | 95 °C |
|---|---|---|---|---|---|
| A | 38.73 ± 2.77 | 38.84 ± 0.72 | 40.82 ± 3.60 | 42.59 ± 0.19 | 45.74 ± 2.07 |
| B | 45.95 ± 4.84 | 47.78 ± 2.61 | 46.18 ±0.62 | 50.13 ± 0.94 | 52.97 ± 2.93 |
| C | 40.97 ± 2.58 | 42.19 ± 1.47 | 43.62 ± 1.31 | 42.76 ± 1.32 | 48.81 ± 3.95 |
| D | 33.67 ± 2.31 | 35.20 ± 0.97 | 39.95 ± 2.94 | 38.77 ± 1.68 | 40.64 ± 3.89 |
| E | 39.51 ± 0.19 | 41.33 ± 3.22 | 42.51 ± 2.85 | 41.63 ± 5.62 | 45.49 ± 1.56 |
| Sample Brand | 55 °C | 65 °C | 75 °C | 85 °C | 95 °C |
|---|---|---|---|---|---|
| A | 4.99 ± 0.71 | 6.26 ± 0.31 | 6.22 ± 0.82 | 7.41 ± 0.28 | 6.86 ± 1.14 |
| B | 5.96 ± 1.64 | 7.44 ± 1.38 | 8.83 ± 0.21 | 8.79 ± 1.44 | 6.69 ± 1.19 |
| C | 6.43 ± 0.57 | 6.15 ± 1.09 | 8.93 ± 0.35 | 7.45 ± 1.99 | 4.74 ± 0.71 |
| D | 4.78 ± 0.31 | 5.37 ± 0.71 | 5.42 ± 1.58 | 5.59 ± 0.53 | 7.71 ± 1.54 |
| E | 4.04 ± 0.41 | 4.57 ± 0.89 | 5.81 ± 0.43 | 5.25 ± 0.85 | 6.60 ± 0.60 |
2.4.4. Crude Fiber Content
3. Experimental Section
3.1. Reagents and Materials
3.2. Preparation of Herbal Extraction
3.3. UPLC-ESI-MS/MS System and Experimental Condition
3.4. Preparation of the Sample
3.5. Preparation of the Samples
3.6. Physical Examination of Additives for Raw Herbal Powder
3.6.1. Scanning Electron Microscope
3.6.2. Light Microscopy Images of Congo Red and Iodine-KI Stained Samples
3.6.3. Solubility and Swelling Experiments
3.6.4. Crude Fiber Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ernst, E. Prevalence of use of complementary/alternative medicine: A systematic review. Bull. World Health Organ. 2000, 78, 252–257. [Google Scholar] [PubMed]
- Grant, S.J.; Bin, Y.S.; Kiat, H.; Chang, D.H. The use of complementary and alternative medicine by people with cardiovascular disease: A systematic review. BMC Public Health 2012, 12. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Liu, W.K.; Deng, L.; Tian, J.X.; Tong, X.L. Clinical efficacy of aconitum-containing traditional Chinese medicine for diabetic peripheral neuropathic pain. Am. J. Chin. Med. 2014, 42, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Hou, H. Effects of Huangqi Guizhi Wuwu Tang on diabetic peripheral neuropathy. J. Altern. Complement. Med. 2006, 12, 506–509. [Google Scholar] [PubMed]
- Zu, Y.; Yan, M.; Fu, Y.; Liu, W.; Zhang, L.; Gu, C.; Efferth, T. Determination and quantification of astragalosides in Radix Astragali and its medicinal products using LC-MS. J. Sep. Sci. 2009, 32, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Du, H.W.; Zhao, X.L.; Zhang, A.H.; Yao, L.; Zhang, Y.Y. Rapid separation, identification and analysis of Astragalus membranaceus Fisch using liquid chromatography-tandem mass spectrometry. J. Chromatogr. Sci. 2014, 52, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.V.; Gan, S.H. Cinnamon: A multifaceted medicinal plant. Evid. -Based Complement. Altern. Med. 2014, 2014, 642942. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Qiao, Y.; Yang, J.; Liu, X.; Song, Y.; Liu, Z.; Li, F. UPLC-MS/MS determination of paeoniflorin, naringin, naringenin and glycyrrhetinic acid in rat plasma and its application to a pharmacokinetic study after oral administration of Si-Ni-San decoction. J. Pharm. Biomed. Anal. 2012, 66, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.G.; Shin, B.S.; Choi, Y.; Ryu, J.K.; Shin, S.W.; Choo, H.W.; Yoo, S.D. Determination and pharmacokinetics of [6]-gingerol in mouse plasma by liquid chromatography-tandem mass spectrometry. Biomed. Chromatogr. 2012, 26, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, Y.; Zhong, Y.; Huang, X.; Zheng, X.; Li, N.; He, S.; Mi, S.; Wang, N. An LC-MS/MS method for determination of jujuboside A in rat plasma and its application to pharmacokinetic studies. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 899, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.P.; Deal, G.; Koo, H.L.; Rees, D.; Sun, H.; Chen, S.; Dou, J.H.; Makarov, V.G.; Pozharitskaya, O.N.; Shikov, A.N.; et al. Future development of global regulations of Chinese herbal products. J. Ethnopharmacol. 2012, 140, 568–586. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Song, Y.; Li, S.L.; Bian, Y.Y.; Guan, J.; Li, P. Simultaneous analysis of seven astragalosides in Radix Astragali and related preparations by liquid chromatography coupled with electrospray ionization time-of-flight mass spectrometry. J. Sep. Sci. 2006, 29, 2012–2022. [Google Scholar] [CrossRef] [PubMed]
- Hussein Al-Amier, S.J.E. Lyle Craker, Evaluation of Extraction Methods for Isolation and Detection of Formononetin in Black Cohosh (Actaea racemosa L.). J. Med. Act. Plants 2012, 1, 6–12. [Google Scholar]
- Jiang, H.; Solyom, A.M.; Timmermann, B.N.; Gang, D.R. Characterization of gingerol-related compounds in ginger rhizome (Zingiber officinale Rosc.) by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. RCM 2005, 19, 2957–2964. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zick, S.; Li, X.; Zou, P.; Wright, B.; Sun, D. Examination of the pharmacokinetics of active ingredients of ginger in humans. AAPS J. 2011, 13, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hu, X.; Yang, Q.; Yu, Z.; Zhao, Z.; Yi, T.; Chen, H. Comparison of the immunoregulatory function of different constituents in radix astragali and radix hedysari. J. Biomed. Biotechnol. 2010, 2010, 479426. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Xia, X.; Chou, G.; Liu, D.; Zuberi, A.; Ye, J.; Liu, Z. Variations in the contents of gingerols and chromatographic fingerprints of ginger root extracts prepared by different preparation methods. J. AOAC Int. 2014, 97, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.M.; Hou, M.L.; Lin, L.C.; Tsai, T.H. Chemical and Physical Methods to Analyze a Multicomponent Traditional Chinese Herbal Prescription Using LC-MS/MS, Electron Microscope, and Congo Red Staining. Evid. -Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Samim, M.; Sandkuijl, D.; Tretyakov, I.; Cisek, R.; Barzda, V. Differential polarization nonlinear optical microscopy with adaptive optics controlled multiplexed beams. Int. J. Mol. Sci. 2013, 14, 18520–18534. [Google Scholar] [CrossRef] [PubMed]
- Herth, W.S.E. The fluorochrome, Calcofluor white, binds oriented to structural polysaccharide fibrils. Protoplasma 1980, 105, 129–133. [Google Scholar] [CrossRef]
- Agasimani, S.; Selvakumar, G.; Joel, A.J.; Ganesh Ram, S. A simple and rapid single kernel screening method to estimate amylose content in rice grains. Phytochem. Anal. 2013, 24, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-Y.; Yeh, A.-I. Relationships between thermal, rheological characteristics and swelling power for various starches. J. Food Eng. 2001, 50, 141–148. [Google Scholar] [CrossRef]
- Ratnayake, W.S.; Jackson, D.S. Gelatinization and solubility of corn starch heatng excess water: New Insights. J. Agric. Food Chem. 2006, 54, 3712–3716. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.H.; Chen, W.M.; Tsai, T.H. Identification of multiple ingredients for a Traditional Chinese Medicine preparation (bu-yang-huan-wu-tang) by liquid chromatography coupled with tandem mass spectrometry. Molecules 2013, 18, 11281–11298. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.L.; Chang, L.W.; Lin, C.H.; Lin, L.C.; Tsai, T.H. Determination of bioactive components in Chinese herbal formulae and pharmacokinetics of rhein in rats by UPLC-MS/MS. Molecules 2014, 19, 4058–4075. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds astragaloside I, astragaloside IV, formononetin, cinnamic acid, paeoniflorin and gingerol are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, K.-M.; Cheng, Y.-Y.; Tsai, T.-H. Integrated LC-MS/MS Analytical Systems and Physical Inspection for the Analysis of a Botanical Herbal Preparation. Molecules 2015, 20, 10641-10656. https://doi.org/10.3390/molecules200610641
Lai K-M, Cheng Y-Y, Tsai T-H. Integrated LC-MS/MS Analytical Systems and Physical Inspection for the Analysis of a Botanical Herbal Preparation. Molecules. 2015; 20(6):10641-10656. https://doi.org/10.3390/molecules200610641
Chicago/Turabian StyleLai, Kuan-Ming, Yung-Yi Cheng, and Tung-Hu Tsai. 2015. "Integrated LC-MS/MS Analytical Systems and Physical Inspection for the Analysis of a Botanical Herbal Preparation" Molecules 20, no. 6: 10641-10656. https://doi.org/10.3390/molecules200610641






