Mesquite Gum as a Novel Reducing and Stabilizing Agent for Modified Tollens Synthesis of Highly Concentrated Ag Nanoparticles
Abstract
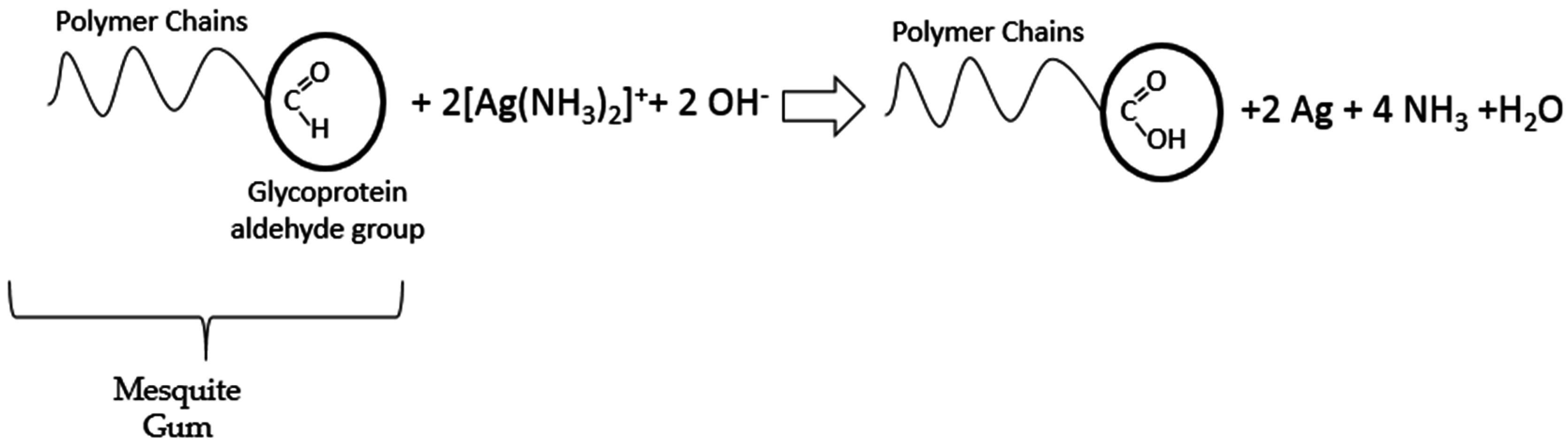
:1. Introduction
2. Results and Discussion
2.1. UV-Vis Absorption Spectroscopy
2.2. Morphological Analysis: SEM and STEM
2.3. Colloidal Stability Assesment: DLS
2.4. Infrared Analysis
2.5. Silver Ion Analysis (ISE Measure)
2.6. Discussion
2.7. Comparison with Other Methods (Production Capacity)
3. Experimental Section
3.1. Materials and Methods
3.2. Mesquite Gum Purification
3.3. Synthesis of Silver Nanoparticles
3.4. Characterization
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mulfinger, L.; Solomon, S.D.; Bahadory, M.; Jeyarajasingam, A.V.; Rutkowsky, S.A.; Boritz, C. Synthesis and Study of Silver Nanoparticles. J. Chem. Educ. 2007, 84, 322–325. [Google Scholar] [CrossRef]
- Abou El-Nour, K.M.M.; Eftaiha, A.; Al-Warthan, A.; Ammar, R.A.A. Synthesis and applications of silver nanoparticles. Arab. J. Chem. 2010, 3, 135–140. [Google Scholar] [CrossRef]
- Zhai, H.-J.; Sun, D.-W.; Wang, H.-S. Catalytic properties of silica/silver nanocomposites. J. Nanosci. Nanotechnol. 2006, 6, 1968–1972. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y. Shape-Controlled Synthesis of Gold and Silver Nanoparticles. Science 2002, 298, 2176–2179. [Google Scholar] [CrossRef] [PubMed]
- Rand, B.P.; Peumans, P.; Forrest, S.R. Long-range absorption enhancement in organic tandem thin-film solar cells containing silver nanoclusters. J. Appl. Phys. 2004, 96, 7519–7526. [Google Scholar] [CrossRef]
- Cole, J.R.; Halas, N.J. Optimized plasmonic nanoparticle distributions for solar spectrum harvesting. Appl. Phys. Lett. 2006, 89, 153120. [Google Scholar] [CrossRef]
- Monteiro, D.R.; Gorup, L.F.; Takamiya, A.S.; Ruvollo-Filho, A.C.; de Camar, E.R.; Barbosa, D.B. The growing importance of materials that prevent microbial adhesion: Antimicrobial effect of medical devices containing silver. Int. J. Antimicrob. Agents 2009, 34, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Tran, Q.H.; Nguyen, V.Q.; Le, A.-T. Silver nanoparticles: Synthesis, properties, toxicology, applications and perspectives. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 033001. [Google Scholar] [CrossRef]
- Tripathi, A.; Chandrasekaran, N.; Raichur, A.M.; Mukherjee, A. Antibacterial applications of silver nanoparticles synthesized by aqueous extract of Azadirachta indica (Neem) leaves. J. Biomed. Nanotechnol. 2009, 5, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-León, E.; Iñiguez-Palomares, R.; Navarro, R.E.; Herrera-Urbina, R.; Tánori, J.; Iñiguez-Palomares, C.; Maldonado, A. Synthesis of silver nanoparticles using reducing agents obtained from natural sources (Rumex hymenosepalus extracts). Nanoscale Res. Lett. 2013, 8, 318. [Google Scholar] [CrossRef] [PubMed]
- Mason, C.; Vivekanandhan, S.; Misra, M.; Mohanty, A.K. Switchgrass (Panicum virgatum) Extract Mediated Green Synthesis of Silver Nanoparticles. World J. Nano Sci. Eng. 2012, 2, 47–52. [Google Scholar] [CrossRef]
- Tai, C.Y.; Wang, Y.-H.; Liu, H.-S. A green process for preparing silver nanoparticles using spinning disk reactor. AIChE J. 2008, 54, 445–452. [Google Scholar] [CrossRef]
- Sintubin, L.; Verstraete, W.; Boon, N. Biologically produced nanosilver: Current state and future perspectives. Biotechnol. Bioeng. 2012, 109, 2422–2436. [Google Scholar] [CrossRef] [PubMed]
- Philip, D. Green synthesis of gold and silver nanoparticles using Hibiscus rosa sinensis. Phys. E Low-Dimens. Syst. Nanostruct. 2010, 42, 1417–1424. [Google Scholar] [CrossRef]
- Ali, Z.A.; Yahya, R.; Sekaran, S.D.; Puteh, R. Green Synthesis of Silver Nanoparticles Using Apple Extract and Its Antibacterial Properties. Adv. Mater. Sci. Eng. 2016, 2016, 6. [Google Scholar] [CrossRef]
- Kumar, V.; Yadav, S.C.; Yadav, S.K. Syzygium cumini leaf and seed extract mediated biosynthesis of silver nanoparticles and their characterization. J. Chem. Technol. Biotechnol. 2010, 85, 1301–1309. [Google Scholar] [CrossRef]
- Shankar, S.S.; Ahmad, A.; Sastry, M. Geranium leaf assisted biosynthesis of silver nanoparticles. Biotechnol. Prog. 2003, 19, 1627–1631. [Google Scholar] [CrossRef] [PubMed]
- Krishnaraj, C.; Jagan, E.G.; Rajasekar, S.; Selvakumar, P.; Kalaichelvan, P.T.; Mohan, N. Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids Surf. B Biointerfaces 2010, 76, 50–56. [Google Scholar] [CrossRef] [PubMed]
- MubarakAli, D.; Thajuddin, N.; Jeganathan, K.; Gunasekaran, M. Plant extract mediated synthesis of silver and gold nanoparticles and its antibacterial activity against clinically isolated pathogens. Colloids Surf. B Biointerfaces 2011, 85, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.P.; Lahtinen, M.; Sarkka, H.; Sillanpaa, M. Bioprospective of Sorbus aucuparia leaf extract in development of silver and gold nanocolloids. Colloids Surf. B Biointerfaces 2010, 80, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Kotakadi, V.S.; Rao, Y.S.; Gaddam, S.A.; Prasad, T.N.V.K.V.; Reddy, A.V.; Gopal, D.V.R.S. Simple and rapid biosynthesis of stable silver nanoparticles using dried leaves of Catharanthus roseus. Linn. G. Donn and its anti microbial activity. Colloids Surf. B Biointerfaces 2013, 105, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Babu, S.A.; Prabu, H.G. Synthesis of AgNPs using the extract of Calotropis procera flower at room temperature. Mater. Lett. 2011, 65, 1675–1677. [Google Scholar] [CrossRef]
- Yin, Y.; Li, Z.-Y.; Zhong, Z.; Gates, B.; Xia, Y.; Venkateswaran, S. Synthesis and characterization of stable aqueous dispersions of silver nanoparticles through the Tollens process. J. Mater. Chem. 2002, 12, 522–527. [Google Scholar] [CrossRef]
- Saito, Y.; Wang, J.J.; Batchelder, D.N.; Smith, D.A. Simple chemical method for forming silver surfaces with controlled grain sizes for surface plasmon experiments. Langmuir 2003, 19, 6857–6861. [Google Scholar] [CrossRef]
- He, Y.; Wu, X.; Lu, G.; Shi, G. A facile route to silver nanosheets. Mater. Chem. Phys. 2006, 98, 178–182. [Google Scholar] [CrossRef]
- Kvitek, L.; Prucek, R.; Panacek, A.; Novotny, R.; Hrbac, J.; Zboril, R. The influence of complexing agent concentration on particle size in the process of SERS active silver colloid synthesis. J. Mater. Chem. 2005, 15, 1099–1105. [Google Scholar] [CrossRef]
- Kvítek, L.; Panáček, A.; Soukupová, J.; Kolář, M.; Večeřová, R.; Prucek, R.; Holecová, M.; Zbořil, R. Effect of Surfactants and Polymers on Stability and Antibacterial Activity of Silver Nanoparticles (NPs). J. Phys. Chem. C 2008, 112, 5825–5834. [Google Scholar] [CrossRef]
- Soukupová, J.; Kvítek, L.; Panáček, A.; Nevěčná, T.; Zbořil, R. Comprehensive study on surfactant role on silver nanoparticles (NPs) prepared via modified Tollens process. Mater. Chem. Phys. 2008, 111, 77–81. [Google Scholar] [CrossRef]
- Yu, D.; Yam, V.W. Hydrothermal-Induced Assembly of Colloidal Silver Spheres into Various Nanoparticles on the Basis of HTAB-Modified Silver Mirror Reaction. J. Phys. Chem. B 2005, 109, 5497–5503. [Google Scholar] [CrossRef] [PubMed]
- Panáček, A.; Kvítek, L.; Prucek, R.; Kolář, M.; Večeřová, R.; Pizúrová, N.; Sharma, V.K.; Tat‘jana, N.; Zbořil, R. Silver Colloid Nanoparticles: Synthesis, Characterization, and Their Antibacterial Activity. J. Phys. Chem. B 2006, 110, 16248–16253. [Google Scholar] [CrossRef] [PubMed]
- López-Franco, Y.L.; Córdova-Moreno, R.E.; Goycoolea, F.M.; Valdez, M.A.; Juárez-Onofre, J.; Lizardi-Mendoza, J. Classification and physicochemical characterization of mesquite gum (Prosopis spp.). Food Hydrocoll. 2012, 26, 159–166. [Google Scholar] [CrossRef]
- Goycoolea, F.M.; de la Barca, A.M.C.; Hernández, G.; Valenzuela, J.R.; Balderrama, J.R. Cell and Developmental Biology of Arabinogalactan-Proteins; Nothnagel, E.A., Bacic, A., Clarke, A.E., Eds.; Springer: Boston, MA, USA, 2000; pp. 263–276. [Google Scholar]
- Goycoolea, F.M.; Morris, E.R.; Richardson, R.K.; Bell, A.E. Solution rheology of mesquite gum in comparison with gum arabic. Carbohydr. Polym. 1995, 27, 37–45. [Google Scholar] [CrossRef]
- Goycoolea, F.M.; Calderón de la Barca, A.M.; Balderrama, J.R.; Valenzuela, J.R.; Hernandez, G. Gums and Stabilisers for the Food Industry 9; Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Qu, L.; Dai, L. Novel silver nanostructures from silver mirror reaction on reactive substrates. J. Phys. Chem. B 2005, 109, 13985–13990. [Google Scholar] [CrossRef] [PubMed]
- Brause, R.; Möltgen, H.; Kleinermanns, K. Characterization of laser-ablated and chemically reduced silver colloids in aqueous solution by UV/VIS spectroscopy and STM/SEM microscopy. Appl. Phys. B 2002, 75, 711–716. [Google Scholar] [CrossRef]
- Vertelov, G.K.; Krutyakov, Y.A.; Efremenkova, O.V.; Olenin, A.Y.; Lisichkin, G.V. A versatile synthesis of highly bactericidal Myramistin® stabilized silver nanoparticles. Nanotechnology 2008, 19, 355707. [Google Scholar] [CrossRef] [PubMed]
- Sinha, C.; Bhattacharyya, S.S.; Indian Association for the Cultivation of Science. Current Topics in Atomic, Molecular and Optical Physics: Invited Lectures Delivered at the Conference on Atomic Molecular and Optical Physics (TC 2005), 13–15th December, 2005, Indian Association for the Cultivation of Science; World Scientific: Kolkata, India, 2007. [Google Scholar]
- Carattino, A.; Khatua, S.; Orrit, M. In situ tuning of gold nanorod plasmon through oxidative cyanide etching. Phys. Chem. Chem. Phys. 2016, 18, 15619–15624. [Google Scholar] [CrossRef] [PubMed]
- Mie, G. Contributions to the optics of turbid media, particularly of colloidal metal solutions. Ann. Phys. (Leipzig) 1976, 1, 377–445. [Google Scholar]
- Sosa, I.O.; Noguez, C.; Barrera, R.G. Optical properties of metal nanoparticles with arbitrary shapes. J. Phys. Chem. B 2003, 107, 6269–6275. [Google Scholar] [CrossRef]
- Skoog, D.A.; Crouch, S.R.; Holler, F.J.; Anzures, M.B. Principles of Instrumental Analysis; Cengage Learning: San Francisco, CA, USA, 2008. [Google Scholar]
- Rea, I.; Giardina, P.; Longobardi, S.; Porro, F.; Casuscelli, V.; Rendina, I.; De Stefano, L. Hydrophobin Vmh2-glucose complexes self-assemble in nanometric biofilms. J. R. Soc. Interface 2012, 9, 2450–2456. [Google Scholar] [CrossRef] [PubMed]
- Lafarga, A.K.S.; Moisés, F.P.P.; Gurinov, A.; Ortiz, G.G.; Arízaga, G.G.C. Dual responsive dysprosium-doped hydroxyapatite particles and toxicity reduction after functionalization with folic and glucuronic acids. Mater. Sci. Eng. C 2015, 48, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Chen, H.; Su, S.; Wang, T.; Zhang, C.; Fida, G.; Cui, S.; Zhao, J. Galactose as Broad Ligand for Multiple Tumor Imaging and Therapy. J. Cancer 2015, 6, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Hövel, H.; Fritz, S.; Hilger, A.; Kreibig, U.; Vollmer, M. Width of cluster plasmon resonancces: Bulk dielectric functions and chemical interface damping. Phys. Rev. B 1993, 48, 178–188. [Google Scholar] [CrossRef]
- Yamamoto, S.; Fujiwara, K.; Watarai, H. Surface-Enhanced Raman Scattering from Oleate-Stabilized Silver Colloids at a Liquid/Liquid Interface. Jpn. Soc. Anal. Sci. 2004, 20, 1347–1352. [Google Scholar] [CrossRef]
- Šileikaite, A.; Prosyevas, I.; Puišo, J.; Juraitis, A.; Guobiene, A. Analysis of Silver Nanoparticles Produced by Chemical Reduction of Silver Salt Solution. Mater. Sci. 2006, 12, 287–291. [Google Scholar]
- Balaji, D.S.; Basavaraja, S.; Deshpande, R.; Mahesh, D.B. Extracellular biosynthesis of functionalized silver nanoparticles by strains of Cladosporium cladosporioides fungus. Colloids Surf. B Bionterfaces 2009, 68, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Mukherjee, P.; Senapati, S.; Mandal, D.; Khan, M.I.; Sastry, M. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf. B Bionterfaces 2003, 28, 313–318. [Google Scholar] [CrossRef]
- Puchalski, M.; Dabrowski, P.; Olejniczak, W.; Krukowski, P.; Kowalczyk, P.; Polański, K. The study of silver nanoparticles by scanning electron microscopy, energy dispersive X-ray analysis and scanning tunnelling microscopy. Mater. Sci. 2007, 25, 473. [Google Scholar]
- López-franco, Y.L.; Cervantes-Montaño, C.I.; Martínez-Robinson, K.G.; Lizardi-Mendoza, J.; Robles-Ozuna, L.E. Physicochemical characterization and functional properties of galactomannans from mesquite seeds (Prosopis spp.). Food Hydrocoll. 2013, 30, 656–660. [Google Scholar] [CrossRef]
- López-Franco, Y.L.; de la Barca, A.M.C.; Valdez, M.A.; Peter, M.G.; Rinaudo, M.; Chambat, G.; Goycoolea, F.M. Structural Characterization of Mesquite (Prosopis velutina) Gum and its Fractions. Macromol. Biosci. 2008, 8, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Prado, B.M.; Kim, S.; Özen, B.F.; Mauer, L.J. Differentiation of Carbohydrate Gums and Mixtures Using Fourier Transform Infrared Spectroscopy and Chemometrics. J. Agric. Food Chem. 2005, 53, 2823–2829. [Google Scholar] [CrossRef] [PubMed]
- Kora, A.; Beedu, S.; Jayaraman, A. Size-controlled green synthesis of silver nanoparticles mediated by gum ghatti (Anogeissus latifolia) and its biological activity. Org. Med. Chem. Lett. 2012, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Mohan, Y.M.; Raju, K.M.; Sambasivudu, K.; Singh, S.; Sreedhar, B. Preparation of acacia-stabilized silver nanoparticles: A green approach. J. Appl. Polym. Sci. 2007, 106, 3375–3381. [Google Scholar] [CrossRef]
- Balderrama, J.R. Caracterización Fisicoquımica y Análisis del Aprovechamiento de la Goma ‘Chúcata’y Galactomanana del Mezquite (Prosopis spp.) como Posibles Hidrocoloides Alimentarios’. Master’s Thesis, Universidad de Sonora, Hermosillo, México, 1998. [Google Scholar]
- Goycoolea, F.M.; de la Barca, A.M.C.; Balderrama, J.R.; Valenzuela, J.R. Immunological and functional properties of the exudate gum from northwestern Mexican mesquite (Prosopis spp.) in comparasion with gum arabic. Int. J. Biol. Macromol. 1997, 21, 29–36. [Google Scholar] [CrossRef]
- Luo, C.; Zhang, Y.; Zeng, X.; Zeng, Y.; Wang, Y. The role of poly(ethylene glycol) in the formation of silver nanoparticles. J. Colloid Interface Sci. 2005, 288, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Juby, K.A.; Dwivedi, C.; Kumar, M.; Kota, S.; Misra, H.S.; Bajaj, P.N. Silver nanoparticle-loaded PVA/gum acacia hydrogel: Synthesis, characterization and antibacterial study. Carbohydr. Polym. 2012, 89, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Alfredsson, V.; Kjellin, P.; Palmqvist, A.E.C. Macroscopic Alignment of Silver Nanoparticles in Reverse Hexagonal Liquid Crystalline Templates. Nano Lett. 2002, 2, 1403–1407. [Google Scholar] [CrossRef]
- Anderson, E.; Sands, L. The composition of mesquite gum; the isolation of d-galactose and l-arabinose. J. Am. Chem. Soc. 1926, 48, 3172–3177. [Google Scholar] [CrossRef]
- Kora, A.J.; Sashidhar, R.B.; Arunachalam, J. Gum kondagogu (Cochlospermum gossypium): A template for the green synthesis and stabilization of silver nanoparticles with antibacterial application. Carbohydr. Polym. 2010, 82, 670–679. [Google Scholar] [CrossRef]
- Dong, C.; Zhang, X.; Cai, H.; Cao, C. Facile and one-step synthesis of monodisperse silver nanoparticles using gum acacia in aqueous solution. J. Mol. Liq. 2014, 196, 135–141. [Google Scholar] [CrossRef]
- Sosa, Y.D.; Rabelero, M.; Treviño, M.E.; Saade, H.; López, R.G. High-yield synthesis of silver nanoparticles by precipitation in a high-aqueous phase content reverse microemulsion. J. Nanomater. 2010, 2010, 392572. [Google Scholar] [CrossRef]
- Meléndrez, M.F.; Medina, C.; Solis-Pomar, F.; Flores, P.; Paulraj, M.; Pérez-Tijerina, E. Quality and high yield synthesis of Ag nanowires by microwave-assisted hydrothermal method. Nanoscale Res. Lett. 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Chen, H.; Guo, Z.; Wang, B.; Tang, C.; Feng, Y. High-concentration silver colloid stabilized by a cationic gemini surfactant. Colloids Surf. A Physicochem. Eng. Asp. 2013, 429, 98–105. [Google Scholar] [CrossRef]
- Li, H.-J.; Zhang, A.-Q.; Hu, Y.; Sui, L.; Qian, D.-J.; Chen, M. Large-scale synthesis and self-organization of silver nanoparticles with Tween 80 as a reductant and stabilizer. Nanoscale Res. Lett. 2012, 7, 612. [Google Scholar] [CrossRef] [PubMed]
- Toisawa, K.; Hayashi, Y.; Takizawa, H. Synthesis of Highly Concentrated Ag Nanoparticles in a Heterogeneous Solid-Liquid System under Ultrasonic Irradiation. Mater. Trans. 2010, 51, 1764–1768. [Google Scholar] [CrossRef]
- Córdova, M.R.E. Clasificación y Caracterización Fisicoquímica de la Goma de Mezquite (Chúcata) Cruda y Ultrafiltrada. Ph.D. Thesis, Universidad de Sonora, Hermosillo, Sonora, Mexico, 2004. [Google Scholar]
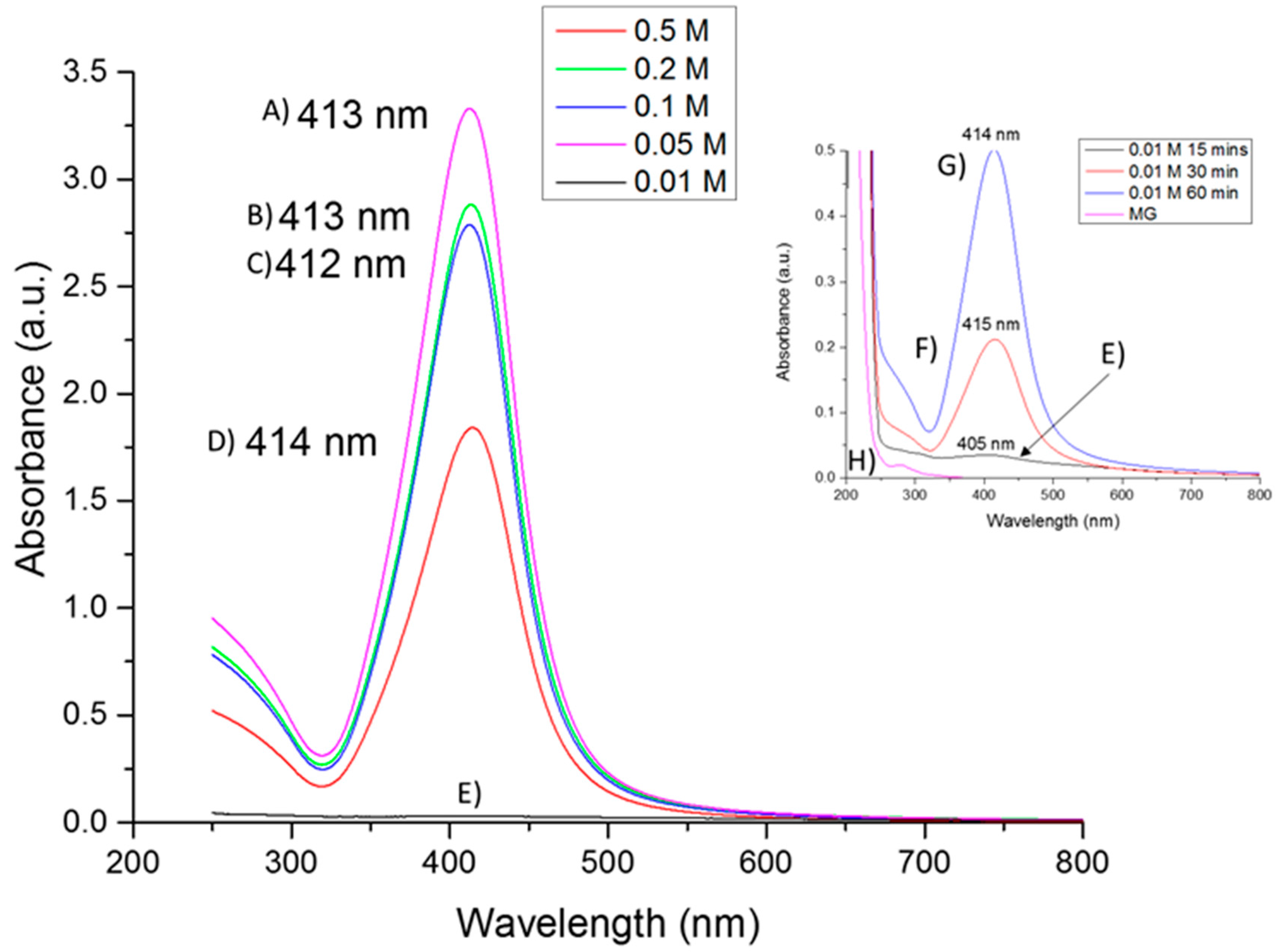
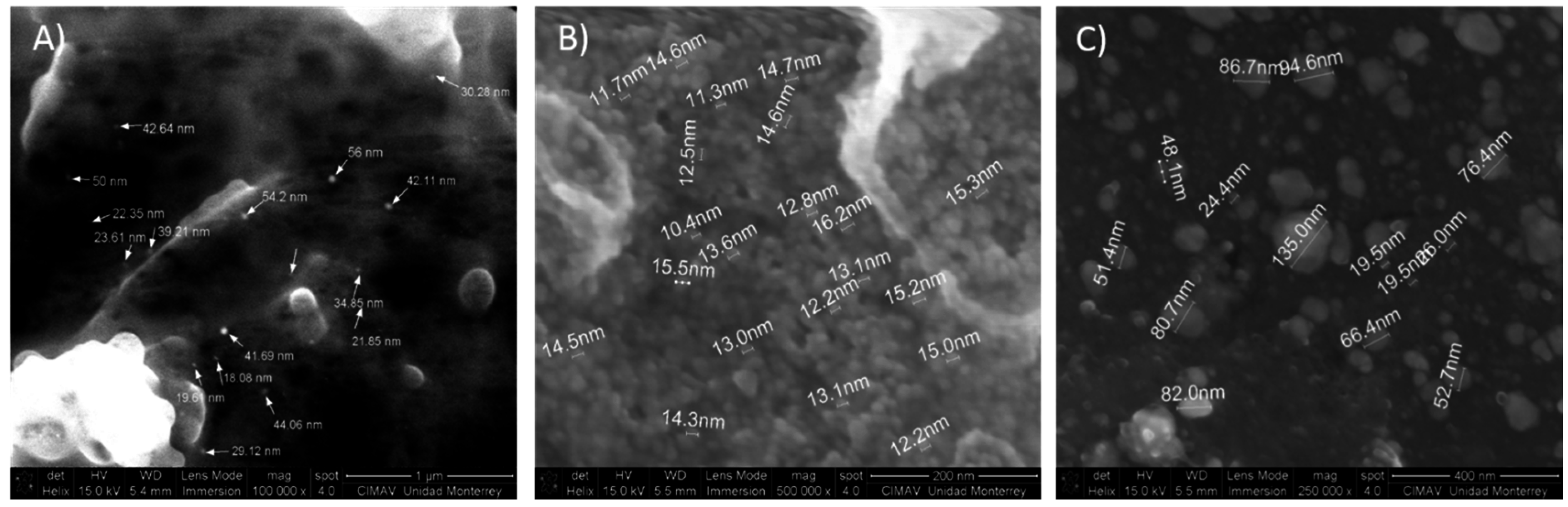
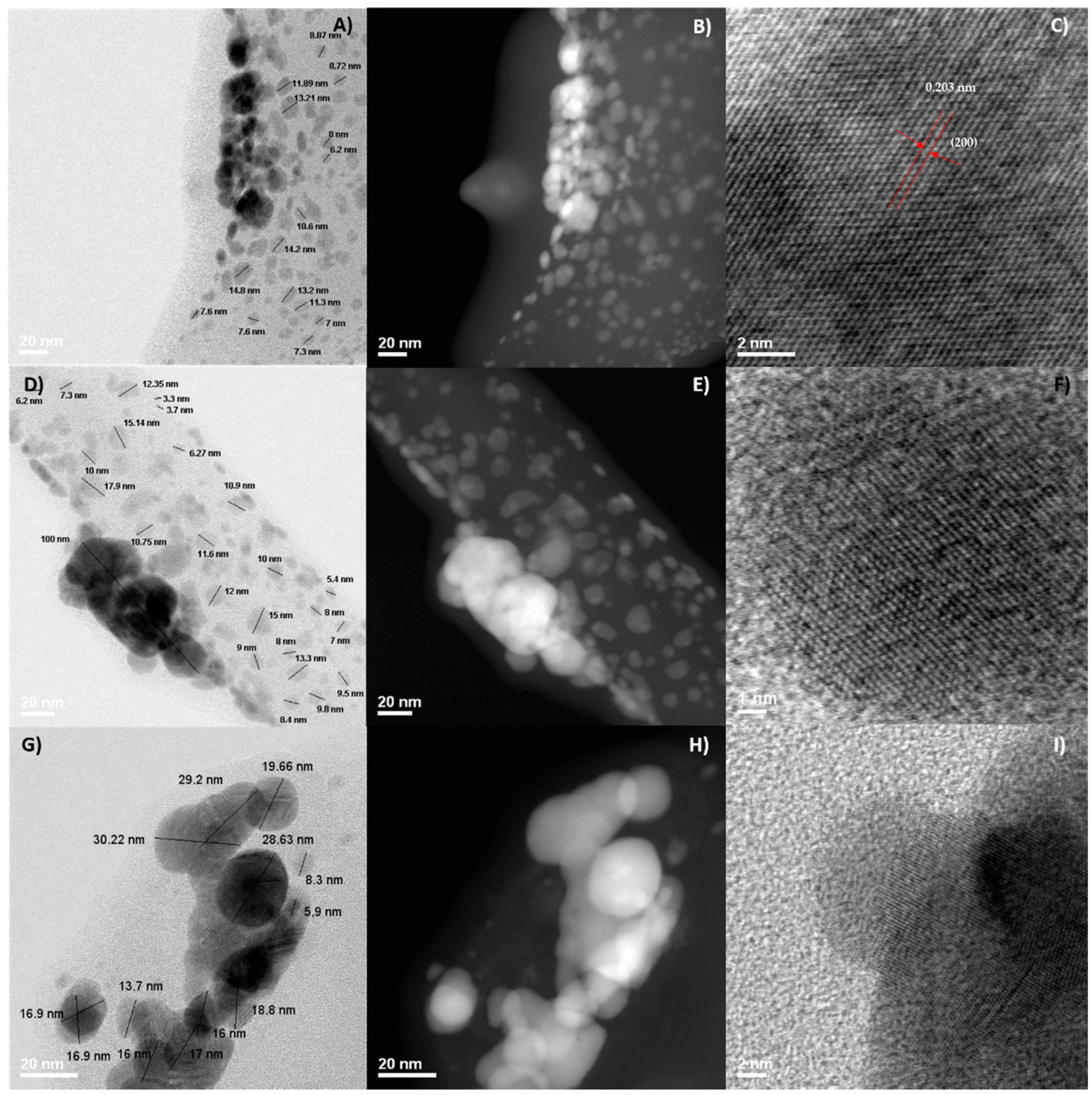

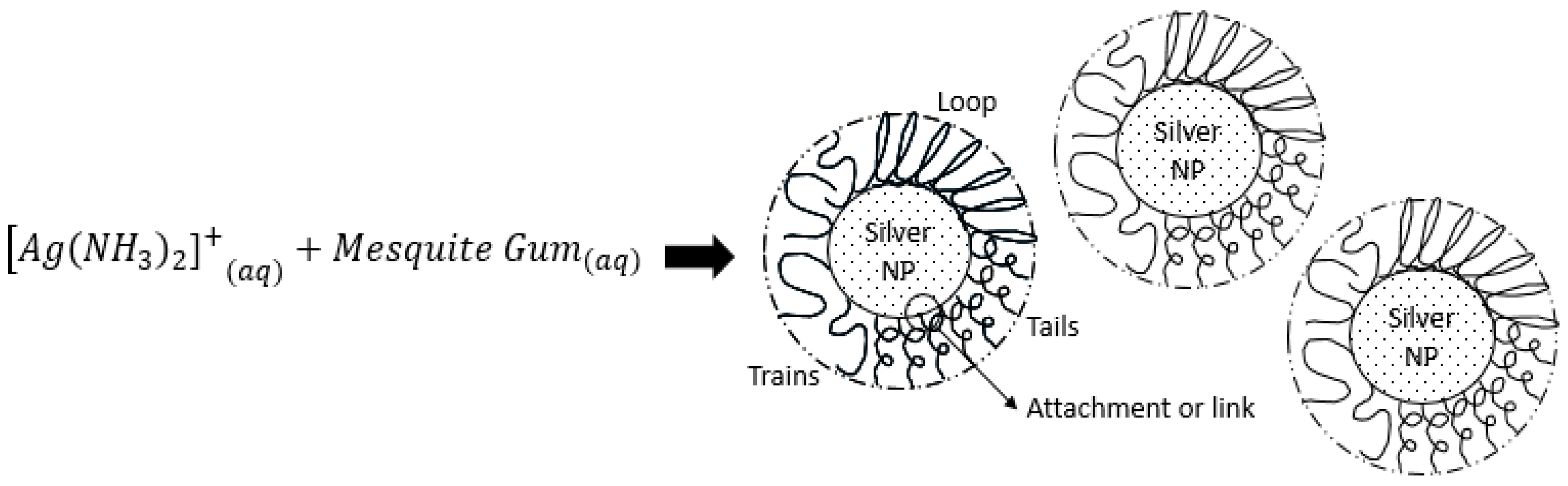

| Sample Silver Concentration (mol/L) | Time in Treatment (min) | AgNO3:Mesquite Gum Ratio | PdI | SIZE (d.nm) ± SE (% Volume) | |
|---|---|---|---|---|---|
| 1st Peak | 2nd Peak | ||||
| 0.01 | 15 | 1:1 | 0.159 | 10.3 ± 3.1 (99.4) | 65.9 ± 20.6 (0.6) |
| 0.05 | 15 | 1:1 | 0.225 | 27.9 ± 11.3 (95.9) | 4674 ± 1009 (4.1) |
| 0.1 | 15 | 1:1 | 0.252 | 35.4 ± 13.9 (86.2) | 4585 ± 1053 (13.8) |
| 0.2 | 15 | 1:1 | 0.205 | 32.8 ± 13.5 (100) | - |
| 0.5 | 15 | 1:1 | 0.221 | 35.9 ± 13.9 (94) | 4920 ± 889.7 (6) |
| 0.01 | 30 | 1:1 | 0.232 | 15.9 ± 3.1 (95.2) | 67.4 ± 18.1 (4.8) |
| 0.01 | 60 | 1:1 | 0.175 | 12.3 ± 2.5 (93.8) | 60.4 ± 21.4 (6.2) |
| 0.05 | 30 | 1:1 | 0.169 | 33.2 ± 7.5 (100) | - |
| 0.05 | 60 | 1:1 | 0.315 | 26.9 ± 4.1 (100) | - |
| 0.2 | 15 | 1:0.3 | 0.276 | 28.9 ± 12.9 (96.1) | 4965 ± 866.3 (3.9) |
| 0.2 | 15 | 1:0.5 | 0.306 | 22.9 ± 14.6 (100) | |
| Sample Silver (mol/L) | Time in Treatment (min) | AgNO3:Mesquite Gum Ratio | PdI | Size (d.nm) ± SE (% Volume) | |
|---|---|---|---|---|---|
| 1st Peak | 2nd Peak | ||||
| 0.01 | 15 | 1:1 | 0.739 | 9.9 ± 7.6 (96.5) | 4329 ± 1172 (3.3) |
| 0.05 | 15 | 1:1 | 0.225 | 27.7 ± 10.1 (92) | 4946 ± 881.1 (8) |
| 0.1 | 15 | 1:1 | 0.252 | 30.4 ± 12.1 (100) | - |
| 0.2 | 15 | 1:1 | 0.205 | 31.6 ± 11.2 (100) | - |
| 0.5 | 15 | 1:1 | 0.221 | 31.4 ± 10.7 (100) | - |
| 0.01 | 30 | 1:1 | 0.396 | 11.4 ± 3.4 (91) | 56.06 ± 37.5(5.6) |
| 0.01 | 60 | 1:1 | 0.391 | 10.3 ± 1.5 (98.1) | 5283 ± 711.4 (1.9) |
| 0.05 | 30 | 1:1 | 0.194 | 31.0 ± 11.5 (100) | - |
| 0.05 | 60 | 1:1 | 0.315 | 28.0 ± 9.9 (99) | 251.5 ± 55.2 (1) |
| 0.2 | 15 | 1:0.3 | 0.321 | 48.9 ± 12.9 (92.8) | 5225 ± 793.5 (5.8) |
| 0.2 | 15 | 1:0.5 | 0.302 | 42.6 ± 21.7 (98) | 3125 ± 85.5 (2) |
| Sample Silver (mol/L) | Time in Treatment (min) | AgNO3:Mesquite Gum Ratio | PdI | Size (d.nm) ± SE (% Volumen) | |
|---|---|---|---|---|---|
| 1st Peak | 2nd Peak | ||||
| 0.01 | 15 | 1:1 | 0.380 | 35.6 ± 17.3 (77.3) | 5462 ± 634.8 (18.7) |
| 0.05 | 15 | 1:1 | 0.141 | 28.4 ± 9.3(100) | - |
| 0.1 | 15 | 1:1 | 0.179 | 31.4 ± 6.5 (100) | - |
| 0.2 | 15 | 1:1 | 0.237 | 30.3 ± 5.8 (100) | - |
| 0.5 | 15 | 1:1 | 0.195 | 32.1 ± 10.5 (100) | - |
| 0.01 | 30 | 1:1 | 0.237 | 51.8 ± 11.7 (100) | - |
| 0.01 | 60 | 1:1 | 0.149 | 10.1 ± 2.1 (94.7) | 53.3 ± 5.3 (5.3) |
| 0.05 | 30 | 1:1 | 0.204 | 31.2 ± 6.3 (100) | - |
| 0.05 | 60 | 1:1 | 0.378 | 26.7 ± 12.4 (94.4) | 5456 ± 636.8 (5.6) |
| 0.2 | 15 | 1:0.3 | 0.402 | 88.9 ± 5.6 (92.8) | 7225 ± 954.7 (8.2) |
| 0.2 | 15 | 1:0.5 | 0.398 | 62.9 ± 18.7 (95) | 3125 ± 85.5 (5) |
| Sample Name, Reaction Time, Diluted | Total Ag (M) | Electrode Potential (mV) | Ag+ (M) | Ag0 (M) | Concentration of Ag0 (%) |
|---|---|---|---|---|---|
| 0.01 30 min 1:10 | 0.00093819 | 201.5 | 7.67183 × 10−6 | 0.000930516 | 99.1822713 |
| 0.01 30 min 1:100 | 0.00009382 | 197 | 2.78488 × 10−7 | 9.35403 × 10−5 | 99.70316447 |
| 0.01 60 min 1:10 | 0.00093819 | 250 | 6.43172 × 10−6 | 0.000931756 | 99.31445266 |
| 0.01 60 min 1:100 | 0.00009382 | 168.6 | 5.17802 × 10−8 | 9.3767 × 10−5 | 99.94480825 |
| 0.05 30 min 1:10 | 0.00416826 | 230.3 | 2.00221 × 10−6 | 0.004166256 | 99.95196538 |
| 0.05 30 min 1:100 | 0.00041683 | 198.3 | 3.00781 × 10−7 | 0.000416525 | 99.92784012 |
| 0.05 60 min 1:10 | 0.00416826 | 243.1 | 4.27378 × 10−6 | 0.004163984 | 99.89746842 |
| 0.05 60 min 1:100 | 0.00041683 | 201.7 | 3.67893 × 10−7 | 0.000416458 | 99.91173937 |
| 0.1 1:10 | 0.00797664 | 180.3 | 1.03554 × 10−7 | 0.007976537 | 99.99870178 |
| 0.1 1:100 | 0.00079766 | 190.6 | 1.90614 × 10−7 | 0.000797473 | 99.97610351 |
| 0.2 1:10 | 0.01519171 | 155 | 2.31355 × 10−8 | 0.015191687 | 99.99984771 |
| 0.2 1:100 | 0.00151917 | 210.2 | 6.08695 × 10−7 | 0.001518562 | 99.95993242 |
| 0.5 1:10 | 0.03312199 | 212.8 | 7.10051 × 10−7 | 0.03312128 | 99.99785626 |
| 0.5 1:100 | 0.0033122 | 221 | 1.15412 × 10−6 | 0.003311045 | 99.96515559 |
| Authors | Method | Estimated Production Capacity (g Ag/100 g) * | Reaction Time | Particle Size | Observations | Reference |
|---|---|---|---|---|---|---|
| Moreno & Sánchez | Aqueous, Tollens with mesquite gum | ~3.0 (with 0.5 M AgNO3) | ~15–30 min | ~30 nm | High colloidal stability in water (tested for 3 months) | This work |
| He et al. | Aqueous, Tollens, glucose, Al(NO3)3 | ~0.43 | 60 min | Nanosheets 27 nm thickness | Dimension of nanosheets, hundreds of nm | [25] |
| Kora et al. | Aqueous, kondagogu gum, Autoclave 121 °C | ~0.01 | ~20–60 min | Various, 3–20 nm | Need high temperature and pressure | [63] |
| Dong et al. | Aqueous, Acacia (Arabic) gum, 60–80 °C | ~0.06 | ~3 h | 2–20 nm | High colloidal stability in water (tested for 1 month) | [64] |
| Kora et al. | Aqueous, ghatti gum, Autoclave 121 °C | ~0.01 | ~20–60 min | Variou, 5–30 nm | Need high temperature and pressure | [55] |
| Sosa et al. | W/O microemulsion, 70 °C, NaBH4 | ~1.5 | ~2.5 h | 9 nm | Toluene, AOT, SDS, 7 washings with water/acetone | [65] |
| Melendrez et al. | Ethyleneglycol, PVP, microwave | ~1.0 | ~10 min | Nanowires 70–110 nm diameter | Need microwave treatment, solvent (ethyleneglycol) | [66] |
| He et al. | Aqueous, 12–2-12 gemini surfactant, NaBH4 | ~0.54 | ~10 h | 11 nm | 12–2-12 surfactant not available commercially | [67] |
| Li et al. | Tween® 80, 100 °C | ~3.2 | 1–3 days | Various, 20–40 nm | High surfactant concentration (about 95%); high temperature and large reaction times | [68] |
| Toisawa et al. | Ethanol, toluene, dodecylamine Ag2O powder, ultrasound | 2–9 | 3–10 h | Various, 10–30 nm | Several hours of ultrasound needed; toluene used | [69] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno‐Trejo, M.B.; Sánchez‐Domínguez, M. Mesquite Gum as a Novel Reducing and Stabilizing Agent for Modified Tollens Synthesis of Highly Concentrated Ag Nanoparticles. Materials 2016, 9, 817. https://doi.org/10.3390/ma9100817
Moreno‐Trejo MB, Sánchez‐Domínguez M. Mesquite Gum as a Novel Reducing and Stabilizing Agent for Modified Tollens Synthesis of Highly Concentrated Ag Nanoparticles. Materials. 2016; 9(10):817. https://doi.org/10.3390/ma9100817
Chicago/Turabian StyleMoreno‐Trejo, Maira Berenice, and Margarita Sánchez‐Domínguez. 2016. "Mesquite Gum as a Novel Reducing and Stabilizing Agent for Modified Tollens Synthesis of Highly Concentrated Ag Nanoparticles" Materials 9, no. 10: 817. https://doi.org/10.3390/ma9100817






