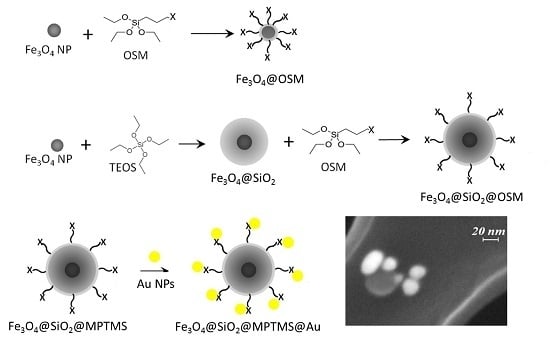
Functionalization of Fe3O4 NPs by Silanization: Use of Amine (APTES) and Thiol (MPTMS) Silanes and Their Physical Characterization
Abstract
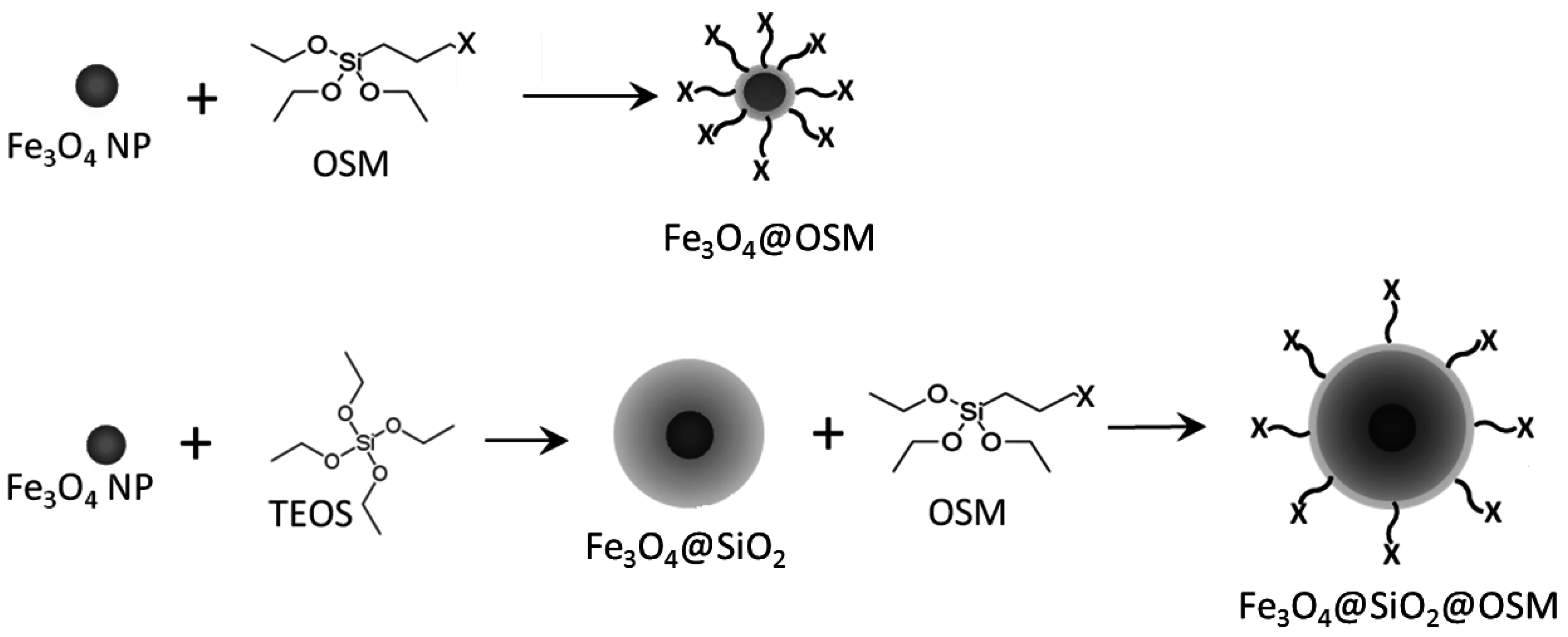
:1. Introduction
2. Results and Discussion
2.1. APTES Functionalization
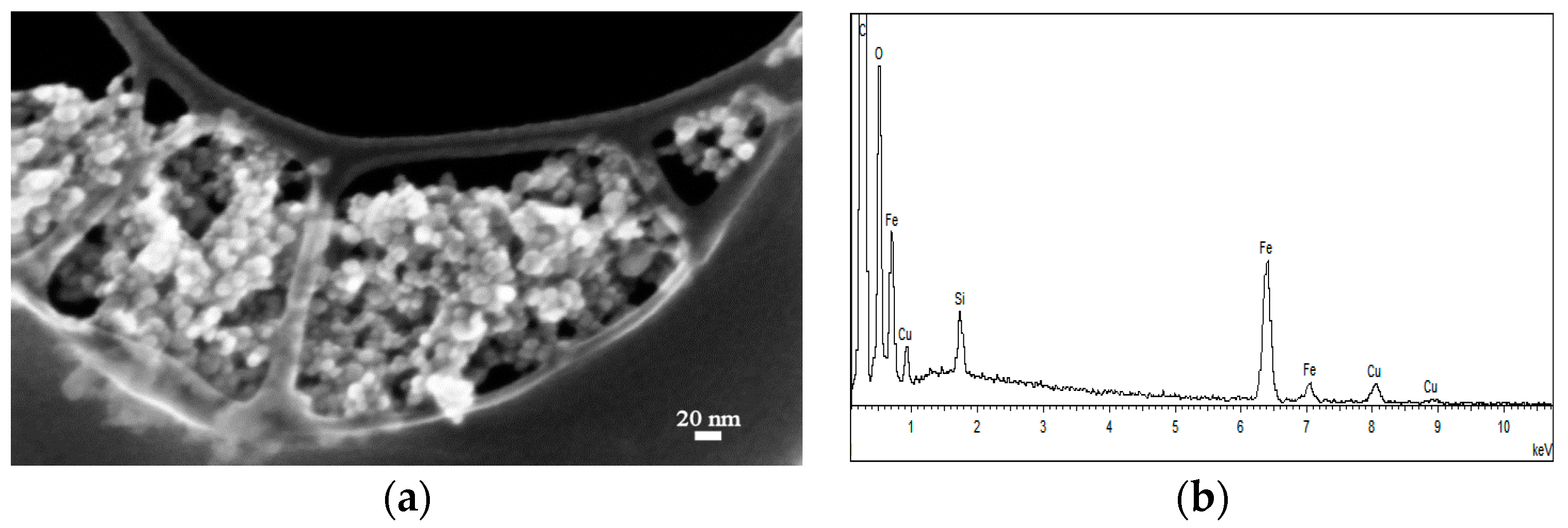
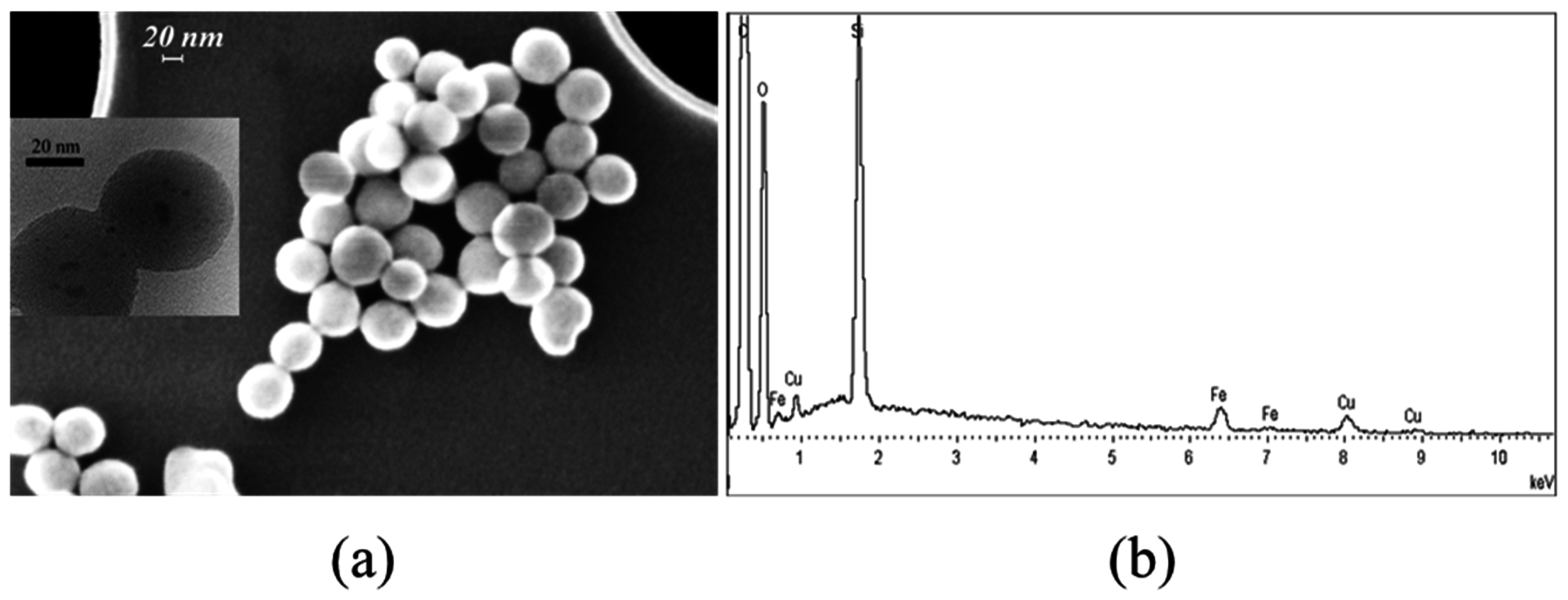
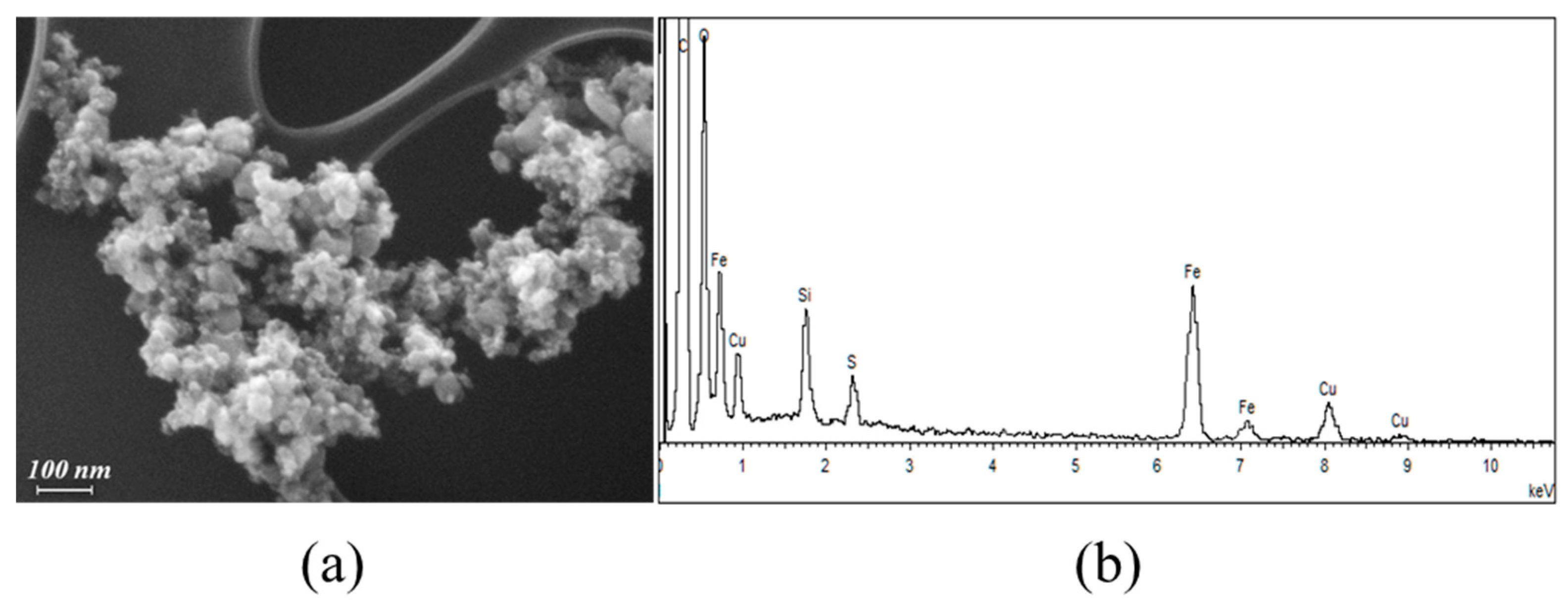
2.1.1. FE-SEM Results
2.1.2. UV-VIS Results
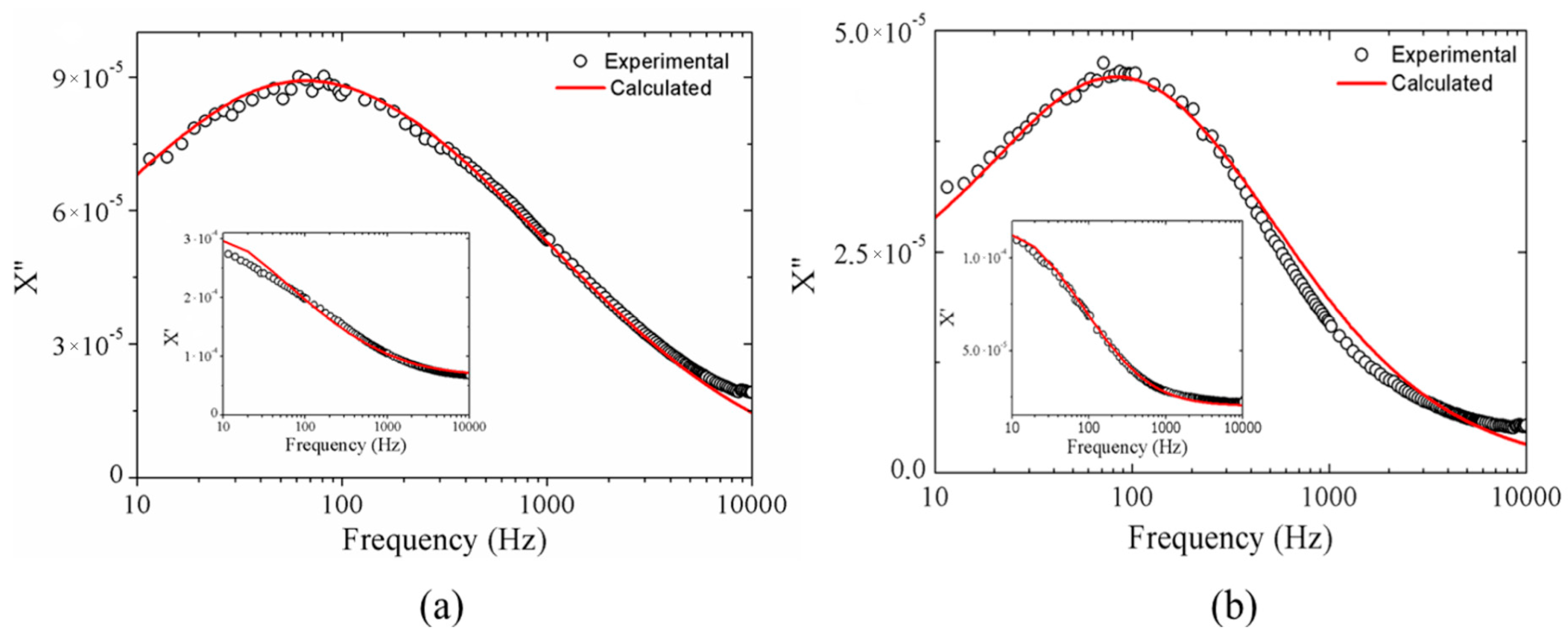
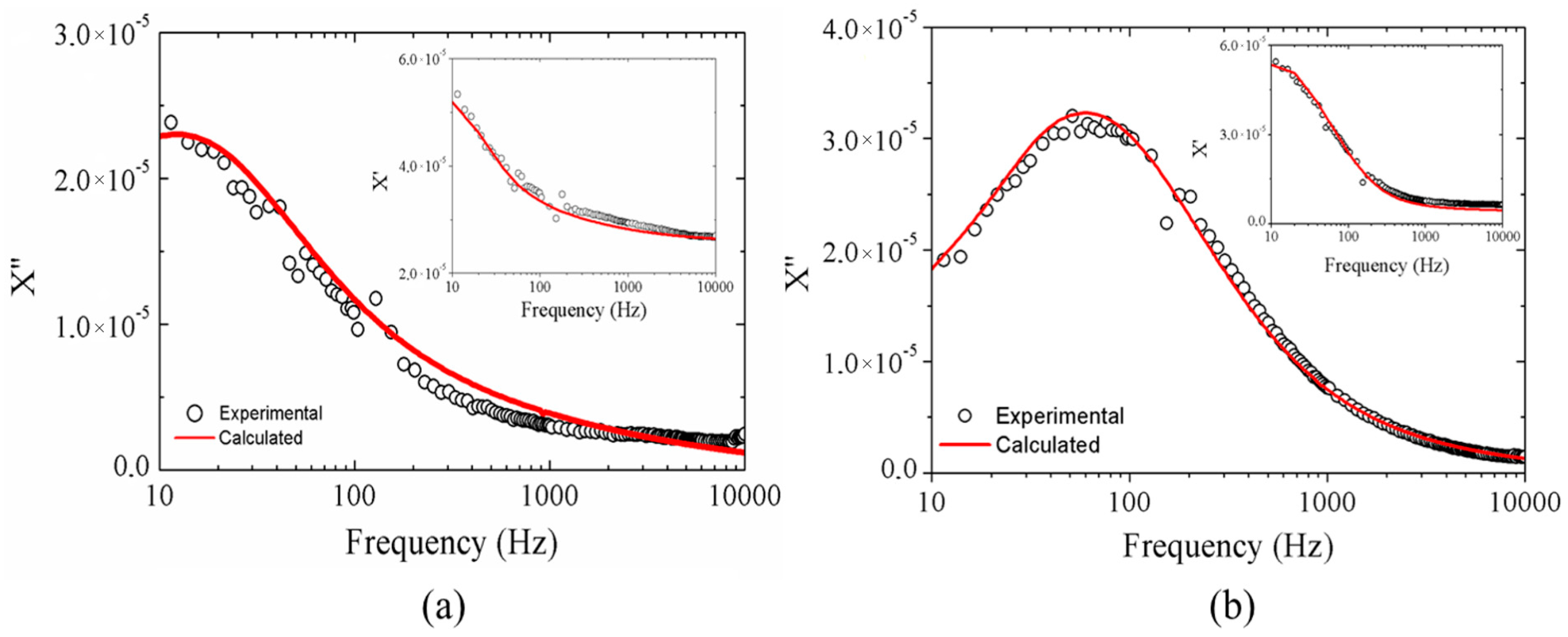
2.1.3. Magnetic Results
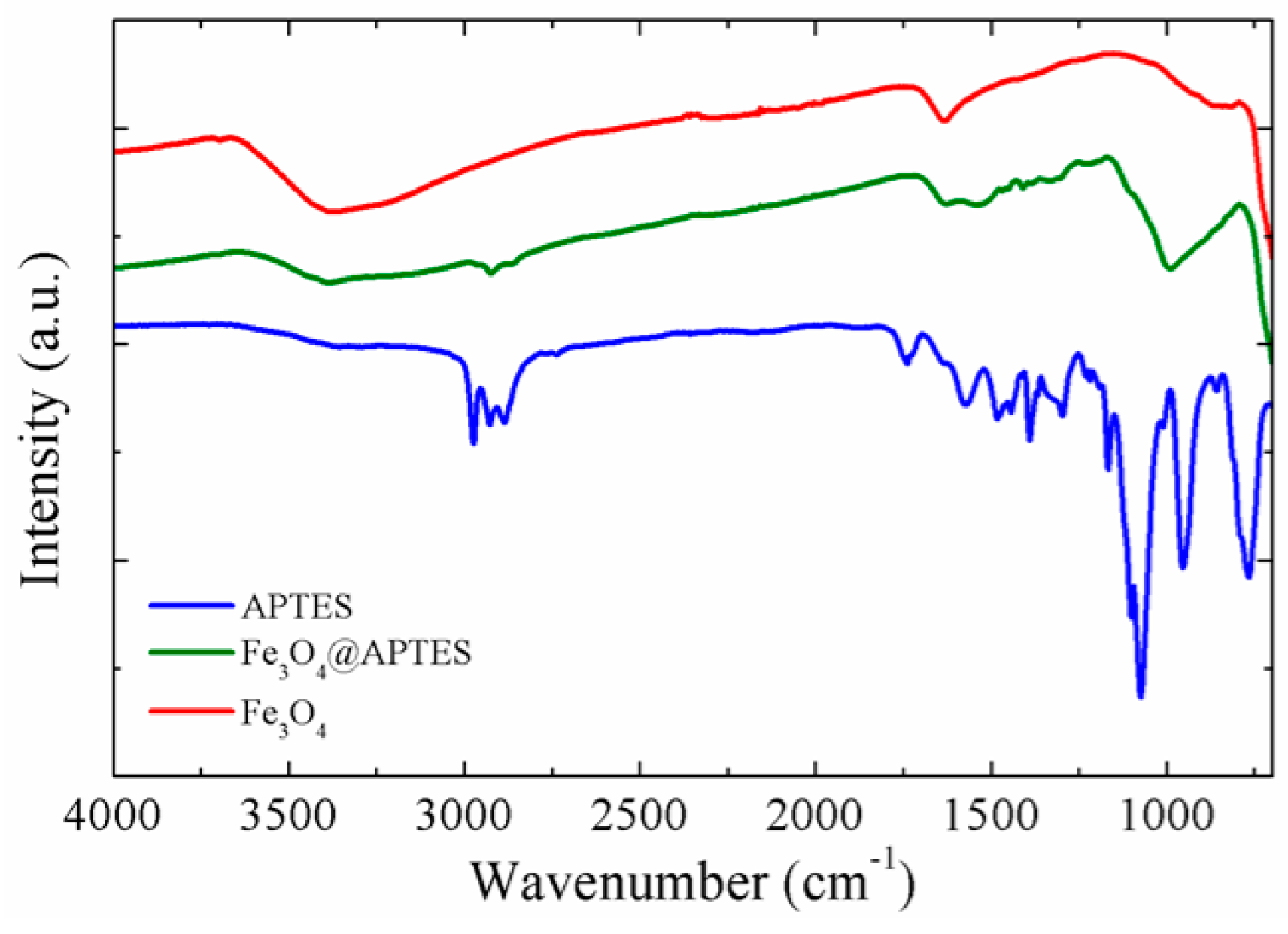
2.1.4. FT-IR Spectroscopy
2.1.5. Thermogravimetric Analyses
2.2. MPTMS Functionalization
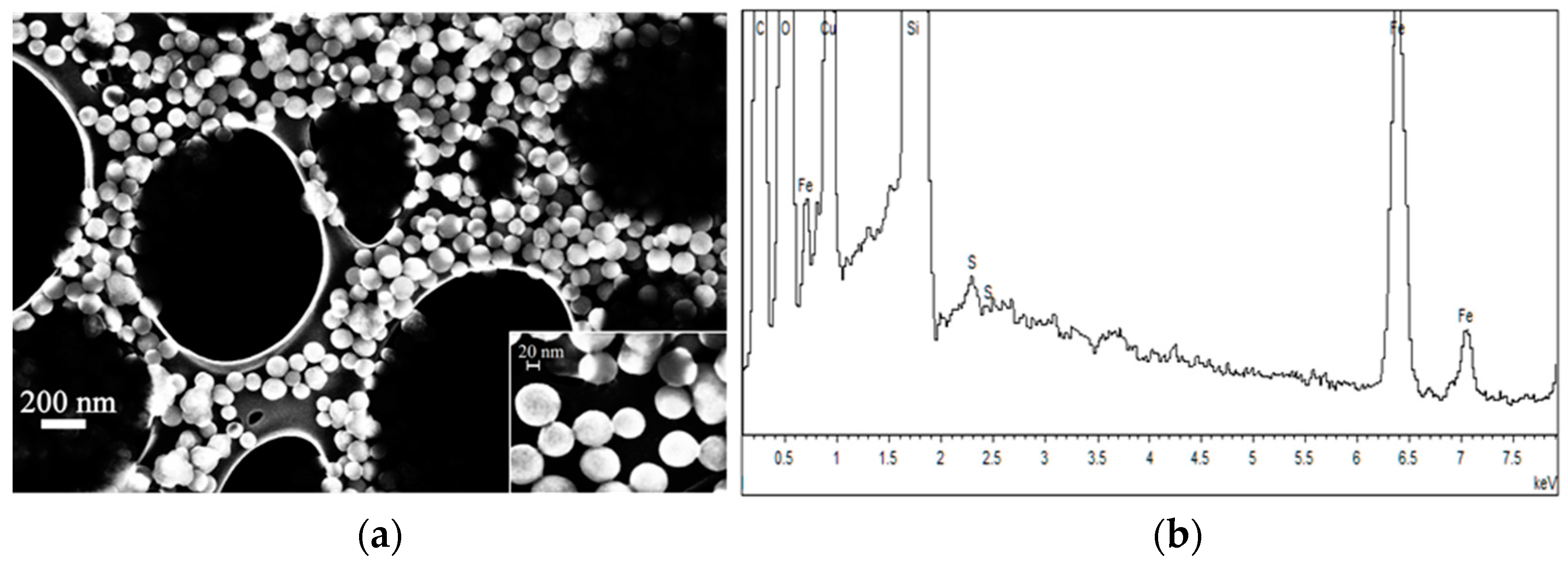
2.2.1. FE-SEM Results
2.2.2. Magnetic Results
2.2.3. FT-IR Spectroscopy
2.2.4. Thermogravimetric Analyses
2.2.5. Au NP Conjugation
3. Materials and Methods
3.1. Materials and Synthetic Method
3.1.1. APTES Functionalization
3.1.2. MPTMS Functionalization
3.1.3. Synthesis of Au NPs
3.1.4. MPTMS-Au Conjugation
3.2. Characterization Techniques
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Rocha-Santos, T.A.P. Sensors and biosensors based on magnetic nanoparticles. TrAC 2014, 62, 28–36. [Google Scholar] [CrossRef]
- Barry, N.P.E.; Sadler, P.J. Challenges for metals in medicine: How nanotechnology may help to shape the future. ACS Nano 2013, 7, 5654–5659. [Google Scholar] [CrossRef] [PubMed]
- Porosoff, M.D.; Chen, J.G. Trends in the catalytic reduction of CO2 by hydrogen over supported monometallic and bimetallic catalysts. J. Catal. 2013, 301, 30–37. [Google Scholar] [CrossRef]
- Parveen, S.; Misra, R.; Sahoo, S.K. Nanoparticles: A boon to drug delivery, therapeutics diagnostic and imaging. Nanomed. NBM 2012, 8, 147–166. [Google Scholar] [CrossRef] [PubMed]
- Pelaz, B.; Jaber, S.; de Aberasturi, D.J.; Wulf, V.; Aida, T.; de la Fuente, J.M.; Feldmann, J.; Gaub, H.E.; Josephson, L.; Kagan, C.R. The state of nanoparticles-based nanoscience and biotechnology: Progress, promises and challenges. ACS Nano 2012, 6, 8468–8483. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.T. The impact of nanoscience on heterogeneous catalysis. Science 2003, 299, 1688–1691. [Google Scholar] [CrossRef] [PubMed]
- Veiseh, O.; Gunn, J.W.; Zhang, M. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv. Drug Deliv. Rev. 2010, 62, 284–304. [Google Scholar] [CrossRef] [PubMed]
- Acklin, B.; Lautens, E. Magnetic Nanoparticles: Properties, Synthesis and Applications; Nova Science Publishers: New York, NY, USA, 2012; ISBN 161942424X,9781619424241. [Google Scholar]
- Tiwari, As.; Tiwari, At.; Tripathi, A.; Melo, J.S.; D’Souza, S.F. Magnetic nanoparticles in tissue regeneration. In Nanomaterials in Drug Delivery, Imaging, and Tissue Engineering; Tiwari, As., Tiwari, At., Eds.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2013; Volume 14. [Google Scholar] [CrossRef]
- Mireles, L.-K.; Sachera, E.; L’Hocine, Y.; Laurent, S.; Stanicki, D. A comparative physicochemical, morphological and magnetic study of silane-functionalized superparamagnetic iron oxide nanoparticles prepared by alkaline coprecipitation. Int. J. Biochem. Cell Biol. 2016, 75, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Sant, S.; Wang, B.; Laurent, S.; Sen, T. Superparamagnetic iron oxide nanoparticles (SPIONs): Development, surface modification and applications in chemotherapy. Adv. Drug Deliv. Rev. 2011, 63, 24–46. [Google Scholar] [CrossRef] [PubMed]
- Bini, R.A.; Marques, R.F.C.; Santos, F.J.; Chaker, J.A.; Jafelicci, M., Jr. Synthesis and functionalization of magnetite nanoparticles with different amino-functional alkoxysilanes. J. Magn. Magn. Mater. 2012, 324, 534–539. [Google Scholar] [CrossRef]
- Gubin, S.P. Magnetic Nanoparticles; Gubin, S.P., Ed.; Wiley VCH-Verlag GmbH and Co.: Weinheim, Germany, 2009; ISBN 978-3-527-40790-3. [Google Scholar]
- Lucchini, M.A.; Riani, P.; Canepa, F. Quantitative analysis of the a.c. susceptibility of core–shell nanoparticles. J. Nanopart. Res. 2013, 15, 1601–1611. [Google Scholar] [CrossRef]
- Friedman, M. Applications of the ninhydrin reaction for analysis of amino acids, peptides, and proteins to agricultural and biomedical sciences. J. Agric. Food Chem. 2004, 52, 385–406. [Google Scholar] [CrossRef] [PubMed]
- Shankara, S.; Ganesh, M.K.; Hemavathi, A. Laboratory Manual for Practical Biochemistry; JAYPEE Brothers Medical Publishers Ltd.: New Delhi, India, 2008; ISBN 978-81-8448-259-1. [Google Scholar]
- Riani, P.; Napoletano, M.; Canepa, F. Synthesis, characterization and a.c. magnetic analysis of magnetite nanoparticles. J. Nanopart. Res. 2011, 13, 7013–7020. [Google Scholar] [CrossRef]
- O’Handley, R.C. Modern Magnetic Materials: Principles and Applications; Wiley: New York, NY, USA, 2000; ISBN 978-0-471-15566-9. [Google Scholar]
- Debye, P. Polar Molecules; The Chemical Catalog Company Inc.: New York, NY, USA, 1929; p. 179. [Google Scholar]
- Dick, K.; Dhanasekaran, T.; Zhang, Z.; Meisel, D. Size-dependent melting of silica-encapsulated gold nanoparticles. J. Am. Chem. Soc. 2002, 124, 2312–2317. [Google Scholar] [CrossRef] [PubMed]
- Varganici, C.D.; Durdureanu-Angheluta, A.; Rosu, D.; Pinteale, M.; Simionescu, B.C. Thermal degradation of magnetite nanoparticles with hydrophilic shell. J. Anal. Appl. Pyrolysis 2012, 96, 63–68. [Google Scholar] [CrossRef]
- Čampelj, S.; Makovec, D.; Drofenik, M. Functionalization of magnetic nanoparticles with 3-aminopropyl silane. J. Magn. Magn. Mater. 2009, 321, 1346–1350. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Massart, R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans. Magn. 1981, 17, 1247–1250. [Google Scholar] [CrossRef]
- Stober, W.; Fink, A.; Bohn, E. Controlled growth of mono-disperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Riani, P.; Lucchini, M.A.; Thea, S.; Alloisio, M.; Bertoni, G.; Canepa, F. New approach for the step by step control of magnetic nanostructure functionalization. Inorg. Chem. 2014, 53, 9166–9173. [Google Scholar] [CrossRef] [PubMed]
- Yamaura, M.; Camilo, R.L.; Sampaio, L.C.; Macedo, M.A.; Nakamura, M.; Toma, H.E. Preparation and characterization of (3-aminopropyl) triethoxysilane-coated magnetite nanoparticles. J. Magn. Magn. Mater. 2004, 279, 210–217. [Google Scholar] [CrossRef]
- Huang, C.; Hu, B. Silica-coated magnetic nanoparticles modified with γ-mercaptopropyl trimethoxysilane for fast and selective solid phase extraction of trace amounts of Cd, Cu, Hg, and Pb in environmental and biological samples prior to their determination by inductively coupled plasma mass spectrometry. Spectrochim. Acta Part B 2008, 63, 437–444. [Google Scholar] [CrossRef]






| Sample | Brownian Distribution | Néel Distribution | ||
|---|---|---|---|---|
| rm (nm) | σ | rm (nm) | σ | |
| Fe3O4@APTES | 115 | 0.6 | 3.8 | 0.03 |
| Fe3O4@SiO2@APTES | 80 | 0.4 | 4 | 0.04 |
| Sample | Brownian Distribution | Néel Distribution | ||
|---|---|---|---|---|
| rm (nm) | σ | rm (nm) | σ | |
| Fe3O4 | 18 | 0.55 | 3.4 | 0.4 |
| Fe3O4@MPTMS | 160 | 0.25 | 4 | 0.05 |
| Fe3O4@SiO2 | 82 | 0.25 | 4 | 0.05 |
| Fe3O4@SiO2@MPTMS | 95 | 0.25 | 4 | 0.05 |
| Sample | Brownian Distribution | Néel Distribution | ||
|---|---|---|---|---|
| rm (nm) | σ | rm (nm) | σ | |
| Fe3O4@MPTMS@Au | 210 | 0.15 | 4 | 0.05 |
| Fe3O4@SiO2@MPTMS@Au | 115 | 0.3 | 4 | 0.1 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villa, S.; Riani, P.; Locardi, F.; Canepa, F. Functionalization of Fe3O4 NPs by Silanization: Use of Amine (APTES) and Thiol (MPTMS) Silanes and Their Physical Characterization. Materials 2016, 9, 826. https://doi.org/10.3390/ma9100826
Villa S, Riani P, Locardi F, Canepa F. Functionalization of Fe3O4 NPs by Silanization: Use of Amine (APTES) and Thiol (MPTMS) Silanes and Their Physical Characterization. Materials. 2016; 9(10):826. https://doi.org/10.3390/ma9100826
Chicago/Turabian StyleVilla, Silvia, Paola Riani, Federico Locardi, and Fabio Canepa. 2016. "Functionalization of Fe3O4 NPs by Silanization: Use of Amine (APTES) and Thiol (MPTMS) Silanes and Their Physical Characterization" Materials 9, no. 10: 826. https://doi.org/10.3390/ma9100826