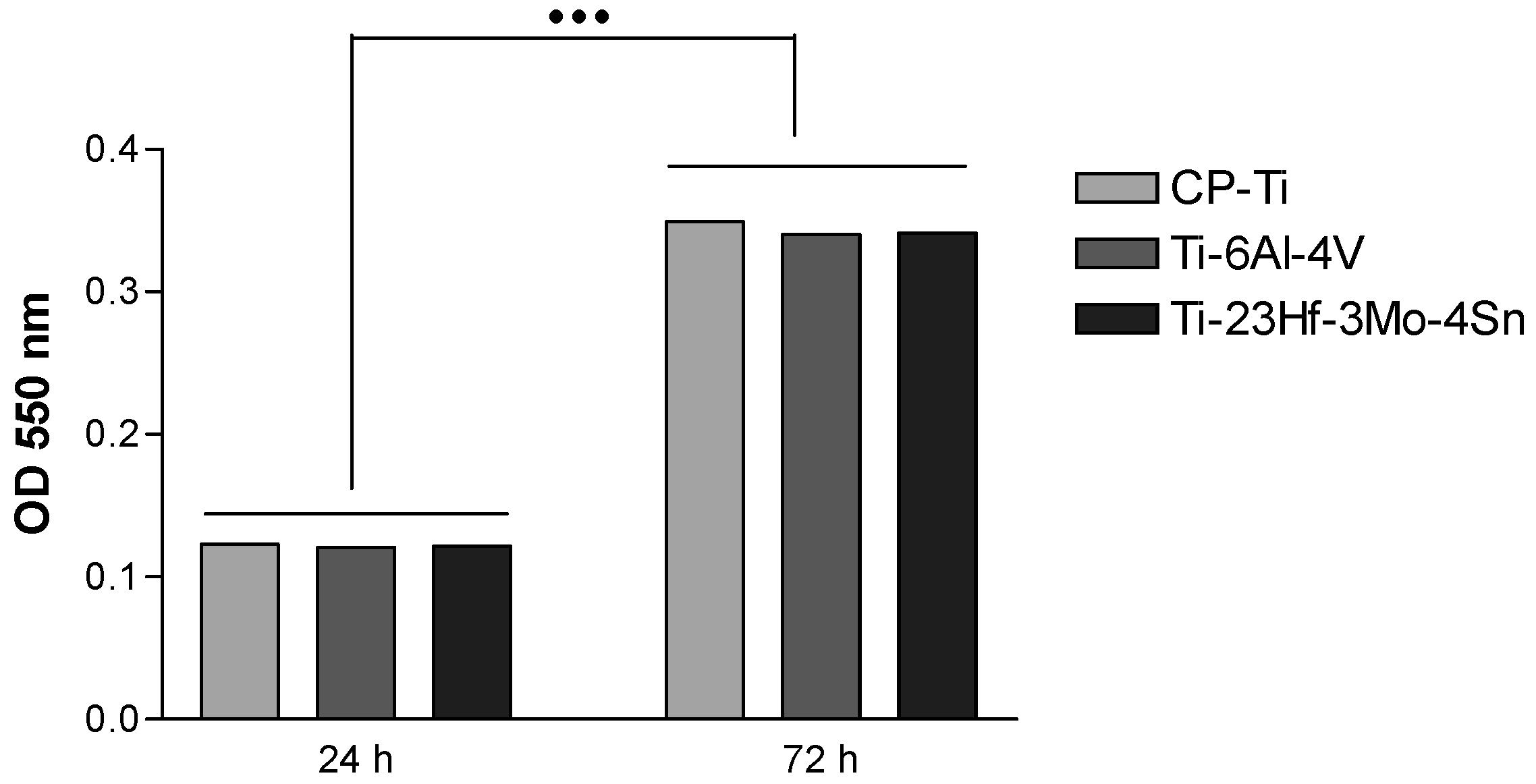
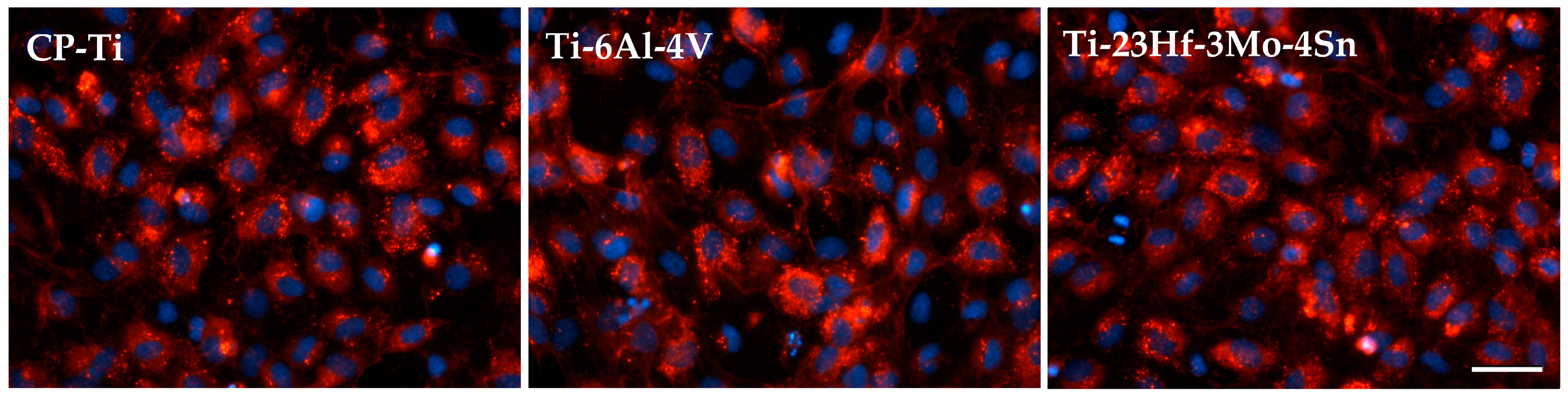
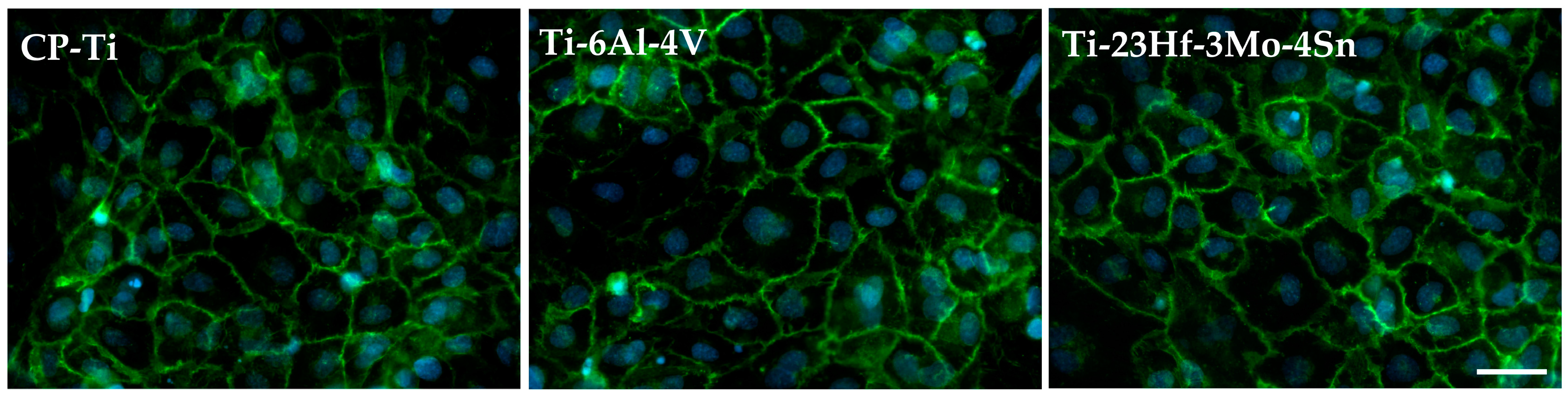
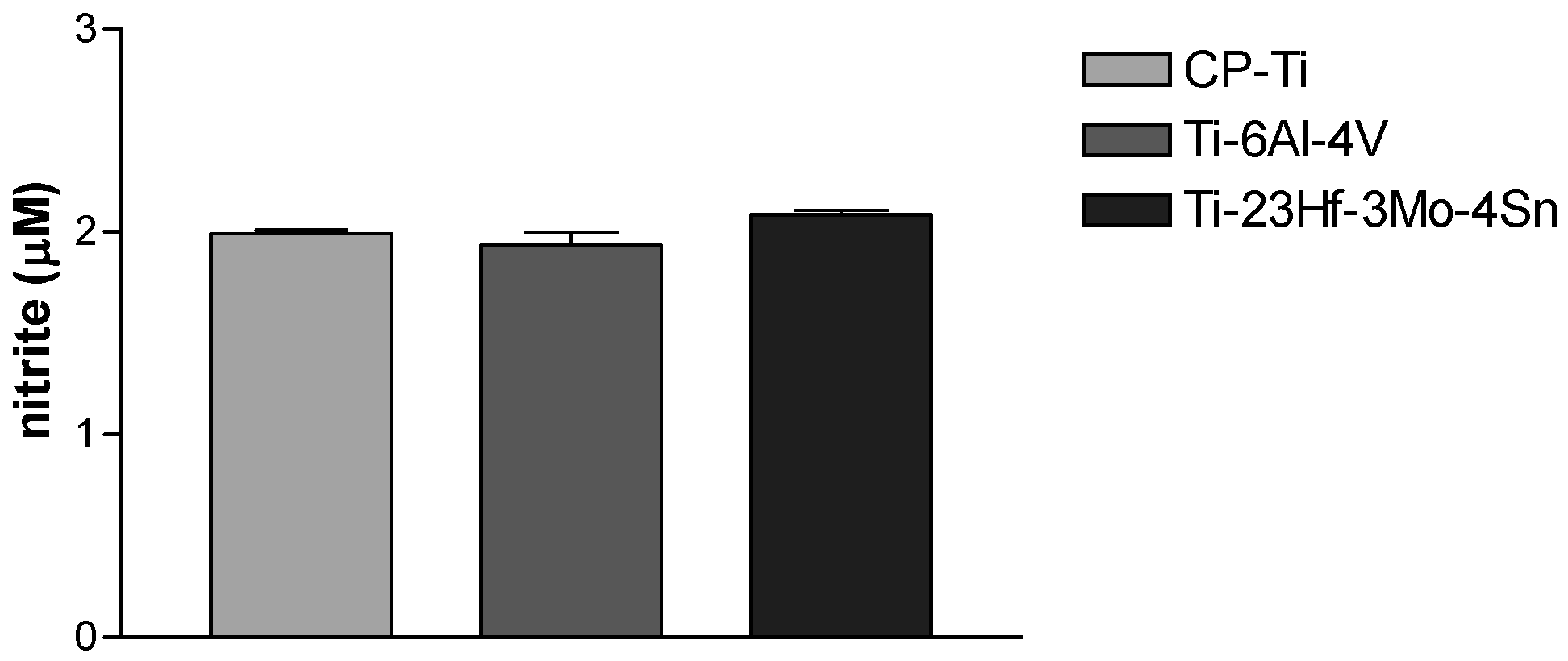
2.2.1. Electrochemical Behavior from Cyclic Potentiodynamic Curves
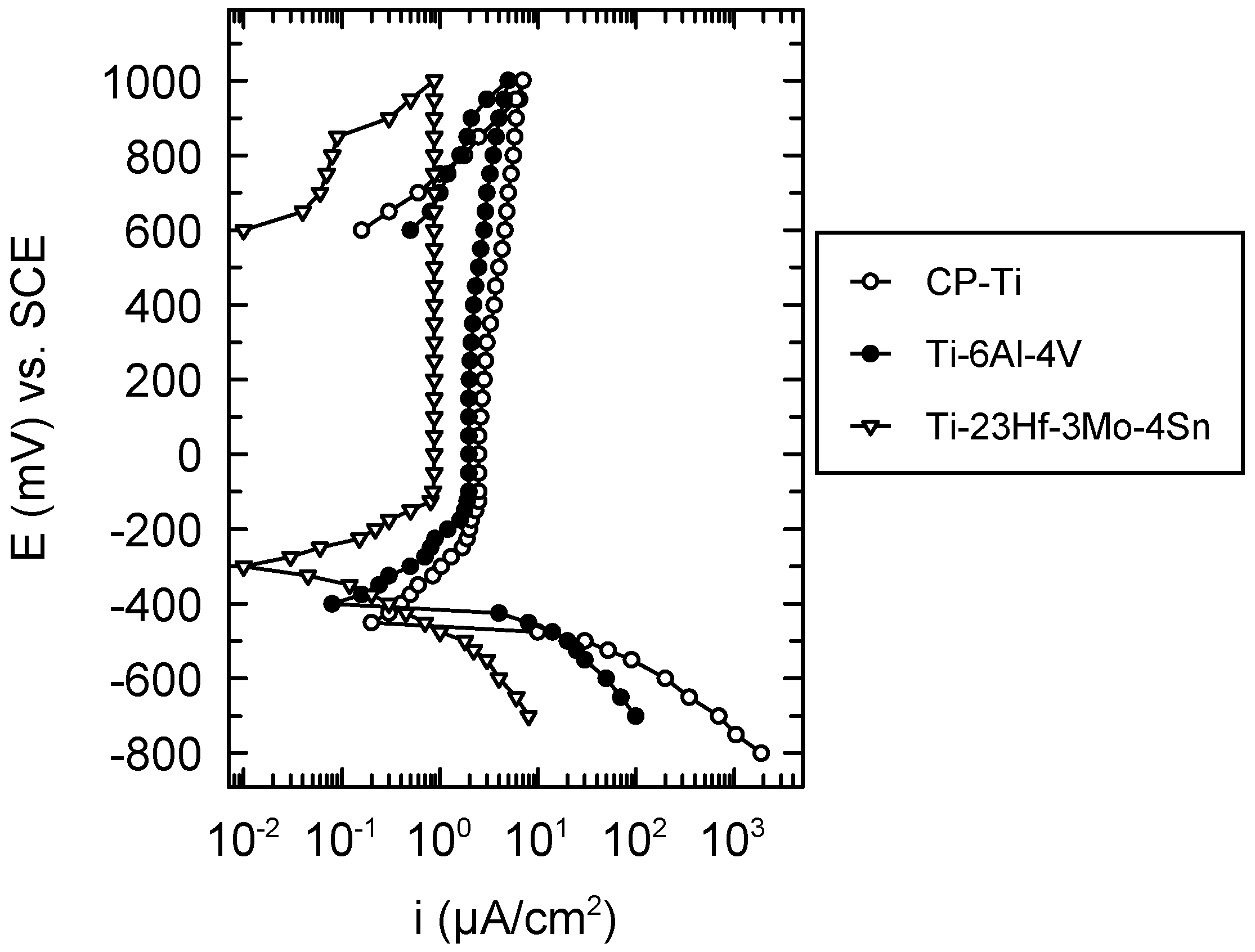
The cyclic potentiodynamic curves presented in
Figure 4 evince a typical passive behavior both for CP-Ti, Ti-6Al-4V and the new Ti-23Hf-3Mo-4Sn alloy. These curves did not display hysteresis loops, namely, no local corrosion took place on the surface of the three studied materials; this fact was confirmed by the microscopic observations. As shown in
Table 3, more favorable values of all the electrochemical parameters were obtained for the newly developed alloy: more electropositive values of the corrosion, E
corr, and passivation, E
p potentials due to the effect of the galvanic couple of the alloying elements; lower values of the tendency to passivation; |E
corr − E
p| and passive current density; i
p which indicates a more rapid, easier, better passivation and a more resistant passive film that prevents the active dissolution of the alloy substrate, respectively [
17,
18]. The values of all the electrochemical parameters prove a nobler electrochemical behavior of the new alloy than that of CP-Ti and Ti-6Al-4V alloy [
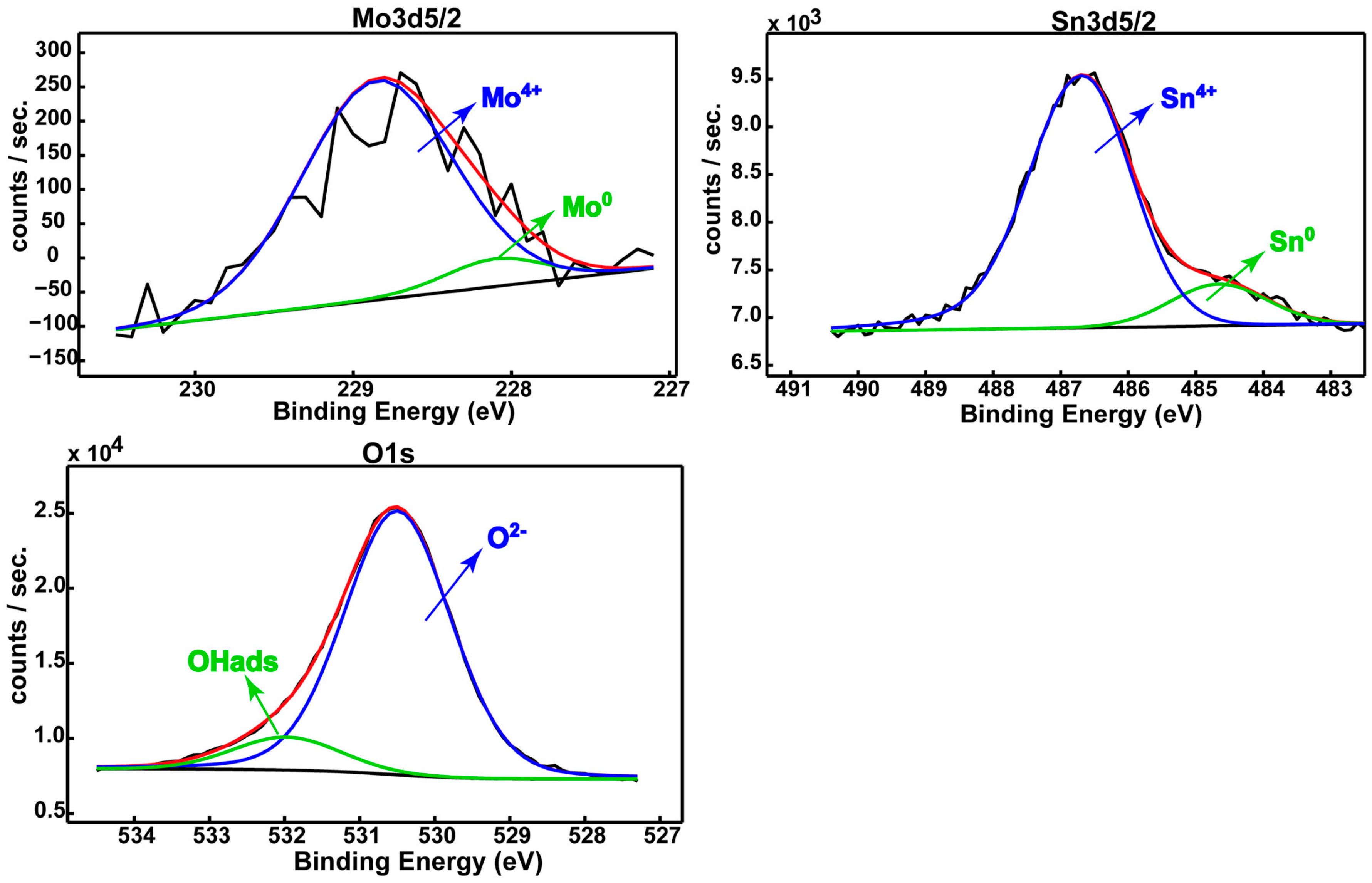
19]. This behavior is ascribed to the presence of a thicker native passive film (18.0 nm ± 1.0 nm) on its surface as compared with that of CP-Ti (1.3–3.7 nm) [
20] and Ti-6Al-4V (5 nm) [
21] alloy; in addition, besides Ti
2O
3, TiO
2 oxides, this film contains HfO
2, SnO
2 and MnO
2 protective oxides that thicken and compact it, conferring very good stability in SBF (see
Section 2.1).
2.2.2. Corrosion Resistance from Linear Polarization Tafel Representations
The corrosion parameters i
corr (corrosion current density) and V
corr (corrosion rate) (
Table 4) for the newly developed alloy have lower values of about 8–9 times than those for CP-Ti and Ti-6Al-4V alloys, a fact that demonstrates a more resistant passive film on the new alloy surface. The total quantity of ions released into SBF by the new alloy is lower than that of the reference materials, showing a much reduced toxicity. In addition, polarization resistance, Rp, for the novel alloy has a higher value of about 6–9 times than those of CP-Ti and Ti-6Al-4V alloys due to more protective passive film existing on the new alloy surface [
17,
18]. The new alloy is placed in the “Perfect Stable” resistance class [
22], a fact that depicts a high resistance to corrosion [
23]. The higher values of the anodic, β
a Tafel slopes than those of the cathodic, β
c Tafel slopes reflect the anodic control of the processes from the interface, namely, the existence of the passive layer [
24] in addition, to the values of the cathodic β
c Tafel slopes around −115 mV/dec. Tafel-like behavior signifies that the cathodic reaction of hydrogen reduction does not depend on the alloy composition [
24]. All corrosion parameters confirm a higher protective capacity of the new alloy passive film in comparison with those of the commercial materials CP-Ti and Ti-6Al-4V alloys.
2.2.3. Electrochemical Behavior from EIS
Nyquist spectra (
Figure 5a) are represented by large, incomplete, depressed semicircles, which show a capacitive behavior, and a passive film like an insulator [
25,
26,
27]. The semicircle diameters increase in the order: Ti < Ti-6Al-4V < Ti-23Hf-3Mo-4Sn, namely, the passive film on the new alloy surface possesses the highest insulating, capacitive, protective properties, confirming the XPS results which describe the thickest film.
Bode phase angle spectra (
Figure 5b) exhibit two phase angles: in the low frequency range, higher phase angles than those from the middle frequency range can be observed. The values of the first phase angle vary between −79° for Ti, to −81° for Ti-6Al-4V alloy, to −85° for Ti-23Hf-3Mo-4Sn alloy. The highest phase angle for the new alloy denotes the highest capacitive, the most protective passive film [
25,
26,
27,
28,
29,
30,
31]; this fact is sustained by the XPS depth profiling analysis that indicates a very thick (18 nm) native passive film. It is known that the thickest passive film assures the highest protection, corrosion resistance [
32]. The second, lower angle from the middle frequency range has values of −76° for Ti, −78° for Ti-6Al-4V alloy and −82° for Ti-23Hf-3Mo-4Sn alloy, indicating a defective capacitor with some pores which permit transfer processes from the substrate to solution and from the solution to substrate. Thus, these two phase angles characterize a passive film with two layers [
25,
26,
27,
28]: the highest phase angle represents the inner, capacitive, barrier, compact layer and the lower one illustrates the outer, less protective, porous layer that confers bioactivity to the alloy [
30,
31].
The EIS results were modeled with an electric equivalent circuit consisting of two time constants (
Figure 6) as other many authors [
25,
26,
27,
28,
29,
30,
31,
33,
34,
35,
36]. The electrical parameters are a resistor, R, to show the film conductance and a capacitor, CPE, for the film dielectric properties. The first time constant associated with the high phase angle describes the inner, dense, barrier layer and is composed by the barrier layer resistance, R
b and capacitance CPE
b. The second time constant is related to the lower phase angle that represents the outer, less insulating, less resistant, porous layer and is formed by the porous layer resistance, R
p and capacitance, CPE
p (the constant phase element, CPE was used instead of capacitance, C to illustrate the non-ideal capacitor).
Fitting parameters from
Table 5 have more favorable values for the new Ti-23Hf-3Mo-4Sn alloy, indicating that its passive film is more resistant. This fact confirms the XPS results that revealed a thicker, denser, more compact passive film on the new alloy surface. The inner, barrier layer resistance R
b has higher values with two orders of magnitude than the resistance of the outer, porous layer R
p, denoting that the resistance of the passive film is conferred by the inner, compact layer [
25,
26]. The barrier layer capacitance, CPE
b, is one order of magnitude lower than that of the porous layer, CPE
p, suggesting that the inner layer is thicker than the outer layer [
25]. The frequency independent parameter, n, shows the non-uniform current distribution on the surface due to its roughness and homogeneity [
25]. For the barrier layer, the n1 values are much closer to 1, namely, an about ideal capacitor; for the porous layer, n2 values are lower, i.e., this layer has a less insulating behavior.
2.2.4. Long-Term Corrosion Resistance from Monitoring of the Open Circuit Potentials
The open circuit potentials (E
oc) were monitored for 1000 exposure hours of the studied materials exposed to the aggressive action of SBF (
Figure 7).
The open circuit potentials for CP-Ti have the most electronegative values from the initial to the final experimental time; the slow increase of E
oc values occurred in the first 50 h and then about stable values of −250 mV (vs. SCE) were maintained; these facts point out the slow growth, a low thickening of the passive film followed by a stable passive state [
17,
18]; the values of E
oc for Ti are placed on a Pourbaix diagram [
9] in the passive potential range; namely, Ti is passive.
For Ti-6Al-4V alloy, the open circuit potentials have some oscillations at the beginning, reflecting that its passive film is not completely stable [
17,
18]; after about 500 immersion hours, E
oc values slowly move to more electropositive values and then stabilize to about −100 mV (vs. SCE), fact that ascertains a stable passive film [
17,
18], taking into account that all its constituent elements Ti, Al, V are placed in their passive potential range on Pourbaix diagrams [
9].
The novel Ti-23Hf-3Mo-4Sn alloy presents the most electropositive values of its open circuit potentials; these E
oc values tend to have nobler values in time, reaching a value of −80 mV (vs. SCE) after 1000 exposure hours in SBF; this behavior reveals a more resistant passive state, namely, the passive film thickened in time and improved its protective properties [
17,
18]; this fact is normal because all alloying elements Ti, Hf, Sn, Mo find out in their passive state on Pourbaix diagrams [
9]. In addition, the new alloy native passive film being thicker than that of the CP-Ti and Ti-6Al-4V alloy confers the best corrosion resistance [
25].
From the monitoring of the open circuit potentials, the results showed that the new Ti-23Hf-3Mo-4Sn alloy has the noblest, stable passive behavior.