Transition Metals Nanocatalysis
A special issue of Applied Nano (ISSN 2673-3501).
Deadline for manuscript submissions: closed (20 July 2022) | Viewed by 7901
Special Issue Editor
Interests: heterogeneous catalysis; surface science; materials science; rational design of metal oxides; nanocatalysis; promotion in catalysis; metal–support interactions; structure–property relationships
Special Issues, Collections and Topics in MDPI journals
Special Issue Information
Dear Colleagues,
In view of the unprecedented energy and environmental issues currently faced, heterogeneous catalysis is expected to have a key role in the near future toward a sustainable development. Heterogeneous catalysis has recently gained considerable attention from both the scientific and industrial community, as it is a field of diverse applications, including, among others, the field of energy production, conversion, and storage, as well as the remediation of the environment through the abatement of hazardous substances, signifying its pivotal role in the world economy.
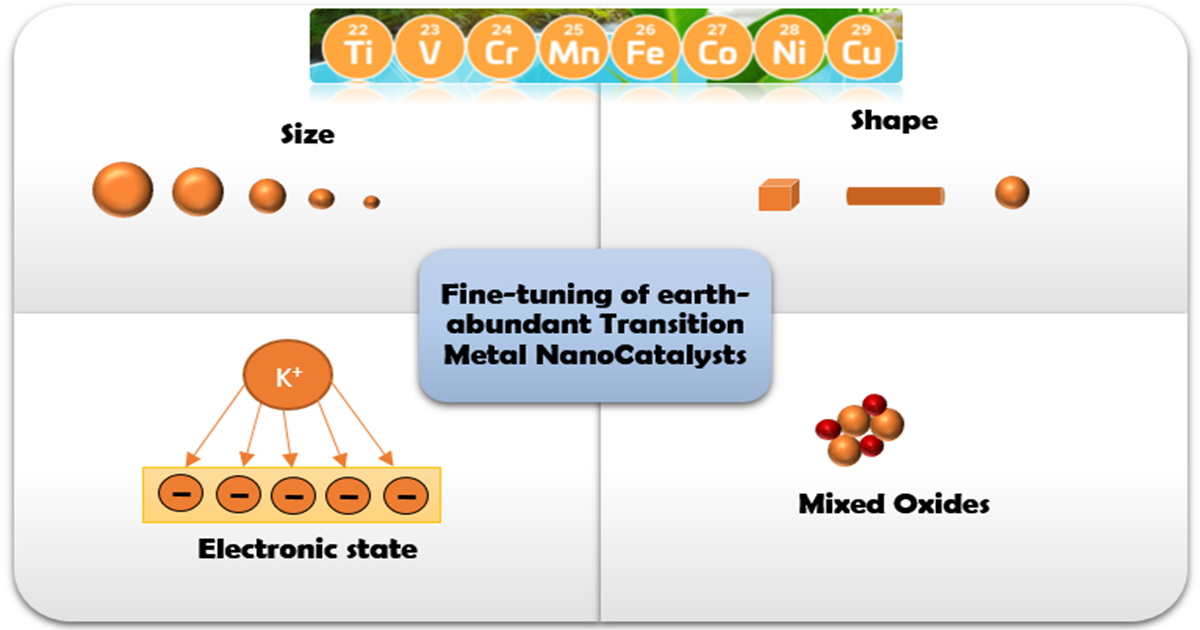
In view of the above aspects, the rational design and development of highly efficient and cost-effective (electro)catalysts are of paramount importance. Among the different types of catalysts, the metal oxides (MOs) of earth-abundant and inexpensive transition metals have gained particular attention due to their particular properties in conjunction to their lower cost. More importantly, the fine-tuning of the local surface structure of MOs through appropriate nanosynthesis and/or promotional/modification routes can adjust the size, shape, and electronic state of different counterparts with great implications on intrinsic reactivity and metal–support interactions. This “fine-tuning” approach is expected to lead to the development of highly active, low-cost nanocomposites for real-life applications.
The present themed Special Issue is mainly focused on recent theoretical and experimental advances in relation to the synthesis, characterization, and fine-tuning of transition metal catalysts at nanoscale. In particular, advanced nanosynthesis and optimization routes toward the development of highly active transition metals nanocatalysts for energy or environmental applications are perfectly matched to this themed issue. In addition, advanced characterization methods and in-depth experimental and computational studies toward a fundamental understanding of metal–support interactions and structure–property relationships are very welcomed.
Dr. Michalis Konsolakis
Guest Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the special issue website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Applied Nano is an international peer-reviewed open access quarterly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 1000 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- Nanomaterials
- Transition metals
- Heterogeneous catalysis/electocatalysis/photocatalysis
- Novel synthetic methods
- Catalysts promotion
- Metal–support interactions
- Ceria-based oxides, perovskites, hexa-aluminates, hydrotalcites, spinels, etc.
- Shape and size effects in catalysis
- Advanced characterization studies



