-
 National Multicenter Study on the Prevalence of Carbapenemase-Producing Enterobacteriaceae in the Post-COVID-19 Era in Argentina: The RECAPT-AR Study
National Multicenter Study on the Prevalence of Carbapenemase-Producing Enterobacteriaceae in the Post-COVID-19 Era in Argentina: The RECAPT-AR Study -
 Infected Fractures and Prosthetic Joints Have Very Similar Microbiology
Infected Fractures and Prosthetic Joints Have Very Similar Microbiology -
 Efflux Pump Inhibitors Enhance Activity of NBTIs Against Gram-Negative Bacteria
Efflux Pump Inhibitors Enhance Activity of NBTIs Against Gram-Negative Bacteria
Journal Description
Antibiotics
Antibiotics
is an international, peer-reviewed, open access journal on all aspects of antibiotics, published monthly online by MDPI. The Croatian Pharmacological Society (CPS) is affiliated with Antibiotics and its members receive discounts on the article processing charges.
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- High Visibility: indexed within Scopus, SCIE (Web of Science), PubMed, PMC, Embase, CAPlus / SciFinder, and other databases.
- Journal Rank: JCR - Q1 (Pharmacology and Pharmacy) / CiteScore - Q1 (General Pharmacology, Toxicology and Pharmaceutics )
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 15.8 days after submission; acceptance to publication is undertaken in 2.5 days (median values for papers published in this journal in the second half of 2024).
- Recognition of Reviewers: reviewers who provide timely, thorough peer-review reports receive vouchers entitling them to a discount on the APC of their next publication in any MDPI journal, in appreciation of the work done.
Impact Factor:
4.3 (2023);
5-Year Impact Factor:
4.6 (2023)
Latest Articles
Prevalence, Antimicrobial Resistance, and Virulence Potential of Staphylococcus aureus in Donkeys from Nigeria
Antibiotics 2025, 14(5), 453; https://doi.org/10.3390/antibiotics14050453 - 29 Apr 2025
Abstract
Background: Animal-associated antimicrobial-resistant staphylococci pose a One Health concern, as they can spread into the environment and cause serious infections. Yet, donkeys in Nigeria have been largely overlooked as potential reservoirs of these pathogens. Aim/Objectives: To isolate Staphylococcus aureus from donkeys in Obollo-Afor,
[...] Read more.
Background: Animal-associated antimicrobial-resistant staphylococci pose a One Health concern, as they can spread into the environment and cause serious infections. Yet, donkeys in Nigeria have been largely overlooked as potential reservoirs of these pathogens. Aim/Objectives: To isolate Staphylococcus aureus from donkeys in Obollo-Afor, southeast Nigeria, assess their antimicrobial resistance profiles, and evaluate their virulence potential. Materials and Methods: Staphylococci were isolated from the nasal swabs of 250 donkeys, using mannitol salt agar, confirmed biochemically, with Staphylococcus aureus identified via a latex agglutination test and mass spectrometry. The resistance profiles of the isolates, including in regard to methicillin, inducible clindamycin, and β-lactamase production, were determined using disc diffusion, while vancomycin resistance was assessed through the use of agar dilution. The virulence factors were evaluated phenotypically. Results: Of the 250 samples, 11 (4.4%) contained S. aureus and 239 (95.6%) grew other Staphylococcus species. The resistance rates of the 11 S. aureus isolates to gentamicin, penicillin, tigecycline, cefoxitin, linezolid, and chloramphenicol were 45.5%, 66.7%, 54.5%, 27.3%, 36.4%, and 18.1%, respectively. The phenotypic methicillin-resistant S. aureus prevalence was 1.2%. Additionally, 23.5% of the S. aureus isolates were multidrug resistant, with a mean antibiotic resistance index of 0.25. All the S. aureus isolates exhibited virulence factors like clumping factor expression, catalase, caseinase, lecithinase, and gelatinase activity, while the occurrence of haemagglutinin, biofilm, pellicle, and hemolysin occurred in 27.3%, 54.5%, 36.4%, 72.2%, respectively. Conclusion: Although a small percentage of donkeys in Nigeria may harbor S. aureus, these animals are potentially spreading antimicrobial resistance, including multidrug and methicillin resistance, to humans and the environment.
Full article
(This article belongs to the Collection Staphylococcus— Molecular Pathogenesis, Virulence Regulation and Antibiotics Resistance)
►
Show Figures
Open AccessArticle
The Influence of the Seasonal Variability of Candida spp. Bloodstream Infections and Antifungal Treatment: A Mediterranean Pilot Study
by
Paola Di Carlo, Nicola Serra, Ornella Collotta, Claudia Colomba, Alberto Firenze, Luigi Aprea, Salvatore Antonino Distefano, Andrea Cortegiani, Giovanni Giammanco, Teresa Maria Assunta Fasciana, Roberta Virruso, Angela Capuano, Consolato M. Sergi and Antonio Cascio
Antibiotics 2025, 14(5), 452; https://doi.org/10.3390/antibiotics14050452 - 29 Apr 2025
Abstract
Background/Objectives: Various factors associated with seasonality, including temperature, humidity, geographical composition, and seasonal fluctuations, can influence the trends of microbes responsible for hospital infections, such as Candida spp. This study evaluates the seasonal variability of Candida spp. bloodstream infections and antifungal resistance
[...] Read more.
Background/Objectives: Various factors associated with seasonality, including temperature, humidity, geographical composition, and seasonal fluctuations, can influence the trends of microbes responsible for hospital infections, such as Candida spp. This study evaluates the seasonal variability of Candida spp. bloodstream infections and antifungal resistance in hospitalized patients in Sicily. Methods: We retrospectively analyzed the demographic and epidemiological characteristics of 175 patients with blood cultures positive for Candida spp. Who were hospitalized at University Hospital Paolo Giaccone (A.U.O.P.), University of Palermo, Italy, from 1 January 2022 to 31 December 2024. Data on Candida species and antifungal resistance were also collected from the hospital’s database system to prevent and control hospital infections in A.U.O.P. Results: A total of 175 patients, 57.7% males, with a mean age of 68.3 years, were included in this study. Candida parapsilosis, Candida albicans, and Candida glabrata were more frequent in ICU (54.5%, p = 0.0001), medical (72.5%, p = 0.0003), and surgical settings (24%, p = 0.0161), respectively. C. parapsilosis was more frequent in dead patients (53.2%, p = 0.005). Among the seasons, we observed a significantly higher presence of C. glabrata in Autumn (20%, p = 0.0436). From the analysis of the seasons, C. parapsilosis and C. albicans were more frequent for each season, except in Spring, where the most frequent isolates were C. glabrata (5.1%, p = 0.0237) and C. parapsilosis (9.7%, p < 0.0001). The antifungal with the most resistance to Candida spp. was fluconazole in all seasons. Conclusions: Our study highlights the seasonal trends in Candida spp. and antifungal resistance, emphasizing climate change’s challenges on fungal diseases. These findings may contribute to improving prevention and treatment strategies for candidemia.
Full article
(This article belongs to the Special Issue Climate Change and Antibiotic Resistance)
Open AccessReview
Delivery of Outpatient Parenteral Antimicrobial Therapy (OPAT) in an Ever-Changing National Health Service (UK): Benefits, Barriers, and Opportunities
by
Oyewole Christopher Durojaiye, Charlotte Fiori and Katharine Cartwright
Antibiotics 2025, 14(5), 451; https://doi.org/10.3390/antibiotics14050451 - 29 Apr 2025
Abstract
Outpatient parenteral antimicrobial therapy (OPAT) is increasingly used to manage a broad range of infections, enabling patients to receive intravenous antibiotics safely outside inpatient settings. In this review, we examine the current landscape of OPAT practice across the United Kingdom (UK), assessing its
[...] Read more.
Outpatient parenteral antimicrobial therapy (OPAT) is increasingly used to manage a broad range of infections, enabling patients to receive intravenous antibiotics safely outside inpatient settings. In this review, we examine the current landscape of OPAT practice across the United Kingdom (UK), assessing its clinical, economic, and operational impact. The benefits of OPAT for patients and the National Health Service (NHS), as well as its associated risks, are discussed. Additionally, we explore the challenges hindering its broader implementation within the UK. Finally, we highlight recent innovations and emerging applications of OPAT relevant to the NHS, underscoring key considerations for its future expansion and emphasising the need for a nationally coordinated strategy to realise its full potential.
Full article
Open AccessArticle
Antimicrobial Susceptibility Profiles of Escherichia coli Isolates from Clinical Cases of Geese in Hungary Between 2022 and 2023
by
Ádám Kerek, Ábel Szabó and Ákos Jerzsele
Antibiotics 2025, 14(5), 450; https://doi.org/10.3390/antibiotics14050450 - 29 Apr 2025
Abstract
Background: Antimicrobial resistance (AMR) poses an increasing threat to animal health and food safety. In the poultry sector, particularly in waterfowl farming, the widespread use of antibiotics may contribute to the dissemination of resistant Escherichia coli strains. This study aims to
[...] Read more.
Background: Antimicrobial resistance (AMR) poses an increasing threat to animal health and food safety. In the poultry sector, particularly in waterfowl farming, the widespread use of antibiotics may contribute to the dissemination of resistant Escherichia coli strains. This study aims to map the antibiotic resistance profiles of E. coli isolates from geese in Hungary, determine the prevalence of multidrug-resistant (MDR) and extensively drug-resistant (XDR) strains, and analyze resistance patterns and co-resistance relationships. Methods: E. coli isolates from clinical cases between 2022 and 2023 were examined using minimum inhibitory concentration (MIC) determination. Susceptibility results were evaluated based on the Clinical Laboratory Standard Institute (CLSI) breakpoints. Cluster analysis and principal component analysis (PCA) were applied to identify resistance patterns. Co-resistance relationships were examined through network analysis, while Monte Carlo simulations were used to estimate the expected prevalence of MDR strains. Results: Among the examined isolates, neomycin resistance was particularly high (86.8%), while florfenicol (73.6%) and amoxicillin (65.9%) resistance levels were also significant. The prevalence of MDR strains was 86.8%, and XDR strains accounted for 38.5%. Co-resistance analysis revealed a strong correlation between neomycin and spectinomycin resistance, as well as amoxicillin and doxycycline resistance. Monte Carlo simulations estimated that the expected range of MDR strain prevalence could vary between 80.2% and 92.3%. Conclusions: The high prevalence of MDR and XDR strains highlights the urgent need to reassess antibiotic usage strategies in goose farming. These findings underscore the importance of targeted antibiotic use, continuous microbiological surveillance, and the exploration of alternative therapeutic approaches to mitigate AMR.
Full article
(This article belongs to the Special Issue Detection of Bacteria and Antibiotics Surveillance in Livestock)
Open AccessArticle
Genetic Variation in the blaZ Gene Leading to the BORSA Phenotype in Staphylococcus aureus
by
Mia Aarris, Frederik Boëtius Hertz, Karen Leth Nielsen, Alexander Sato, Helle Krogh Johansen, Henrik Westh, Michael Kemp, Svend Ellermann-Eriksen, Anders Løbner-Olesen, Niels Frimodt-Møller and Godefroid Charbon
Antibiotics 2025, 14(5), 449; https://doi.org/10.3390/antibiotics14050449 - 29 Apr 2025
Abstract
Background/Objectives: Staphylococcus aureus is a leading cause of bacteraemia in Danish hospitals. Approximately 70% of clinical S. aureus isolates are penicillin-resistant, which is predominantly due to blaZ-mediated β-lactamase production. Methods: A collection of 489 S. aureus strains derived from bacteraemia were cultured
[...] Read more.
Background/Objectives: Staphylococcus aureus is a leading cause of bacteraemia in Danish hospitals. Approximately 70% of clinical S. aureus isolates are penicillin-resistant, which is predominantly due to blaZ-mediated β-lactamase production. Methods: A collection of 489 S. aureus strains derived from bacteraemia were cultured and their genomes sequenced. Results: From this collection, 71% of isolates were methicillin-susceptible S. aureus (MSSA) harbouring blaZ. While most isolates contained the blaZ gene belonging to the well-characterised A, B, C and D variants, three strains (1%) produced a BlaZ protein characterised by having threonine residues on both positions 128 and 216 and, therefore, belonged to neither of the established blaZ variants. We named this variant, variant F. We report that clinical isolates expressing blaZ variant F were resistant to oxacillin. The β-lactamase production phenotype in isolates carrying either of the A, B, C or D variants was only weakly discernible on MIC gradient strip and disk diffusion tests. When the β-lactamases were expressed either from a T7 promoter or from their endogenous promoters in Escherichia coli, variant F was significantly better at degrading ampicillin than variant A. We also showed that variant F conferred oxacillin resistance when expressed in an isogenic S. aureus strain, while variant A did not. Finally, we demonstrated that the F variant threonine 216 played a role in the enzyme’s superior activity. Conclusions: Our findings demonstrate that the new F variant of BlaZ is sufficient to render S. aureus a BORSA strain, which is superior in the degradation of common anti-staphylococcal β-lactam antibiotics, such as benzylpenicillin, cloxacillin, and oxacillin. It is sensitive to β-lactamase inhibitors and rapidly degrades nitrocefin. We provide a genetic explanation for the borderline oxacillin-resistant S. aureus (BORSA) phenotype.
Full article
(This article belongs to the Section Genetic and Biochemical Studies of Antibiotic Activity and Resistance)
►▼
Show Figures

Graphical abstract
Open AccessArticle
Bacterivorous Ciliate Tetrahymena pyriformis Facilitates vanA Antibiotic Resistance Gene Transfer in Enterococcus faecalis
by
Temilola O. Olanrewaju, James S. G. Dooley, Heather M. Coleman, Chris McGonigle and Joerg Arnscheidt
Antibiotics 2025, 14(5), 448; https://doi.org/10.3390/antibiotics14050448 - 28 Apr 2025
Abstract
Background: Wastewater treatment plants (WWTPs) are hotspots for the emergence and spread of antibiotic resistance genes (ARGs). In activated sludge treatment systems, bacterivorous protozoa play a crucial role in biological processes, yet their impact on the horizontal gene transfer in Gram-positive enteric bacteria
[...] Read more.
Background: Wastewater treatment plants (WWTPs) are hotspots for the emergence and spread of antibiotic resistance genes (ARGs). In activated sludge treatment systems, bacterivorous protozoa play a crucial role in biological processes, yet their impact on the horizontal gene transfer in Gram-positive enteric bacteria remains largely unexplored. This study investigated whether the ciliate Tetrahymena pyriformis facilitates the transfer of antibiotic resistance genes between Enterococcus faecalis strains. Methods: Conjugation assays were conducted under laboratory conditions using a vanA-carrying donor and a rifampicin-resistant recipient at an initial bacterial concentration of 109 CFU/mL and ciliate density of 105 N/mL. Results: Transconjugant numbers peaked at 2 h when experiments started with recipient bacteria harvested in the exponential growth phase, and at 24 h when bacteria were in the stationary phase. In both cases, vanA gene transfer frequency was highest at 24 h (10−4–10−5 CFU/mL), and the presence of energy sources increased gene transfer frequency by one order of magnitude. Conclusions: These findings suggest that ciliate grazing may contribute to vanA gene transfer in WWTP effluents, potentially facilitating its dissemination among permissive bacteria. Given the ecological and public health risks associated with vanA gene persistence in wastewater systems, understanding protozoan-mediated gene transfer is crucial for mitigating the spread of antibiotic resistance in aquatic environments.
Full article
(This article belongs to the Special Issue Tracking Reservoirs of Antimicrobial Resistance Genes in Environment)
►▼
Show Figures
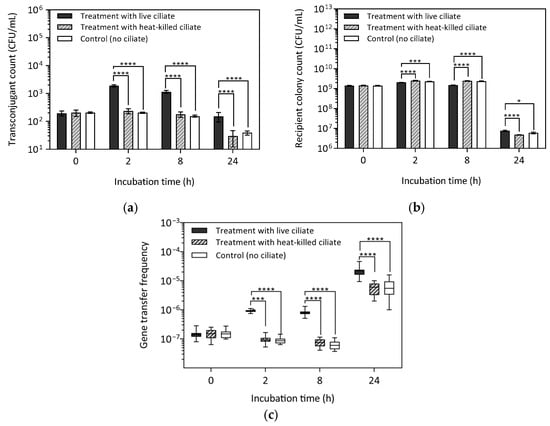
Figure 1
Open AccessArticle
The Accuracy of Empirical Antibiotic Treatment for Periprosthetic Joint Infections in Total Shoulder and Knee Arthroplasties
by
Katrin Freller, Hannah Punz, Clemens Schopper, Tobias Gotterbarm, Antonio Klasan and Stella Stevoska
Antibiotics 2025, 14(5), 447; https://doi.org/10.3390/antibiotics14050447 - 28 Apr 2025
Abstract
Introduction: Periprosthetic joint infections (PJIs) remain a major challenge in orthopedic and trauma surgeries. The microbial resistance profiles and the optimal choice of empirical antibiotic therapy in shoulder arthroplasty revision are less well characterized compared to those in knee or hip arthroplasty revision.
[...] Read more.
Introduction: Periprosthetic joint infections (PJIs) remain a major challenge in orthopedic and trauma surgeries. The microbial resistance profiles and the optimal choice of empirical antibiotic therapy in shoulder arthroplasty revision are less well characterized compared to those in knee or hip arthroplasty revision. Materials and Methods: This retrospective study constitutes a novel comparative analysis, providing valuable insights into the presence of joint-specific pathogen resistance and the empirical treatment accuracy of shoulder and knee arthroplasties. A review of all the revision cases following primary shoulder and knee arthroplasties conducted between January 2012 and December 2023 was performed. Cases that required revision because of PJIs were identified, and microbial cultures were analyzed to determine the presence of pathogens and their resistance profiles. Results: The most administered postoperative empirical antibiotics were cefuroxime and amoxicillin–sulbactam. A statistically significant difference in the prevalence of anerobic pathogens was observed between total shoulder arthroplasty and knee arthroplasty. Furthermore, a statistically significant difference was observed in the sensitivities of pathogens to metronidazole (p < 0.001) and erythromycin (p = 0.014). Conclusions: This study demonstrates microbiological and antimicrobial resistance differences between PJI TSA and TKA cases.
Full article
(This article belongs to the Special Issue Single-Stage Management of Musculo-Skeletal Infections: Local Antibacterial Protection, Antibiotics, and Surgical Treatments)
►▼
Show Figures
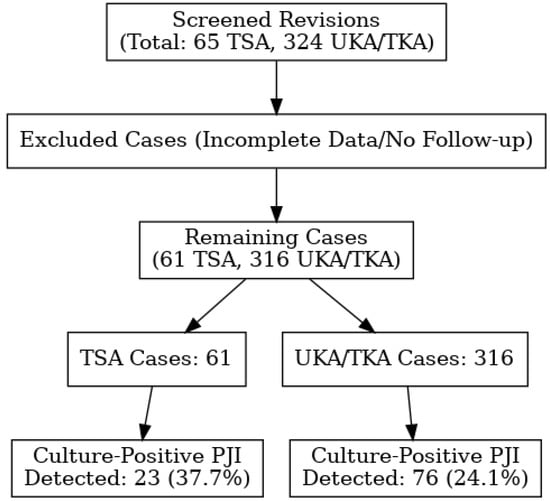
Figure 1
Open AccessArticle
Genomic Insights of Antibiotic-Resistant Escherichia coli Isolated from Intensive Pig Farming in South Africa Using ‘Farm-to-Fork’ Approach
by
Shima E. Abdalla, Linda A. Bester, Akebe L. K. Abia, Mushal Allam, Arshad Ismail, Sabiha Y. Essack and Daniel G. Amoako
Antibiotics 2025, 14(5), 446; https://doi.org/10.3390/antibiotics14050446 - 28 Apr 2025
Abstract
Background/Objectives: Intensive pig farming is a critical component of food security and economic activity in South Africa; however, it also presents a risk of amplifying antimicrobial resistance (AMR). This study provides genomic insights into antibiotic-resistant Escherichia coli (E. coli) circulating
[...] Read more.
Background/Objectives: Intensive pig farming is a critical component of food security and economic activity in South Africa; however, it also presents a risk of amplifying antimicrobial resistance (AMR). This study provides genomic insights into antibiotic-resistant Escherichia coli (E. coli) circulating across the pork production chain, using a ‘farm-to-fork’ approach. Methods: A total of 417 samples were collected from various points along the production continuum, including the farm (n = 144), transport (n = 60), and abattoir (n = 213). E. coli isolates were identified using the Colilert-18 system, and their phenotypic resistance was tested against 20 antibiotics. Thirty-one isolates were selected for further characterization based on their resistance profiles and sampling sources, utilizing whole-genome sequencing and bioinformatic analysis. Results: The isolates exhibited varying resistance to critical antibiotics used in both human and animal health, including ampicillin (31/31, 100%), tetracycline (31/31, 100%), amoxicillin–clavulanate (29/31, 94%), chloramphenicol (25/31, 81%), and sulfamethoxazole–trimethoprim (10/31, 33%). Genetic analysis revealed the presence of resistance genes for β-lactams (blaEC, blaTEM), trimethoprim/sulfonamides (dfrA1, dfrA5, dfrA12, sul2, sul3), tetracyclines (tetA, tetB, tetR, tet34), aminoglycosides (aadA, strA, aph variants), and phenicols (catB4, floR, cmlA1), most of which were plasmid-borne. Virulome analysis identified 24 genes, including toxins and adhesion factors. Mobile genetic elements included 24 plasmid replicons, 43 prophages, 19 insertion sequence families, and 7 class 1 integrons. The E. coli isolates belonged to a diverse range of sequence types, demonstrating significant genetic variability. Further phylogenomic analysis revealed eight major clades, with isolate clustering by sequence type alongside South African environmental and clinical E. coli strains, regardless of their sampling source. Conclusions: The genetic complexity observed across the pork production continuum threatens food safety and may impact human health. These findings underscore the need for enhanced AMR monitoring in livestock systems and support the integration of AMR surveillance into food safety policy frameworks.
Full article
(This article belongs to the Special Issue Genomic Characterization of Antimicrobial Resistance and Evolution Mechanism of Bacteria)
►▼
Show Figures
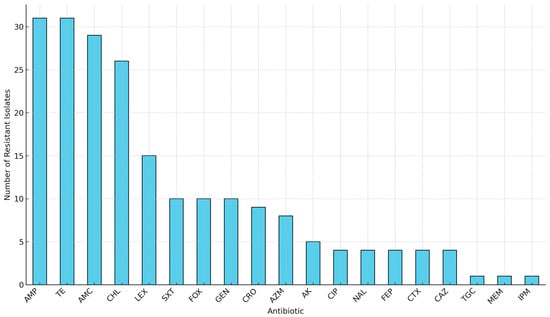
Figure 1
Open AccessArticle
The α-Helical Amphipathic Peptide Alleviates Colistin-Induced Nephrotoxicity by Maintaining Mitochondrial Function in Both In Vitro and In Vivo Infection Models
by
Min Soo Kook, Heeseung Kim, Yoonhwa Choi, Seong Man Bae, Jaehoon Yu and Yang Soo Kim
Antibiotics 2025, 14(5), 445; https://doi.org/10.3390/antibiotics14050445 - 28 Apr 2025
Abstract
►▼
Show Figures
Background/Objective: Colistin is the primary treatment for carbapenem-resistant Gram-negative bacteria (CR-GNB) infections, but its use is limited by nephrotoxicity, which reduces its effectiveness. There is an urgent need for nephroprotective agents to address this toxicity. This study investigated the potential of CMP3029,
[...] Read more.
Background/Objective: Colistin is the primary treatment for carbapenem-resistant Gram-negative bacteria (CR-GNB) infections, but its use is limited by nephrotoxicity, which reduces its effectiveness. There is an urgent need for nephroprotective agents to address this toxicity. This study investigated the potential of CMP3029, an α-helical peptide, to protect against colistin-induced nephrotoxicity. Methods: In vitro, CMP3029 was applied to HK-2 cells before colistin exposure, and cell viability and reactive oxygen species (ROS) levels were measured. In infected mice, CMP3029 was administered before colistin treatment, and urinary kidney injury molecule-1 (KIM-1), cystatin C levels, neutrophil gelatinase-associated lipocalin (NGAL), and renal damage were assessed. Results: CMP3029 preserved cell viability and significantly reduced mitochondrial ROS in HK-2 cells exposed to colistin. CMP3029 lowered urinary biomarkers and mitigated tubular injury in mice, demonstrating significant nephroprotective effects. Conclusions: These findings suggest that CMP3029 mitigates colistin-induced nephrotoxicity. Given the increasing threat of CR-GNB infections, CMP3029 could be a crucial clinical solution for improving patient outcomes in treating colistin-associated nephrotoxicity.
Full article

Figure 1
Open AccessArticle
In Vivo Antimicrobial Activity of Nisin Z Against S. aureus and Polyurea Pharmadendrimer PUREG4OEI48 Against P. aeruginosa from Diabetic Foot Infections
by
Isa Serrano, Dalila Mil-Homens, Rita F. Pires, Vasco D. B. Bonifácio, Joana F. Guerreiro, Eva Cunha, Sofia S. Costa, Luís Tavares and Manuela Oliveira
Antibiotics 2025, 14(5), 444; https://doi.org/10.3390/antibiotics14050444 - 28 Apr 2025
Abstract
Background/Objectives: Diabetic foot infections (DFIs) are commonly associated with frequent hospitalizations, limb amputations, and premature death due to the profile of the bacteria infecting foot ulcers. DFIs are generally colonized by a polymicrobial net of bacteria that grows in biofilms, developing an increased
[...] Read more.
Background/Objectives: Diabetic foot infections (DFIs) are commonly associated with frequent hospitalizations, limb amputations, and premature death due to the profile of the bacteria infecting foot ulcers. DFIs are generally colonized by a polymicrobial net of bacteria that grows in biofilms, developing an increased antimicrobial resistance to multiple antibiotics. DFI treatment is a hurdle, and the need to develop new therapies that do not promote resistance is urgent. Therefore, the antibacterial efficacy of Nisin Z (antimicrobial peptide), a core–shell polycationic polyurea pharmadendrimer (PUREG4OEI48) (antimicrobial polymer), and amlodipine (antihypertensive drug) was evaluated against S. aureus and P. aeruginosa isolated from a DFI and previously characterized. Methods: The antibacterial activity was analyzed in vitro by determining the minimal inhibitory concentration (MIC) and in vivo in a Galleria mellonella model by assessing the larvae survival and health index. Results: The results indicate that Nisin Z exhibited antibacterial activity against S. aureus in vivo, allowing larvae full survival, and no antibacterial activity against P. aeruginosa. Nisin Z may have reduced the antibacterial effectiveness of both PUREG4OEI48 and amlodipine. PUREG4OEI48 significantly increased the survival of the larvae infected with P. aeruginosa, while amlodipine showed no activity against both bacteria in vivo. Conclusions: These findings suggest that both Nisin Z and PUREG4OEI48 could potentially be used individually as adjunct treatments for mild DFIs. However, further studies are needed to confirm these findings and assess the potential toxicity and efficacy of PUREG4OEI48 in more complex models.
Full article
(This article belongs to the Special Issue Strategies to Combat Antibiotic Resistance and Microbial Biofilms)
►▼
Show Figures
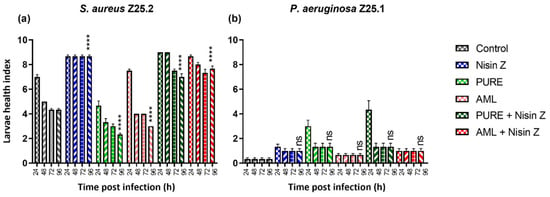
Figure 1
Open AccessReview
Antimicrobial Resistance in Diverse Ecological Niches—One Health Perspective and Food Safety
by
Nedjeljko Karabasil, Milica Mirković, Ivan Vićić, Ivana Perić, Nevena Zlatković, Bojana Luković and Ina Gajić
Antibiotics 2025, 14(5), 443; https://doi.org/10.3390/antibiotics14050443 - 28 Apr 2025
Abstract
Antimicrobial resistance (AMR) is a multi-sectoral, systemic, and global issue worldwide. Antimicrobial use (AMU) is a key factor in the selection of resistant bacteria within different ecological niches, from agriculture to food-producing animals to humans. There is a question regarding the extent to
[...] Read more.
Antimicrobial resistance (AMR) is a multi-sectoral, systemic, and global issue worldwide. Antimicrobial use (AMU) is a key factor in the selection of resistant bacteria within different ecological niches, from agriculture to food-producing animals to humans. There is a question regarding the extent to which the use of antibiotics in livestock production and the primary food production sector influences the selection and transmission of resistant bacteria and/or resistant genes throughout the food chain and thus contributes to the complexity in the development of AMR in humans. Although the trends in the prevalence of foodborne pathogens have changed over time, the burden of ecological niches with resistance genes, primarily in commensal microorganisms, is of concern. The implementation of the harmonized surveillance of AMU and AMR would provide comprehensive insights into the actual status of resistance and further interventions leading to its reduction. Tracking AMR in different ecological niches by applying advanced genome-based techniques and developing shared AMR data repositories would strengthen the One Health concept.
Full article
(This article belongs to the Special Issue Comprehensively Addressing Antimicrobial Resistance, Improving Food Safety and Achieving the One Health Goals: Global Challenges)
Open AccessArticle
Antibiotic Cocktail Exacerbates Esomeprazole-Induced Intestinal Dysmotility While Ameliorating Gastric Dyspepsia in Mice
by
Jing-Hua Wang, Song-Yi Han, Kyungjae Lee, Uijeong Han, Si-Kyung Cho and Hojun Kim
Antibiotics 2025, 14(5), 442; https://doi.org/10.3390/antibiotics14050442 - 27 Apr 2025
Abstract
►▼
Show Figures
Background/Objectives: Esomeprazole, a proton pump inhibitor (PPI), is commonly prescribed for gastric-acid-related disorders but has been associated with impaired gastrointestinal (GI) motility with long-term use. However, the effect of concurrent antibiotic administration on this dysfunction remains unclear. Therefore, this study aimed to investigate
[...] Read more.
Background/Objectives: Esomeprazole, a proton pump inhibitor (PPI), is commonly prescribed for gastric-acid-related disorders but has been associated with impaired gastrointestinal (GI) motility with long-term use. However, the effect of concurrent antibiotic administration on this dysfunction remains unclear. Therefore, this study aimed to investigate the effects of antibiotics on esomeprazole-induced GI motility dysfunction and explore the underlying mechanisms in a mouse model. Methods: Male C57BL/6 mice were orally administered esomeprazole (160 mg/kg) five times per week for 4 weeks. Three days before initiating esomeprazole treatment, a broad-spectrum antibiotic cocktail (ABX) consisting of ampicillin (1 g/kg), neomycin (1 g/kg), metronidazole (1 g/kg), and vancomycin (0.5 g/kg) was provided in drinking water and maintained throughout the experimental period. Mosapride (3 mg/kg), a prokinetic agent, was used as a positive control. Results: Neither esomeprazole alone nor in combination with ABX affected body weight or food intake. Compared to normal controls, esomeprazole treatment significantly delayed both intestinal transit and gastric emptying. However, ABX co-administration further pronounced intestinal transit time and improved gastric motility. The potential mechanisms may involve interactions among gastric H+/K+-ATPase, CYP3A11, gastrointestinal hormones (secretin and motilin), and the gut microbiome. Conclusions: Long-term esomeprazole use can impair both gastric and intestinal motility, and ABX co-treatment further exacerbates intestinal transit delay while paradoxically enhancing gastric emptying. These findings highlight the critical role of the gut microbiota in esomeprazole-induced GI motility dysfunction and suggest that antibiotic use should be approached with caution, particularly when combined with PPI therapy.
Full article
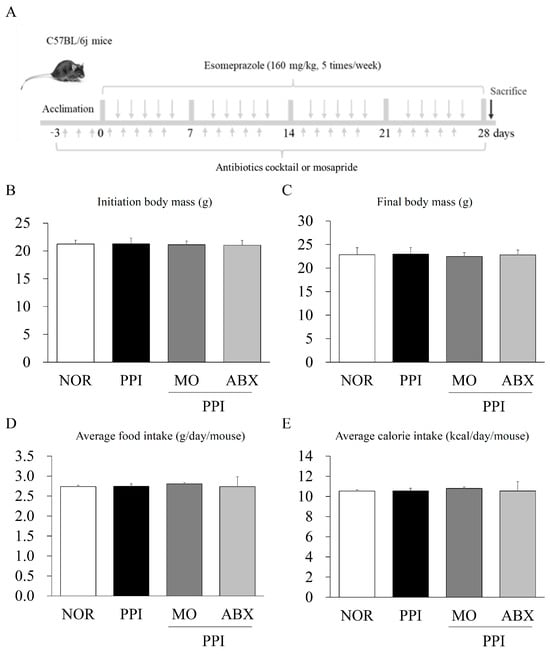
Figure 1
Open AccessArticle
Thermoplastic Zinc-Infused Polymer for Chairside Socket Seal Abutments Enhances Antimicrobial and Tissue-Integrative Properties
by
Wannes Van Holm, Katleen Vandamme, Jill Hadisurya, Ferda Pamuk, Naiera Zayed, Merve Kübra Aktan, Annabel Braem, Andy Temmerman and Wim Teughels
Antibiotics 2025, 14(5), 441; https://doi.org/10.3390/antibiotics14050441 - 27 Apr 2025
Abstract
Background/Objectives: The essential trace element zinc (Zn) has a pivotal role in wound healing and can show antibacterial activity, but its application in oral implant materials is underexplored. Customized healing abutments can modulate the peri-implant tissue health when appropriate bioactive materials promoting
[...] Read more.
Background/Objectives: The essential trace element zinc (Zn) has a pivotal role in wound healing and can show antibacterial activity, but its application in oral implant materials is underexplored. Customized healing abutments can modulate the peri-implant tissue health when appropriate bioactive materials promoting mucosal healing are used. The present study investigated a novel Zn-containing polymer for its potential in soft-tissue engineering applications. Methods: Four traditional materials—titanium, glass ionomer, a composite, and the novel Zn-containing polymer—were tested in vitro for bacterial growth using a multispecies oral bacterial model compared to hydroxyapatite. The biocompatibility of the materials was also evaluated by evaluating the adhesion, proliferation, and cytotoxicity of human oral keratinocytes (HOK-18A) onto these materials, compared to tissue culture plastic. Results: The Zn-containing polymer exhibited a significantly lower biofilm formation compared to conventional materials as it was composed of less pathogenic bacteria. The Zn-containing material also demonstrated a superior biocompatibility towards HOK-18A, approximating the adhesion and proliferation of the keratinocytes to optimal tissue culture conditions. Moreover, these properties did not seem to degrade and were maintained over a period of 31 days. The cytotoxicity assessment revealed no significant reduction in metabolic activity for any material. Conclusions: This study highlights the potential of the novel Zn-containing polymer in soft-tissue engineering, owing to its antimicrobial and biocompatible assets. These properties, combined with the ease of chairside modeling, position the material as a promising alternative for creating customized healing abutments. Further research is needed to explore its mechanism of wound healing modulation and its clinical performance.
Full article
(This article belongs to the Special Issue Biofilm-Associated Oral Diseases: Advances in Diagnosis and Challenges in Treatment)
►▼
Show Figures
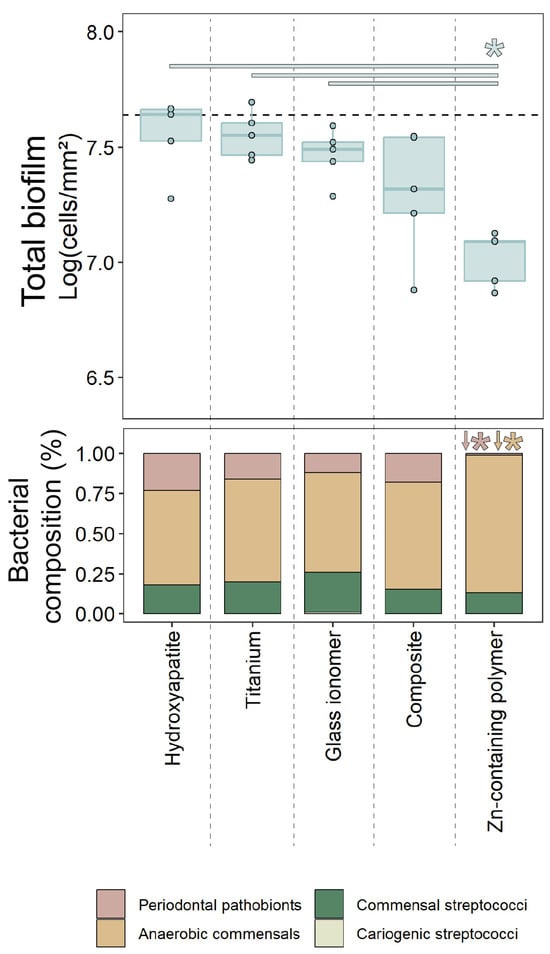
Figure 1
Open AccessArticle
A Cross-Sectional Study Assessing Antibiotic Resistance Awareness Among University Students in Samborondón, Greater Guayaquil, Ecuador
by
Norka Michelle Mora Pincay, José Luis Villegas, César Marcelo Larrea-Álvarez, Daniela Beatriz Briones Caiminagua, Lilibeth Torres-Elizalde, Miroslava Anna Šefcová and Marco Larrea-Álvarez
Antibiotics 2025, 14(5), 440; https://doi.org/10.3390/antibiotics14050440 - 27 Apr 2025
Abstract
Background/Objectives: Education on antibiotic use has the potential to positively shape the practices and perspectives of future professionals. Assessing awareness levels of antibiotic resistance among university students is, therefore, critical, as they represent a vital demographic capable of influencing public health outcomes, especially
[...] Read more.
Background/Objectives: Education on antibiotic use has the potential to positively shape the practices and perspectives of future professionals. Assessing awareness levels of antibiotic resistance among university students is, therefore, critical, as they represent a vital demographic capable of influencing public health outcomes, especially in low- and middle-income countries. Methods: This cross-sectional study employed the World Health Organization’s Antibiotic Resistance: Multi-Country Public Awareness Survey, which examines demographics, antibiotic use, knowledge, perspectives, and sources of information. A total of 922 surveys were collected from students across various disciplines at two universities in Greater Guayaquil. Results: Most participants reported obtaining antibiotics through healthcare professionals, adhering to proper usage instructions, and purchasing them primarily from pharmacies. However, only 56% of the responses were correct, with many students incorrectly associating antibiotic use with conditions where they are typically ineffective. Despite these gaps, the students expressed positive attitudes toward proposed measures to address antibiotic resistance. While the participants demonstrated familiarity with terms related to antibiotic resistance and identified doctors and educators as their main sources of information, educational campaigns were not widely recognized as important. Conclusions: These findings evidence knowledge gaps among an essential group, suggesting the need for targeted health programs, preventive strategies, and educational initiatives to combat misinformation regarding antimicrobial resistance.
Full article
(This article belongs to the Special Issue Antibiotic Use in the Communities—2nd Edition)
►▼
Show Figures
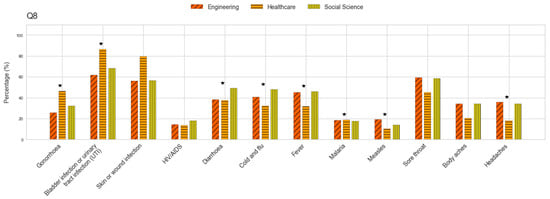
Figure 1
Open AccessSystematic Review
Effectiveness of Bacillus clausii (O/C, N/R, SIN, T) in the Prevention of Antibiotic-Associated Diarrhea and Gastrointestinal Symptoms: A Systematic Review
by
Ana Teresa Abreu, Rodrigo Vázquez Frías, Christian Boggio Marzet, Juan Pablo Stefanolo, Alejandro Concha Mejía, Luis Bustos Fernández, Oscar Laudanno, Dimas Rosa, Maria Claudia Cruz Serrano, Karen Cárdenas and Julio Zuluaga
Antibiotics 2025, 14(5), 439; https://doi.org/10.3390/antibiotics14050439 - 27 Apr 2025
Abstract
►▼
Show Figures
Background: Dysbiosis and antibiotic-associated diarrhea (AAD) are significant concerns in clinical settings. Probiotics, such as Bacillus clausii (O/C, N/R, SIN, T), a spore-forming bacterium resistant to gastrointestinal conditions and most commonly used antibiotics, emerge as a promising approach. We aim to assess
[...] Read more.
Background: Dysbiosis and antibiotic-associated diarrhea (AAD) are significant concerns in clinical settings. Probiotics, such as Bacillus clausii (O/C, N/R, SIN, T), a spore-forming bacterium resistant to gastrointestinal conditions and most commonly used antibiotics, emerge as a promising approach. We aim to assess the role of B. clausii in preventing AAD in children and adults during antibiotic therapy. Materials and methods: A systematic literature search was conducted across multiple databases, including MEDLINE, EMBASE, Cochrane Library, LILACS, and SciELO, up to May 2024. Studies were included if they involved B. clausii (O/C, N/R, SIN, T) administration during antibiotic treatment and reported AAD-related outcomes. Results: A total of four studies were included in the review. The studies comprised two randomized controlled trials (RCTs), one meta-analysis of RCTs, and one expert consensus. The primary outcome was the effectiveness of B. clausii (O/C, N/R, SIN, T) in reducing the incidence of diarrhea. Results showed a significant reduction in the risk of AAD and gastrointestinal symptoms in patients receiving it at a dosage of 4 × 109 CFU/day for children and 6 × 109 CFU/day for adolescents and adults for up to 14 days. Conclusions: B. clausii (O/C, N/R, SIN, T) appears to be an effective probiotic for preventing AAD in adults and children. It significantly improves gastrointestinal symptoms associated with antibiotic treatment, including diarrhea, nausea, and epigastric pain. Future studies are recommended to further elucidate its effectiveness in diverse populations, especially in low- and middle-income countries.
Full article
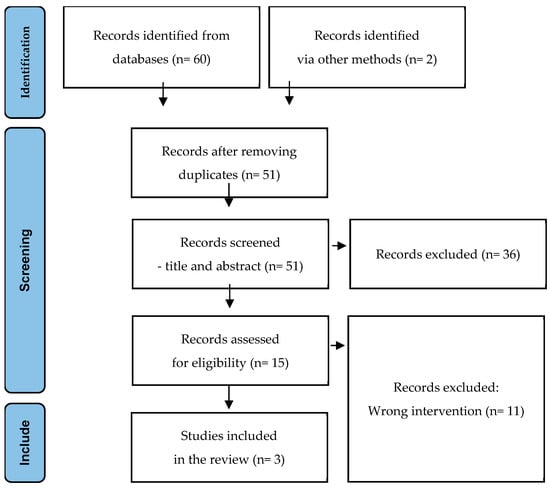
Figure 1
Open AccessArticle
Navigating Vancomycin and Acute Kidney Injury: AUC- vs. Trough-Guided Monitoring in Initial and Steady-State Therapy
by
Astrid Marovič, Tomaž Vovk and Maja Petre
Antibiotics 2025, 14(5), 438; https://doi.org/10.3390/antibiotics14050438 - 27 Apr 2025
Abstract
►▼
Show Figures
Background/Objectives: Vancomycin, a glycopeptide antibiotic used for gram-positive infections, is associated with acute kidney injury (AKI). Therapeutic drug monitoring (TDM) is recommended to minimize this risk while ensuring therapeutic efficacy. This study evaluated whether AUC-guided monitoring improved patient safety compared to traditional
[...] Read more.
Background/Objectives: Vancomycin, a glycopeptide antibiotic used for gram-positive infections, is associated with acute kidney injury (AKI). Therapeutic drug monitoring (TDM) is recommended to minimize this risk while ensuring therapeutic efficacy. This study evaluated whether AUC-guided monitoring improved patient safety compared to traditional trough-guided monitoring. Methods: A retrospective observational cohort study was conducted at the University Medical Centre Maribor, Slovenia, involving patients receiving intravenous vancomycin. One cohort was managed using trough-guided monitoring (n = 85), while the other was monitored using the AUC-guided approach (n = 139). The primary outcome was AKI incidence, and secondary outcomes included renal replacement therapy and mortality. Risk factors for AKI were identified, and pharmacokinetic parameters were evaluated at vancomycin therapy initiation and steady state. Results: The incidence of AKI was 20% in the trough-guided group and 18% in the AUC-guided group (p = 0.727). Secondary outcomes were similar in both cohorts. Risk factors for AKI included older age (OR 1.04; p = 0.042), higher steady-state AUC (OR 1.01; p < 0.001), longer duration of concomitant nephrotoxic therapy (OR 1.06; p = 0.019), and concomitant use of loop diuretics (OR 2.46; p = 0.045). Steady-state AUC values and trough levels (AUC0–24ss, AUC24–48ss, AUC0–48ss, and Cmin48ss) were significantly lower in the AUC-guided group, which was further reflected in the lower percentage of patients exceeding the AUC > 600 mg·h/L threshold at steady state. Conclusions: Although AKI incidence was lower in the AUC-guided group, the difference did not reach statistical significance. However, lower AUC values and trough levels in the AUC-guided group at steady state suggest a trend toward reduced vancomycin exposure and toxicity.
Full article
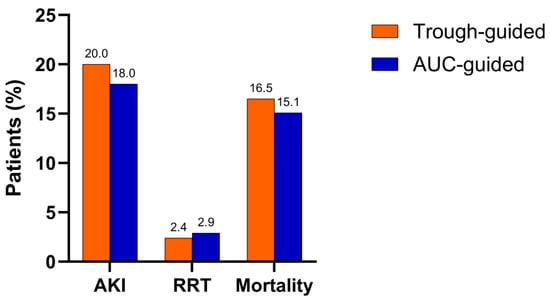
Figure 1
Open AccessArticle
Anti-Staphylococcus aureus Activity and Structural Characterization of Rationally Designed Peptides
by
Lorenza Artesani, Mariana Gallo, Laura Giovati, Francesca Maria Bisignano, Elena Ferrari, Lara M. Castronovo, Stefania Conti, Francesco Santoro, Thelma A. Pertinhez and Tecla Ciociola
Antibiotics 2025, 14(5), 437; https://doi.org/10.3390/antibiotics14050437 - 26 Apr 2025
Abstract
Background/Objectives: Microbial infections represent a significant threat to public health due to the emergence and spread of antimicrobial resistance. Adjunctive and alternative therapeutic strategies are explored to tackle this issue, including the use of natural or synthetic antimicrobial peptides. Previous research showed
[...] Read more.
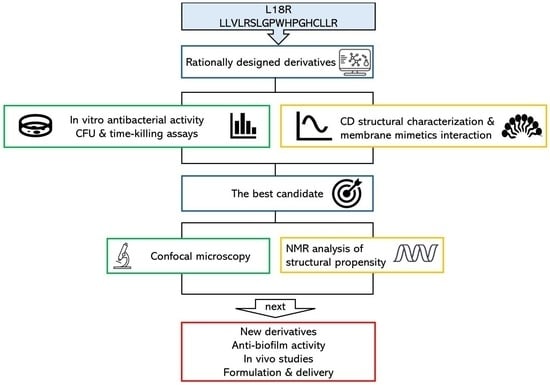
Background/Objectives: Microbial infections represent a significant threat to public health due to the emergence and spread of antimicrobial resistance. Adjunctive and alternative therapeutic strategies are explored to tackle this issue, including the use of natural or synthetic antimicrobial peptides. Previous research showed that antibody-derived peptides possess antimicrobial, antiviral, and immunomodulatory properties. This study aimed to characterize newly designed antibody-derived peptides and evaluate their effectiveness against representative strains of Staphylococcus aureus, including drug-resistant isolates. Methods: Colony-forming unit assays and confocal microscopy studies were performed to evaluate peptide activity against planktonic microbial cells. Cytotoxicity tests were performed on THP-1 human monocytic cells. Circular dichroism (CD) and nuclear magnetic resonance (NMR) were employed for the conformational characterization of peptides. Results: The half-maximal effective concentrations of the peptides against bacterial reference strains and drug-resistant isolates ranged from 0.17 to 18.05 µM, while cytotoxic effects were not observed against mammalian cells. A killing kinetics analysis and observation by confocal microscopy of the interaction between peptides and bacteria suggested a mechanism of action involving membrane perturbation. CD studies showed that all peptides predominantly exhibit a random coil arrangement in aqueous solution. NMR spectroscopy revealed that the most active peptide adopts a helical conformation in the presence of membrane mimetics. Conclusions: The structural characterization and evaluation of the newly designed peptides’ antimicrobial activity may lead to the selection of a candidate to be further studied to develop an alternative treatment against microbial infections caused by drug-resistant strains.
Full article
(This article belongs to the Section Antimicrobial Peptides)
►▼
Show Figures
Graphical abstract
Open AccessReview
A Scoping Review Unveiling Antimicrobial Resistance Patterns in the Environment of Dairy Farms Across Asia
by
Yuvaneswary Veloo, Syahidiah Syed Abu Thahir, Zunita Zakaria, Salina Abdul Rahman, Rozaihan Mansor and Sakshaleni Rajendiran
Antibiotics 2025, 14(5), 436; https://doi.org/10.3390/antibiotics14050436 - 26 Apr 2025
Abstract
Antimicrobial resistance (AMR) poses a significant “One Health” challenge in the farming industry attributed to antimicrobial misuse and overuse, affecting the health of humans, animals, and the environment. Recognizing the crucial role of the environment in facilitating the transmission of AMR is imperative
[...] Read more.
Antimicrobial resistance (AMR) poses a significant “One Health” challenge in the farming industry attributed to antimicrobial misuse and overuse, affecting the health of humans, animals, and the environment. Recognizing the crucial role of the environment in facilitating the transmission of AMR is imperative for addressing this global health issue. Despite its urgency, there remains a notable gap in understanding resistance levels in the environment. This scoping review aims to consolidate and summarize available evidence of AMR prevalence and resistance genes in dairy farm settings. This study was conducted following the PRISMA Extension checklist to retrieve relevant studies conducted in Asian countries between 2013 and 2023. An electronic literature search involving PubMed, ScienceDirect, Embase, and Scopus resulted in a total of 1126 unique articles that were identified. After a full-text eligibility assessment, 39 studies were included in this review. The findings indicate that AMR studies in dairy farm environments have primarily focused on selective bacteria, especially Escherichia coli and other bacteria such as Staphylococcus aureus, Klebsiella spp., and Salmonella spp. Antimicrobial resistance patterns were reported across 24 studies involving 78 antimicrobials, which predominantly consisted of gentamicin (70.8%), ampicillin (58.3%), and tetracycline (58.3%). This review emphasizes the current state of AMR in the environmental aspects of dairy farms across Asia, highlighting significant gaps in regional coverage and bacterial species studied. It highlights the need for broader surveillance, integration with antimicrobial stewardship, and cross-sector collaboration to address AMR through a One Health approach.
Full article
(This article belongs to the Special Issue A One Health Approach to Antimicrobial Resistance, 2nd Edition)
►▼
Show Figures
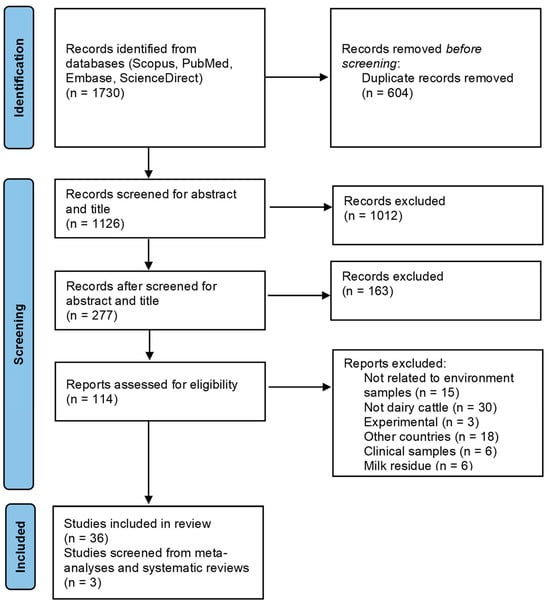
Figure 1
Open AccessArticle
Distribution, Antibiotic Resistance, and Virulence Factors of Vibrio parahaemolyticus in the Southern Coastal Waters of Republic of Korea
by
Hyunwoo Zin, Intae Ham, Soonbum Shin, Hongsik Yu, Tae-Jin Choi, Kwangsoo Ha and Jong Soo Mok
Antibiotics 2025, 14(5), 435; https://doi.org/10.3390/antibiotics14050435 - 26 Apr 2025
Abstract
►▼
Show Figures
Background/Objectives: Vibrio parahaemolyticus is a marine bacterium and a major cause of food poisoning worldwide, primarily associated with gastric illnesses such as gastroenteritis. This study aimed to investigate the distribution, antibiotic resistance, and virulence genes of V. parahaemolyticus present in shellfish and
[...] Read more.
Background/Objectives: Vibrio parahaemolyticus is a marine bacterium and a major cause of food poisoning worldwide, primarily associated with gastric illnesses such as gastroenteritis. This study aimed to investigate the distribution, antibiotic resistance, and virulence genes of V. parahaemolyticus present in shellfish and seawater of the southern coast of Korea, a major shellfish harvesting area. Methods: Shellfish and seawater samples were collected monthly in 2023 from 24 coastal sites in Korea. V. parahaemolyticus was isolated and identified using the MPN method, biochemical tests, MALDI-TOF mass spectrometry, and 16S rRNA sequencing. Antimicrobial susceptibility was tested for 673 isolates using the Sensititre MIC system, and virulence genes (tdh and trh) were detected by PCR. Results:V. parahaemolyticus had a detection rate of 18.2–58.3% in shellfish and 8.3–50% in seawater samples. Among the isolates, 97.9% and 97.3% were resistant to ampicillin and colistin, respectively, while 8.3% showed resistance to four or more antibiotics. The virulence genes tdh and trh were detected in 0.45% and 3.34% of shellfish samples and 1.23% and 4.46% of seawater samples, respectively. Conclusions: These findings will help implement appropriate precautionary measures to prevent potential human health risks arising from exposure to multidrug-resistant or pathogenic V. parahaemolyticus.
Full article
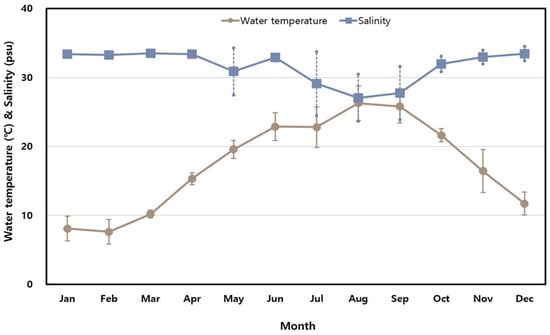
Figure 1
Open AccessArticle
Impact of Parenteral Ceftiofur on Developmental Dynamics of Early Life Fecal Microbiota and Antibiotic Resistome in Neonatal Lambs
by
Mohamed Donia, Nasr-Eldin Aref, Mohamed Zeineldin, Ameer Megahed, Benjamin Blair, James Lowe and Brian Aldridge
Antibiotics 2025, 14(5), 434; https://doi.org/10.3390/antibiotics14050434 - 25 Apr 2025
Abstract
►▼
Show Figures
Background: Early gut microbiome development is critical for neonatal health, and its dysbiosis may impact long-term animal productivity. This study examined the effects of parenteral Ceftiofur Crystalline Free Acid (CCFA) on the composition and diversity of the neonatal lamb fecal microbiome. The emergence
[...] Read more.
Background: Early gut microbiome development is critical for neonatal health, and its dysbiosis may impact long-term animal productivity. This study examined the effects of parenteral Ceftiofur Crystalline Free Acid (CCFA) on the composition and diversity of the neonatal lamb fecal microbiome. The emergence of antimicrobial resistance genes associated with CCFA exposure was also investigated. Results: There were distinct microbial populations in the CCFA-treated lambs compared to the control group at each time point, with a highly significant decrease in alpha and beta diversity. The CCFA treatment showed a reduction in several key microbial taxa during nursing, but these differences were diminished by day 56. Unlike the control group, CCFA-treated lambs had core microbes potentially carrying multiple antibiotic resistance genes, including those for beta-lactam, fosfomycin, methicillin, and multidrug resistance. Methods: Twenty-four healthy neonatal lambs were randomly assigned to CCFA-treated (n = 12) and control (n = 12) groups. Fecal samples were collected on days 0, 7, 14, 28, and 56. Genomic DNA was extracted and sequenced using the Illumina MiSeq platform. Microbial composition was analyzed using the MG-RAST pipeline with the RefSeq database. Conclusions: Despite temporary reductions in critical bacterial populations during nursing, the early sheep fecal microbiome demonstrated resilience by repopulating after CCFA antibiotic disruption. While this highlights microbiota stability after short-course antibiotic exposure, the transient disturbance underscores potential risks to early gut health. Importantly, persistent CCFA resistance poses environmental dissemination risks, emphasizing the need for cautious antibiotic use in livestock to mitigate ecological impacts.
Full article
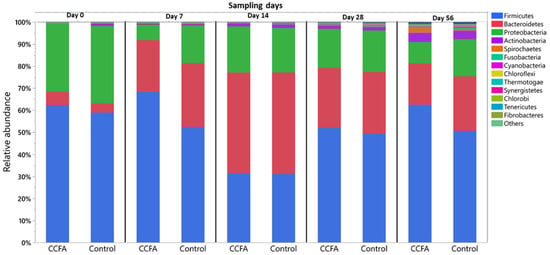
Figure 1

Journal Menu
► ▼ Journal Menu-
- Antibiotics Home
- Aims & Scope
- Editorial Board
- Reviewer Board
- Topical Advisory Panel
- Instructions for Authors
- Special Issues
- Topics
- Sections & Collections
- Article Processing Charge
- Indexing & Archiving
- Editor’s Choice Articles
- Most Cited & Viewed
- Journal Statistics
- Journal History
- Journal Awards
- Society Collaborations
- Conferences
- Editorial Office
Journal Browser
► ▼ Journal BrowserHighly Accessed Articles
Latest Books
E-Mail Alert
News
Topics
Topic in
Antibiotics, Biomedicines, JCM, Pharmaceuticals, Pharmaceutics
Challenges and Future Prospects of Antibacterial Therapy
Topic Editors: Kwang-sun Kim, Zehra EdisDeadline: 30 June 2025
Topic in
Antibiotics, Antioxidants, JoF, Microbiology Research, Microorganisms
Redox in Microorganisms, 2nd Edition
Topic Editors: Michal Letek, Volker BehrendsDeadline: 31 July 2025
Topic in
Agriculture, Animals, Veterinary Sciences, Antibiotics, Zoonotic Diseases
Animal Diseases in Agricultural Production Systems: Their Veterinary, Zoonotic, and One Health Importance, 2nd Edition
Topic Editors: Ewa Tomaszewska, Beata Łebkowska-Wieruszewska, Tomasz Szponder, Joanna Wessely-SzponderDeadline: 30 September 2025
Topic in
Antibiotics, JPM, Pharmaceuticals, Pharmaceutics, Medicines
Pharmacokinetic and Pharmacodynamic Modelling in Drug Discovery and Development
Topic Editors: Inaki F. Troconiz, Victor Mangas Sanjuán, Maria Garcia-Cremades MiraDeadline: 31 October 2025

Conferences
26–29 August 2025
The 5th International Symposium on Frontiers in Molecular Science
Molecular Regulatory Mechanisms of Biological Function and Drug Discovery based on Protein Structure/Function Analysis
Molecular Regulatory Mechanisms of Biological Function and Drug Discovery based on Protein Structure/Function Analysis

21–23 May 2025
The 4th International Electronic Conference on Antibiotics: Challenges and Strategies for the Antibiotic Resistance Crisis

Special Issues
Special Issue in
Antibiotics
Staphylococcus aureus Skin and Soft Tissue Infections Pathogenesis and Therapeutic Approaches, 2nd Edition
Guest Editors: Marisa I. Gómez, Cintia González, Constanza GiaiDeadline: 30 April 2025
Special Issue in
Antibiotics
Environmental Fate of Antibiotics: Monitoring, Toxicity, Resistance, and Removal Methods
Guest Editor: Takashi AzumaDeadline: 30 April 2025
Special Issue in
Antibiotics
Urinary Tract Infections and Antibiotic Intervention, 2nd Edition
Guest Editor: Majid MirzazadehDeadline: 30 April 2025
Special Issue in
Antibiotics
Zoonotic Bacterial Infections: Genetic Studies and Antibiotic Resistance
Guest Editors: Sónia Silva, Daniela Araújo, Joana CastroDeadline: 30 April 2025
Topical Collections
Topical Collection in
Antibiotics
Antibiotics in Ophthalmology Practice
Collection Editor: Sanjay Marasini
Topical Collection in
Antibiotics
Staphylococcus— Molecular Pathogenesis, Virulence Regulation and Antibiotics Resistance
Collection Editor: Ewa Szczuka
Topical Collection in
Antibiotics
Editorial Board Members' Collection Series: Structural Aspects of AMPs and Antimicrobials
Collection Editors: J. Michael Conlon, Marc Maresca, Bong-Jin Lee, Aurélie Tasiemski
Topical Collection in
Antibiotics
Synthetic and Natural Products-Based Antimicrobial and Antiparasitic Agents
Collection Editor: Antonio Eduardo Miller Crotti








