An Update on Hypomagnesemia and Hypermagnesemia
Abstract
:1. Introduction
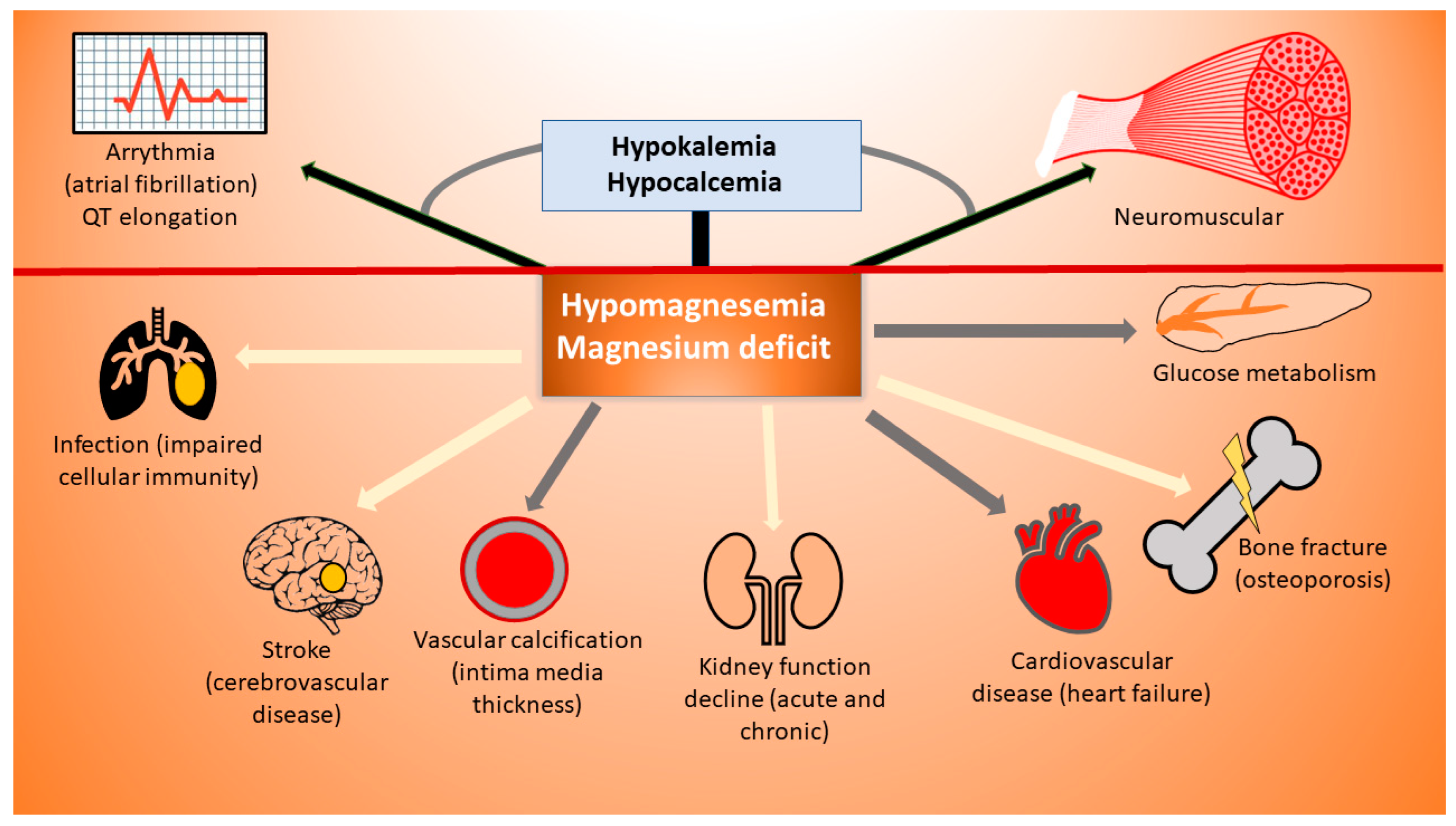
2. Hypomagnesemia
2.1. Pathophysiology
2.2. Hereditary Etiologies of Hypomagnesemia
2.3. Hypomagnesemia and Glucose Metabolism
3. Symptoms
3.1. Hypomagnesemia and Its Clinical Correlates
3.2. When and How to Treat Hypomagnesemia?
3.3. Magnesium and Pregnancy
3.4. Magnesium and Critical Illness
3.5. Efficacy of Magnesium Supplementation
4. Hypermagnesemia
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Baaij, J.H.; Hoenderop, J.G.; Bindels, R.J. Magnesium in man: Implications for health and disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Castañeda, J.R.; de Mier, M.V.P.-R.; Rodríguez, M.; Rodríguez-Ortiz, M.E. Magnesium Replacement to Protect Cardiovascular and Kidney Damage? Lack of Prospective Clinical Trials. Int. J. Mol. Sci. 2018, 19, 664. [Google Scholar] [CrossRef] [PubMed]
- Tangvoraphonkchai, K.; Davenport, A. Magnesium and Cardiovascular Disease. Adv. Chronic Kidney Dis. 2018, 25, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Tseng, M.-H.; Yang, S.-S.; Sung, C.-C.; Ding, J.-J.; Hsu, Y.-J.; Chu, S.-M.; Lin, S.-H. Novel CNNM2 Mutation Responsible for Autosomal-Dominant Hypomagnesemia With Seizure. Front. Genet. 2022, 13, 875013. [Google Scholar] [CrossRef] [PubMed]
- de Baaij, J.H.F. Magnesium reabsorption in the kidney. Am. J. Physiol. Physiol. 2023, 324, F227–F244. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Chen, L.; Gutin, B.; Huang, Y.; Dong, Y.; Zhu, H. Magnesium Intake, C-Reactive Protein, and Muscle Mass in Adolescents. Nutrients 2022, 14, 2882. [Google Scholar] [CrossRef]
- Schimatschek, H.F.; Rempis, R. Prevalence of hypomagnesemia in an unselected German population of 16,000 individuals. Magnes. Res. 2001, 14, 283–290. [Google Scholar]
- Ma, J.; Folsom, A.R.; Melnick, S.L.; Eckfeldt, J.H.; Sharrett, A.; Nabulsi, A.A.; Hutchinson, R.G.; Metcalf, P.A. Associations of serum and dietary magnesium with cardiovascular disease, hypertension, diabetes, insulin, and carotid arterial wall thickness: The ARIC study. Atherosclerosis Risk in Communities Study. J. Clin. Epidemiol. 1995, 48, 927–940. [Google Scholar] [CrossRef]
- Cunha, A.R.; D’el-Rei, J.; Medeiros, F.; Umbelino, B.; Oigman, W.; Touyz, R.M.; Neves, M.F. Oral magnesium supplementation improves endothelial function and attenuates subclinical atherosclerosis in thiazide-treated hypertensive women. J. Hypertens. 2017, 35, 89–97. [Google Scholar] [CrossRef]
- Sakaguchi, Y.; Hamano, T.; Obi, Y.; Monden, C.; Oka, T.; Yamaguchi, S.; Matsui, I.; Hashimoto, N.; Matsumoto, A.; Shimada, K.; et al. A Randomized Trial of Magnesium Oxide and Oral Carbon Adsorbent for Coronary Artery Calcification in Predialysis CKD. J. Am. Soc. Nephrol. 2019, 30, 1073–1085. [Google Scholar] [CrossRef]
- Xu, C.; Smith, E.R.; Tiong, M.K.; Ruderman, I.; Toussaint, N.D. Interventions To Attenuate Vascular Calcification Progression in Chronic Kidney Disease: A Systematic Review of Clinical Trials. J. Am. Soc. Nephrol. 2022, 33, 1011–1032. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Zhang, R.; Li, G. Effect of magnesium on vascular calcification in chronic kidney disease patients: A systematic review and meta-analysis. Ren. Fail. 2023, 45, 2182603. [Google Scholar] [CrossRef] [PubMed]
- Pethő, A.G.; Tapolyai, M.; Browne, M.; Fülöp, T. Hypomagnesemia as a Risk Factor and Accelerator for Vascular Aging in Diabetes Mellitus and Chronic Kidney Disease. Metabolites 2023, 13, 306. [Google Scholar] [CrossRef] [PubMed]
- Oost, L.J.; van der Heijden, A.A.; Vermeulen, E.A.; Bos, C.; Elders, P.J.; Slieker, R.C.; Kurstjens, S.; van Berkel, M.; Hoenderop, J.G.; Tack, C.J.; et al. Serum Magnesium Is Inversely Associated with Heart Failure, Atrial Fibrillation, and Microvascular Complications in Type 2 Diabetes. Diabetes Care 2021, 44, 1757–1765. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-W.; Huang, Y.-T.; Jiang, M.-Y. Association of dietary magnesium intake and glycohemoglobin with mortality risk in diabetic patients. PLoS ONE 2022, 17, e0277180. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-W.; Chen, Y.-Y.; Chen, W.-L. Association between oral intake magnesium and sarcopenia: A cross-sectional study. BMC Geriatr. 2022, 22, 816. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, H.S.; Burdmann, E.A.; Vieira, E.A.; Ferreira, M.L.; Ferreira, A.P.; Inda-Filho, A.J. Association of magnesium abnormalities at intensive care unit admission with kidney outcomes and mortality: A prospective cohort study. Clin. Exp. Nephrol. 2022, 26, 997–1004. [Google Scholar] [CrossRef]
- Koh, H.B.; Jung, C.-Y.; Kim, H.W.; Kwon, J.Y.; Kim, N.H.; Kim, H.J.; Jhee, J.H.; Han, S.H.; Yoo, T.-H.; Kang, S.-W.; et al. Preoperative Ionized Magnesium Levels and Risk of Acute Kidney Injury After Cardiac Surgery. Am. J. Kidney Dis. 2022, 80, 629–637.e1. [Google Scholar] [CrossRef]
- Correa, S.; Guerra-Torres, X.E.; Waikar, S.S.; Mc Causland, F.R. Serum Magnesium, Blood Pressure, and Risk of Hypertension and Chronic Kidney Disease Progression in the CRIC Study. Hypertension 2021, 78, 1771–1780. [Google Scholar] [CrossRef]
- Van Laecke, S.; Vermeiren, P.; Nagler, E.V.; Caluwe, R.; De Wilde, M.; Van der Vennet, M.; Peeters, P.; Randon, C.; Vermassen, F.; Vanholder, R.; et al. Magnesium and infection risk after kidney transplantation: An observational cohort study. J. Infect. 2016, 73, 8–17. [Google Scholar] [CrossRef]
- Odler, B.; Deak, A.T.; Pregartner, G.; Riedl, R.; Bozic, J.; Trummer, C.; Prenner, A.; Söllinger, L.; Krall, M.; Höflechner, L.; et al. Hypomagnesemia Is a Risk Factor for Infections after Kidney Transplantation: A Retrospective Cohort Analysis. Nutrients 2021, 13, 1296. [Google Scholar] [CrossRef] [PubMed]
- Groenendijk, I.; van Delft, M.; Versloot, P.; van Loon, L.J.; de Groot, L.C. Impact of magnesium on bone health in older adults: A systematic review and meta-analysis. Bone 2022, 154, 116233. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Stubbs, B.; Solmi, M.; Noale, M.; Vaona, A.; Demurtas, J.; Maggi, S. Dietary magnesium intake and fracture risk: Data from a large prospective study. Br. J. Nutr. 2017, 117, 1570–1576. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Wang, K.; Han, D.; He, X.; Wei, J.; Zhao, L.; Imam, M.U.; Ping, Z.; Li, Y.; Xu, Y.; et al. Dietary magnesium intake and the risk of cardiovascular disease, type 2 diabetes, and all-cause mortality: A dose–response meta-analysis of prospective cohort studies. BMC Med. 2016, 14, 210. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-C.; Pang, Z.; Liu, Q.-F. Magnesium intake and risk of colorectal cancer: A meta-analysis of prospective studies. Eur. J. Clin. Nutr. 2012, 66, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Del Gobbo, L.C.; Imamura, F.; Wu, J.H.; Otto, M.C.d.O.; Chiuve, S.E.; Mozaffarian, D. Circulating and dietary magnesium and risk of cardiovascular disease: A systematic review and meta-analysis of prospective studies. Am. J. Clin. Nutr. 2013, 98, 160–173. [Google Scholar] [CrossRef]
- Schuchardt, J.P.; Hahn, A. Intestinal Absorption and Factors Influencing Bioavailability of Magnesium- An Update. Curr. Nutr. Food Sci. 2017, 13, 260–278. [Google Scholar] [CrossRef]
- Lin, T.; Bi, C.; Song, Y.; Guo, H.; Liu, L.; Zhou, Z.; Wang, B.; Tang, G.; Liu, C.; Yang, Y.; et al. Plasma Magnesium Concentrations and Risk of Incident Cancer in Adults with Hypertension: A Nested Case-Control Study. Ann. Nutr. Metab. 2020, 76, 304–312. [Google Scholar] [CrossRef]
- Sakaguchi, Y.; Fujii, N.; Shoji, T.; Hayashi, T.; Rakugi, H.; Isaka, Y. Hypomagnesemia is a significant predictor of cardiovascular and non-cardiovascular mortality in patients undergoing hemodialysis. Kidney Int. 2014, 85, 174–181. [Google Scholar] [CrossRef]
- Panthofer, A.M.; Lyu, B.; Astor, B.C.; Singh, T.; Aziz, F.; Mandelbrot, D.; Parajuli, S.; Mohamed, M.; Djamali, A.; Garg, N. Post-kidney transplant serum magnesium exhibits a U-shaped association with subse-quent mortality: An observational cohort study. Transpl. Int. Off. J. Eur. Soc. Organ Transplant. 2021, 34, 1853–1861. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Veronese, N.; Barbagallo, M. Magnesium and Hypertension in Old Age. Nutrients 2020, 13, 139. [Google Scholar] [CrossRef] [PubMed]
- Vetter, T.; Lohse, M.J. Magnesium and the parathyroid. Curr. Opin. Nephrol. Hypertens. 2002, 11, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Van Laecke, S.; Van Biesen, W. Hypomagnesaemia in kidney transplantation. Transplant. Rev. 2015, 29, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Groenestege, W.M.T.; Hoenderop, J.G.; van den Heuvel, L.; Knoers, N.; Bindels, R.J. The Epithelial Mg2+Channel Transient Receptor Potential Melastatin 6 Is Regulated by Dietary Mg2+Content and Estrogens. J. Am. Soc. Nephrol. 2006, 17, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Gommers, L.M.M.; Hoenderop, J.G.J.; de Baaij, J.H.F. Mechanisms of proton pump inhibitor-induced hypomagnesemia. Acta Physiol. 2022, 235, e13846. [Google Scholar] [CrossRef] [PubMed]
- Kieboom, B.C.T.; Kiefte–de Jong, J.C.; Eijgelsheim, M.; Franco, O.H.; Kuipers, E.J.; Hofman, A.; Zietse, R.; Stricker, B.H.; Hoorn, E.J. Proton Pump Inhibitors and Hypomagnesemia in the General Population: A Population-Based Cohort Study. Am. J. Kidney Dis. 2015, 66, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Meaney, C.J.; Beccari, M.V.; Yang, Y.; Zhao, J. Systematic Review and Meta-Analysis of Patiromer and Sodium Zirconium Cyclosilicate: A New Armamentarium for the Treatment of Hyperkalemia. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2017, 37, 401–411. [Google Scholar] [CrossRef]
- Weir, M.R.; Bakris, G.L.; Bushinsky, D.A.; Mayo, M.R.; Garza, D.; Stasiv, Y.; Wittes, J.; Christ-Schmidt, H.; Berman, L.; Pitt, B. Patiromer in Patients with Kidney Disease and Hyperkalemia Receiving RAAS Inhibitors. New Engl. J. Med. 2015, 372, 211–221. [Google Scholar] [CrossRef]
- Oka, T.; Hamano, T.; Sakaguchi, Y.; Yamaguchi, S.; Kubota, K.; Senda, M.; Yonemoto, S.; Shimada, K.; Matsumoto, A.; Hashimoto, N.; et al. Proteinuria-associated renal magnesium wasting leads to hypomagnesemia: A common electrolyte abnormality in chronic kidney disease. Nephrol. Dial. Transplant. 2019, 34, 1154–1162. [Google Scholar] [CrossRef]
- Gagliano, V.; Schäffeler, F.; Del Giorno, R.; Bianchetti, M.; Canarte, C.F.C.; Regueira, J.J.C.; Gabutti, L. Does Ionized Magnesium Offer a Different Perspective Exploring the Association between Magnesemia and Targeted Cardiovascular Risk Factors? J. Clin. Med. 2022, 11, 4015. [Google Scholar] [CrossRef]
- Macian, N.; Dualé, C.; Voute, M.; Leray, V.; Courrent, M.; Bodé, P.; Giron, F.; Sonneville, S.; Bernard, L.; Joanny, F.; et al. Short-Term Magnesium Therapy Alleviates Moderate Stress in Patients with Fibromyalgia: A Randomized Double-Blind Clinical Trial. Nutrients 2022, 14, 2088. [Google Scholar] [CrossRef] [PubMed]
- Salehidoost, R.; Boroujeni, G.T.; Feizi, A.; Aminorroaya, A.; Amini, M. Effect of oral magnesium supplement on cardiometabolic markers in people with prediabetes: A double blind randomized controlled clinical trial. Sci. Rep. 2022, 12, 18209. [Google Scholar] [CrossRef] [PubMed]
- Schutten, J.C.; Joris, P.J.; Groendijk, I.; Eelderink, C.; Groothof, D.; van der Veen, Y.; Westerhuis, R.; Goorman, F.; Danel, R.M.; de Borst, M.H.; et al. Effects of Magnesium Citrate, Magnesium Oxide, and Magnesium Sulfate Supplementation on Arterial Stiffness: A Randomized, Double-Blind, Placebo-Controlled Intervention Trial. J. Am. Heart Assoc. 2022, 11, e021783. [Google Scholar] [CrossRef]
- Schutten, J.C.; Joris, P.J.; Minović, I.; Post, A.; van Beek, A.P.; de Borst, M.H.; Mensink, R.P.; Bakker, S.J.L. Long-term magnesium supplementation improves glucocorticoid metabolism: A post-hoc analysis of an intervention trial. Clin. Endocrinol. 2021, 94, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Micke, O.; Vormann, J.; Kraus, A.; Kisters, K. Serum magnesium: Time for a standardized and evidence-based reference range. Magnes. Res. 2021, 34, 84–89. [Google Scholar] [PubMed]
- Viering, D.H.H.M.; de Baaij, J.H.F.; Walsh, S.B.; Kleta, R.; Bockenhauer, D. Genetic causes of hypomagnesemia, a clinical overview. Pediatr. Nephrol. 2017, 32, 1123–1135. [Google Scholar] [CrossRef]
- Figueres, L.; Bruneau, S.; Prot-Bertoye, C.; Brideau, G.; Néel, M.; Griveau, C.; Cheval, L.; Bignon, Y.; Dimitrov, J.; Dejoie, T.; et al. Hypomagnesemia, Hypocalcemia, and Tubulointerstitial Nephropathy Caused by Claudin-16 Autoantibodies. J. Am. Soc. Nephrol. 2022, 33, 1402–1410. [Google Scholar] [CrossRef]
- Blanchard, A.; Bockenhauer, D.; Bolignano, D.; Calò, L.A.; Cosyns, E.; Devuyst, O.; Ellison, D.H.; Frankl, F.E.K.; Knoers, N.V.; Konrad, M.; et al. Gitelman syndrome: Consensus and guidance from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2017, 91, 24–33. [Google Scholar] [CrossRef]
- Schlingmann, K.P.; de Baaij, J.H. The genetic spectrum of Gitelman(-like) syndromes. Curr. Opin. Nephrol. Hypertens. 2022, 31, 508–515. [Google Scholar] [CrossRef]
- Viering, D.; Schlingmann, K.P.; Hureaux, M.; Nijenhuis, T.; Mallett, A.; Chan, M.M.; van Beek, A.; van Eerde, A.M.; Coulibaly, J.-M.; Vallet, M.; et al. Gitelman-Like Syndrome Caused by Pathogenic Variants in mtDNA. J. Am. Soc. Nephrol. 2022, 33, 305–325. [Google Scholar] [CrossRef]
- Blanchard, A.; Vallet, M.; Dubourg, L.; Hureaux, M.; Allard, J.; Haymann, J.-P.; de la Faille, R.; Arnoux, A.; Dinut, A.; Bergerot, D.; et al. Resistance to Insulin in Patients with Gitelman Syndrome and a Subtle Intermediate Phenotype in Heterozygous Carriers: A Cross-Sectional Study. J. Am. Soc. Nephrol. 2019, 30, 1534–1545. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.D.R.; Antonelou, M.; Sathiananthamoorthy, S.; Rega, M.; Henderson, S.; Ceron-Gutierrez, L.; Barcenas-Morales, G.; Müller, C.A.; Doffinger, R.; Walsh, S.B.; et al. Inherited salt-losing tubulopathies are associated with immunodeficiency due to impaired IL-17 responses. Nat. Commun. 2020, 11, 4368. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, M.; Di Bella, G.; Brucato, V.; D’angelo, D.; Damiani, P.; Monteverde, A.; Belvedere, M.; Dominguez, L.J. Serum ionized magnesium in diabetic older persons. Metabolism 2014, 63, 502–509. [Google Scholar] [CrossRef]
- Kieboom, B.C.T.; Ligthart, S.; Dehghan, A.; Kurstjens, S.; de Baaij, J.H.F.; Franco, O.H.; Hofman, A.; Zietse, R.; Stricker, B.H.; Hoorn, E.J. Serum magnesium and the risk of prediabetes: A population-based cohort study. Diabetologia 2017, 60, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Van Laecke, S.; Van Biesen, W.; Verbeke, F.; De Bacquer, D.; Peeters, P.; Vanholder, R. Posttransplantation Hypomagnesemia and Its Relation with Immunosuppression as Predictors of New-Onset Diabetes after Transplantation. Am. J. Transplant. 2009, 9, 2140–2149. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.W.; Famure, O.; Li, Y.; Kim, S.J. Hypomagnesemia and the Risk of New-Onset Diabetes Mellitus after Kidney Transplantation. J. Am. Soc. Nephrol. 2016, 27, 1793–1800. [Google Scholar] [CrossRef] [PubMed]
- Oost, L.J.; Kurstjens, S.; Ma, C.; Hoenderop, J.G.J.; Tack, C.J.; de Baaij, J.H.F. Magnesium increases insulin-dependent glucose uptake in adipocytes. Front. Endocrinol. 2022, 13, 986616. [Google Scholar] [CrossRef]
- Lecube, A.; Baena-Fustegueras, J.A.; Fort, J.M.; Pelegrí, D.; Hernández, C.; Simó, R. Diabetes Is the Main Factor Accounting for Hypomagnesemia in Obese Subjects. PLoS ONE 2012, 7, e30599. [Google Scholar] [CrossRef]
- Nielsen, F.H.; Lukaski, H.C. Update on the relationship between magnesium and exercise. Magnes. Res. 2006, 19, 180–189. [Google Scholar]
- Scherr, J.; Schuster, T.; Pressler, A.; Roeh, A.; Christle, J.; Wolfarth, B.; Halle, M. Repolarization Perturbation and Hypomagnesemia after Extreme Exercise. Med. Sci. Sports Exerc. 2012, 44, 1637–1643. [Google Scholar] [CrossRef]
- Hansen, B.A.; Bruserud, Ø. Hypomagnesemia in critically ill patients. J. Intensive Care 2018, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Agus, Z.S. Hypomagnesemia. J. Am. Soc. Nephrol. 1999, 10, 1616–1622. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-L.; Kuo, E. Mechanism of Hypokalemia in Magnesium Deficiency. J. Am. Soc. Nephrol. 2007, 18, 2649–2652. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-C.; Wahlqvist, M.L.; Kao, M.-D.; Wang, J.-L.; Lee, M.-S. Optimal Dietary and Plasma Magnesium Statuses Depend on Dietary Quality for a Reduction in the Risk of All-Cause Mortality in Older Adults. Nutrients 2015, 7, 5664–5683. [Google Scholar] [CrossRef] [PubMed]
- Van Laecke, S.; Nagler, E.V.; Verbeke, F.; Van Biesen, W.; Vanholder, R. Hypomagnesemia and the Risk of Death and GFR Decline in Chronic Kidney Disease. Am. J. Med. 2013, 126, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Lacson, E.; Wang, W.; Ma, L.; Passlick-Deetjen, J. Serum Magnesium and Mortality in Hemodialysis Patients in the United States: A Cohort Study. Am. J. Kidney Dis. 2015, 66, 1056–1066. [Google Scholar] [CrossRef]
- Lutsey, P.L.; Alonso, A.; Michos, E.D.; Loehr, L.R.; Astor, B.C.; Coresh, J.; Folsom, A.R. Serum magnesium, phosphorus, and calcium are associated with risk of incident heart failure: The Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Clin. Nutr. 2014, 100, 756–764. [Google Scholar] [CrossRef]
- Ferrè, S.; Li, X.; Adams-Huet, B.; Maalouf, N.M.; Sakhaee, K.; Toto, R.D.; Moe, O.W.; Neyra, J.A. Association of serum magnesium with all-cause mortality in patients with and without chronic kidney disease in the Dallas Heart Study. Nephrol. Dial. Transplant. 2018, 33, 1389–1396. [Google Scholar] [CrossRef]
- Azem, R.; Daou, R.; Bassil, E.; Anvari, E.M.; Taliercio, J.J.; Arrigain, S.; Schold, J.D.; Vachharajani, T.; Nally, J.; Na Khoul, G.N. Serum magnesium, mortality and disease progression in chronic kidney disease. BMC Nephrol. 2020, 21, 49. [Google Scholar] [CrossRef]
- Lötscher, J.; i Líndez, A.-A.M.; Kirchhammer, N.; Cribioli, E.; Attianese, G.M.P.G.; Trefny, M.P.; Lenz, M.; Rothschild, S.I.; Strati, P.; Künzli, M.; et al. Magnesium sensing via LFA-1 regulates CD8+ T cell effector function. Cell 2022, 185, 585–602.e29. [Google Scholar] [CrossRef]
- Chaigne-Delalande, B.; Li, F.-Y.; O’connor, G.M.; Lukacs, M.J.; Jiang, P.; Zheng, L.; Shatzer, A.; Biancalana, M.; Pittaluga, S.; Matthews, H.F.; et al. Mg2+ Regulates Cytotoxic Functions of NK and CD8 T Cells in Chronic EBV Infection Through NKG2D. Science 2013, 341, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, S.D.; Price, S.; Zou, J.; Hunsberger, S.; Brofferio, A.; Matthews, H.; Similuk, M.; Rosenzweig, S.D.; Su, H.C.; Cohen, J.I.; et al. A Double-Blind, Placebo-Controlled, Crossover Study of Magnesium Supplementation in Patients with XMEN Disease. J. Clin. Immunol. 2022, 42, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Lian, X.; Chen, H. The association of serum magnesium with infection in new-onset systemic lupus erythematosus patients. Lupus 2023, 32, 9612033221149884. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-L.; Wang, S.-Q.; Ji, M.-J.; Wang, X.-M.; Sun, J.; Zhang, M.-M.; Ma, C.-M. Hypomagnesemia is associated with increased mortality in the short-term but not the long-term in community-acquired pneumonia patients with type 2 diabetes. Magnes. Res. 2022, 35, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Juan, R.; Otim, I.; Nabalende, H.; Legason, I.D.; Reynolds, S.J.; Ogwang, M.D.; Ndugwa, C.M.; Marshall, V.; Whitby, D.; Goedert, J.J.; et al. Plasma magnesium is inversely associated with Epstein-Barr virus load in peripheral blood and Burkitt lymphoma in Uganda. Cancer Epidemiol. 2018, 52, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Ravell, J.; Chaigne-Delalande, B.; Lenardo, M. X-linked immunodeficiency with magnesium defect, Epstein-Barr virus infection, and neoplasia disease: A combined immune deficiency with magnesium defect. Curr. Opin. Pediatr. 2014, 26, 713–719. [Google Scholar] [CrossRef]
- Alam, A.B.; Thomas, D.S.; Lutsey, P.L.; Shrestha, S.; Alonso, A. Associations of Serum Magnesium with Brain Morphology and Subclinical Cerebrovascular Disease: The Atherosclerosis Risk in Communities-Neurocognitive Study. Nutrients 2021, 13, 4496. [Google Scholar] [CrossRef]
- Kieboom, B.C.; Licher, S.; Wolters, F.J.; Ikram, M.K.; Hoorn, E.J.; Zietse, R.; Stricker, B.H. Serum magnesium is associated with the risk of dementia. Neurology 2017, 89, 1716–1722. [Google Scholar] [CrossRef]
- Sakaguchi, Y.; Hamano, T.; Wada, A.; Hoshino, J.; Masakane, I. Magnesium and Risk of Hip Fracture among Patients Undergoing Hemodialysis. J. Am. Soc. Nephrol. 2018, 29, 991–999. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Whitehouse, M.R.; Blom, A.W.; Laukkanen, J.A. Low serum magnesium levels are associated with increased risk of fractures: A long-term prospective cohort study. Eur. J. Epidemiol. 2017, 32, 593–603. [Google Scholar] [CrossRef]
- Soliman, H.M.; Mercan, D.; Lobo, S.S.M.; Mélot, C.; Vincent, J.-L. Development of ionized hypomagnesemia is associated with higher mortality rates. Crit. Care Med. 2003, 31, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Upala, S.; Jaruvongvanich, V.; Wijarnpreecha, K.; Sanguankeo, A. Hypomagnesemia and mortality in patients admitted to intensive care unit: A systematic review and meta-analysis. QJM 2016, 109, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Masuyama, S.; Mizui, M.; Maeda, K.; Shimamura, K.; Sakaguchi, Y.; Morita, M.; Kuratani, T.; Mizote, I.; Nakamura, D.; Sakata, Y.; et al. Preoperative hypomagnesemia as a possible predictive factor for postoperative increase of transvalvular pressure gradient in hemodialysis patients treated with transcatheter aortic valve implantation. Ren. Fail. 2022, 44, 1083–1089. [Google Scholar] [CrossRef]
- Ferrè, S.; Li, X.; Adams-Huet, B.; Maalouf, N.M.; Sakhaee, K.; Toto, R.D.; Moe, O.W.; Neyra, J.A. Low Serum Magnesium is associated with Faster Decline in Kidney Function: The Dallas Heart Study Experience. J. Investig. Med. 2019, 67, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Joosten, M.M.; Gansevoort, R.T.; Bakker, S.J. Low plasma magnesium and risk of developing chronic kidney disease: Results from the PREVEND Study. Kidney Int. 2015, 87, 1262–1263. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, Y.; Iwatani, H.; Hamano, T.; Tomida, K.; Kawabata, H.; Kusunoki, Y.; Shimomura, A.; Matsui, I.; Hayashi, T.; Tsubakihara, Y.; et al. Magnesium modifies the association between serum phosphate and the risk of progression to end-stage kidney disease in patients with non-diabetic chronic kidney disease. Kidney Int. 2015, 88, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Rebholz, C.M.; Tin, A.; Liu, Y.; Kuczmarski, M.F.; Evans, M.K.; Zonderman, A.B.; Crews, D.C. Dietary Magnesium and Kidney Function Decline: The Healthy Aging in Neighborhoods of Diversity across the Life Span Study. Am. J. Nephrol. 2016, 44, 381–387. [Google Scholar] [CrossRef]
- Brown, R.S. Magnesium Sulfate: Another Cause of a Solute Diuresis. Am. J. Kidney Dis. 2017, 69, 550–551. [Google Scholar] [CrossRef]
- Veronese, N.; Pizzol, D.; Smith, L.; Dominguez, L.J.; Barbagallo, M. Effect of Magnesium Supplementation on Inflammatory Parameters: A Meta-Analysis of Randomized Controlled Trials. Nutrients 2022, 14, 679. [Google Scholar] [CrossRef]
- Ng, H.Y.; Kuo, W.H.; Tain, Y.L.; Leung, F.F.; Lee, W.C.; Lee, C.T. Effect of Dapagliflozin and Magnesium Supplementation on Renal Magnesium Handling and Magnesium Homeostasis in Metabolic Syndrome. Nutrients 2021, 13, 4088. [Google Scholar] [CrossRef]
- Tang, H.; Zhang, X.; Zhang, J.; Li, Y.; Del Gobbo, L.C.; Zhai, S.; Song, Y. Elevated serum magnesium associated with SGLT2 inhibitor use in type 2 diabetes patients: A meta-analysis of randomised controlled trials. Diabetologia 2016, 59, 2546–2551. [Google Scholar] [CrossRef] [PubMed]
- Palmer, B.F.; Clegg, D.J. Kidney-Protective Effects of SGLT2 Inhibitors. Clin. J. Am. Soc. Nephrol. 2023, 18, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.M.; Li, J.; Bhalla, V.; Jardine, M.J.; Neal, B.; de Zeeuw, D.; Fulcher, G.; Perkovic, V.; Mahaffey, K.W.; Chang, T.I. Canagliflozin, serum magnesium and cardiovascular outcomes—Analysis from the CANVAS Program. Endocrinol. Diabetes Metab. 2021, 4, e00247. [Google Scholar] [CrossRef] [PubMed]
- Elasy, A.N.; Nafea, O.E. Critical Hypermagnesemia in Preeclamptic Women Under a Magnesium Sulfate Regimen: Incidence and Associated Risk Factors. Biol. Trace Element Res. 2022, 201, 3670–3678. [Google Scholar] [CrossRef] [PubMed]
- Qu, Q.; Rong, R.; Yu, J. Effect of magnesium supplementation on pregnancy outcome in gestational diabetes mellitus patients: A meta-analysis of randomized controlled trials. Food Sci. Nutr. 2022, 10, 3193–3202. [Google Scholar] [CrossRef] [PubMed]
- Garrison, S.R.; Allan, G.M.; Sekhon, R.K.; Musini, V.M.; Khan, K.M. Magnesium for skeletal muscle cramps. Cochrane Database Syst. Rev. 2020, 9, Cd009402. [Google Scholar] [CrossRef] [PubMed]
- Vesterlund, G.K.; Jensen, T.S.; Ellekjaer, K.L.; Møller, M.H.; Thomsen, T.; Perner, A. Effects of magnesium, phosphate, or zinc supplementation in intensive care unit patients—A systematic review and meta-analysis. Acta Anaesthesiol. Scand. 2023, 67, 264–276. [Google Scholar] [CrossRef]
- Ramesh, T.; Lee, P.Y.K.; Mitta, M.; Allencherril, J. Intravenous magnesium in the management of rapid atrial fibrillation: A systematic review and meta-analysis. J. Cardiol. 2021, 78, 375–381. [Google Scholar] [CrossRef]
- Fairley, J.L.; Zhang, L.; Glassford, N.J.; Bellomo, R. Magnesium status and magnesium therapy in cardiac surgery: A systematic review and meta-analysis focusing on arrhythmia prevention. J. Crit. Care 2017, 42, 69–77. [Google Scholar] [CrossRef]
- Jahangir, A.; Zia, Z.; Niazi, M.R.K.; Sahra, S.; Jahangir, A.; Sharif, M.A.; Chalhoub, M.N. Efficacy of Magnesium Sulfate in the Chronic Obstructive Pulmonary Disease Population: A Systematic Review and Meta-Analysis. Adv. Respir. Med. 2022, 90, 125–133. [Google Scholar] [CrossRef]
- Ng, K.T.; Yap, J.L.; Izham, I.N.; Teoh, W.Y.; Kwok, P.E.; Koh, W.J. The effect of intravenous magnesium on postoperative morphine consumption in noncardiac surgery: A systematic review and meta-analysis with trial sequential analysis. Eur. J. Anaesthesiol. 2020, 37, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.N.; Drury, A.S.; Gupta, N. Continuous Magnesium Sulfate Infusions for Status Asthmaticus in Children: A Systematic Review. Front. Pediatr. 2022, 10, 853574. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; Pfister, K.; Schwabe, R.; Vormann, J.; Stahlmann, R. Ultrastructure of Achilles Tendons of Rats Treated with Ofloxacin and Fed a Normal or Magnesium-Deficient Diet. Antimicrob. Agents Chemother. 2000, 44, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Oren, S.; Rapoport, J.; Zlotnik, M.; Brami, J.; Heimer, D.; Chaimovitz, C. Extreme Hypermagnesemia Due to Ingestion of Dead Sea Water. Nephron 1987, 47, 199–201. [Google Scholar] [CrossRef]
- Wyskida, K.; Witkowicz, J.; Chudek, J.; Więcek, A. Daily Magnesium Intake and Hypermagnesemia in Hemodialysis Patients With Chronic Kidney Disease. J. Ren. Nutr. 2012, 22, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.-M.; Chen, S.-Y.; Chen, H.-C.; Yu, J.-H.; Wang, S.-H. Hypermagnesemia in a Constipated Female. J. Emerg. Med. 2013, 44, e57–e60. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Shimada, H.; Yoshita, K.; Tsubata, Y.; Ikarashi, K.; Morioka, T.; Saito, N.; Sakai, S.; Narita, I. Severe hypermagnesemia induced by magnesium oxide ingestion: A case series. CEN Case Rep. 2019, 8, 31–37. [Google Scholar] [CrossRef]
- Wakai, E.; Ikemura, K.; Sugimoto, H.; Iwamoto, T.; Okuda, M. Risk factors for the development of hypermagnesemia in patients prescribed magnesium oxide: A retrospective cohort study. J. Pharm. Health Care Sci. 2019, 5, 4. [Google Scholar] [CrossRef]
- Tuttle, A.; Fitter, S.; Hua, H.; Moussavi, K. The Effects of Magnesium Coadminstration During Treatment of Hypokalemia in the Emergency Department. J. Emerg. Med. 2022, 63, 399–413. [Google Scholar] [CrossRef]
- Naksuk, N.; Hu, T.; Krittanawong, C.; Thongprayoon, C.; Sharma, S.; Park, J.Y.; Rosenbaum, A.N.; Gaba, P.; Killu, A.M.; Sugrue, A.M.; et al. Association of Serum Magnesium on Mortality in Patients Admitted to the Intensive Cardiac Care Unit. Am. J. Med. 2017, 130, 229.e5–229.e13. [Google Scholar] [CrossRef]
- Dibaba, D.T.; Xun, P.; Song, Y.; Rosanoff, A.; Shechter, M.; He, K. The effect of magnesium supplementation on blood pressure in individuals with insulin resistance, prediabetes, or noncommunicable chronic diseases: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2017, 106, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Simental-Mendia, L.E.; Sahebkar, A.; Rodriguez-Moran, M.; Zambrano-Galvan, G.; Guerrero-Romero, F. Effect of Magnesium Supplementation on Plasma C-reactive Protein Concentrations: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Curr. Pharm. Des. 2017, 23, 4678–4686. [Google Scholar] [CrossRef] [PubMed]
- Van Laecke, S.; Van Biesen, W.; Vanholder, R. Hypomagnesaemia, the kidney and the vessels. Nephrol. Dial. Transplant. 2012, 27, 4003–4010. [Google Scholar] [CrossRef] [PubMed]
- Leenders, N.H.J.; Bos, C.; Hoekstra, T.; Schurgers, L.J.; Vervloet, M.G.; Hoenderop, J.G.J. Dietary magnesium supplementation inhibits abdominal vascular calcification in an experimental animal model of chronic kidney disease. Nephrol. Dial. Transplant. 2022, 37, 1049–1058. [Google Scholar] [CrossRef]
- Ter Braake, A.D.; Vervloet, M.G.; de Baaij, J.H.F.; Hoenderop, J.G.J. Magnesium to prevent kidney disease-associated vascular calcification: Crystal clear? Nephrol. Dial. Transplant. 2022, 37, 421–429. [Google Scholar] [CrossRef]
- Sakaguchi, Y.; Hamano, T.; Kubota, K.; Oka, T.; Yamaguchi, S.; Matsumoto, A.; Hashimoto, N.; Mori, D.; Obi, Y.; Matsui, I.; et al. Anion Gap as a Determinant of Ionized Fraction of Divalent Cations in Hemodialysis Patients. Clin. J. Am. Soc. Nephrol. 2018, 13, 274–281. [Google Scholar] [CrossRef]
- Carrillo, I.G.; Vega, A.; Goicoechea, M.; Shabaka, A.; Gatius, S.; Abad, S.; López-Gómez, J.M. Impact of Serum Magnesium Levels on Kidney and Cardiovascular Prognosis and Mortality in CKD Patients. J. Ren. Nutr. 2021, 31, 494–502. [Google Scholar] [CrossRef]
| Proven | Probable | Uncertain or Unproven |
|---|---|---|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Laecke, S. An Update on Hypomagnesemia and Hypermagnesemia. Kidney Dial. 2024, 4, 1-14. https://doi.org/10.3390/kidneydial4010001
Van Laecke S. An Update on Hypomagnesemia and Hypermagnesemia. Kidney and Dialysis. 2024; 4(1):1-14. https://doi.org/10.3390/kidneydial4010001
Chicago/Turabian StyleVan Laecke, Steven. 2024. "An Update on Hypomagnesemia and Hypermagnesemia" Kidney and Dialysis 4, no. 1: 1-14. https://doi.org/10.3390/kidneydial4010001





