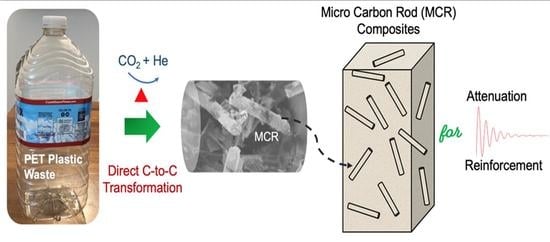
Unusual Micro Carbon Rods Formed from PET Plastic via Pyrolysis and Annealing in CO2/He Co-Gas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of PET-Derived Activated Carbon (PET-AC) Powder
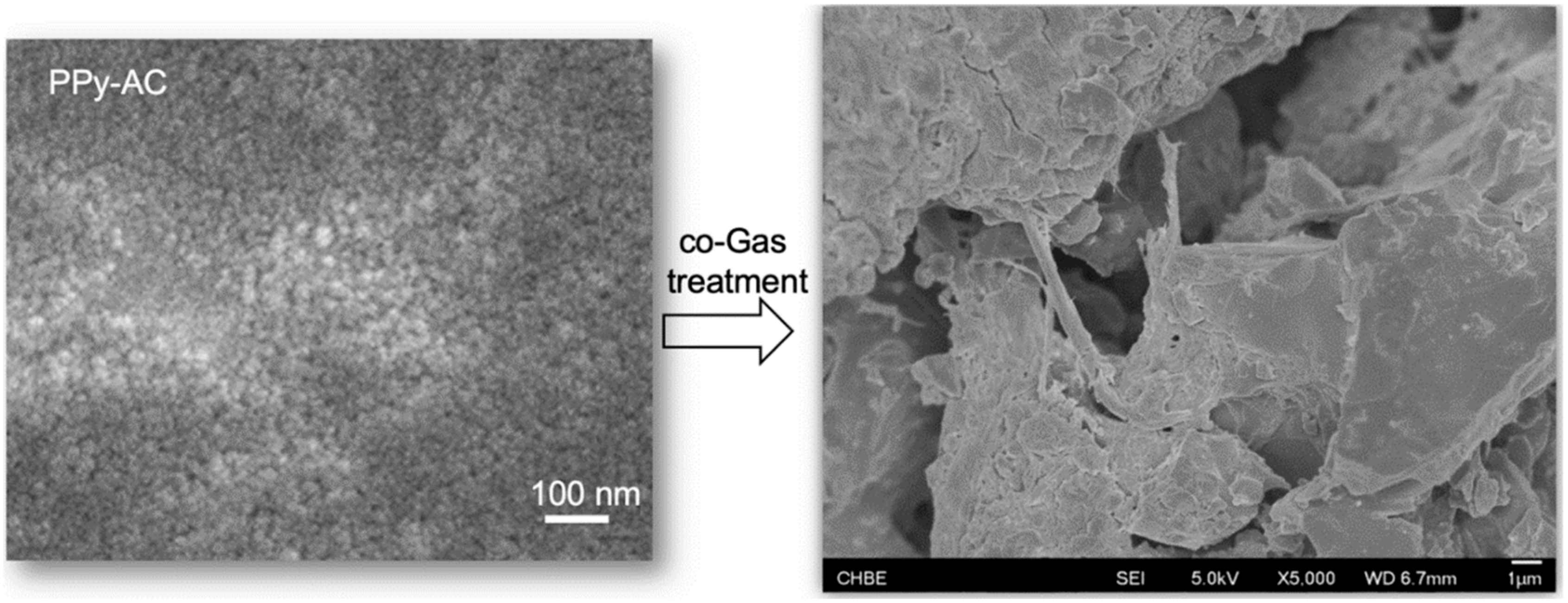
2.2. Formation of Micro Carbon Rods (MCR) via the C-C Transformation
3. Results
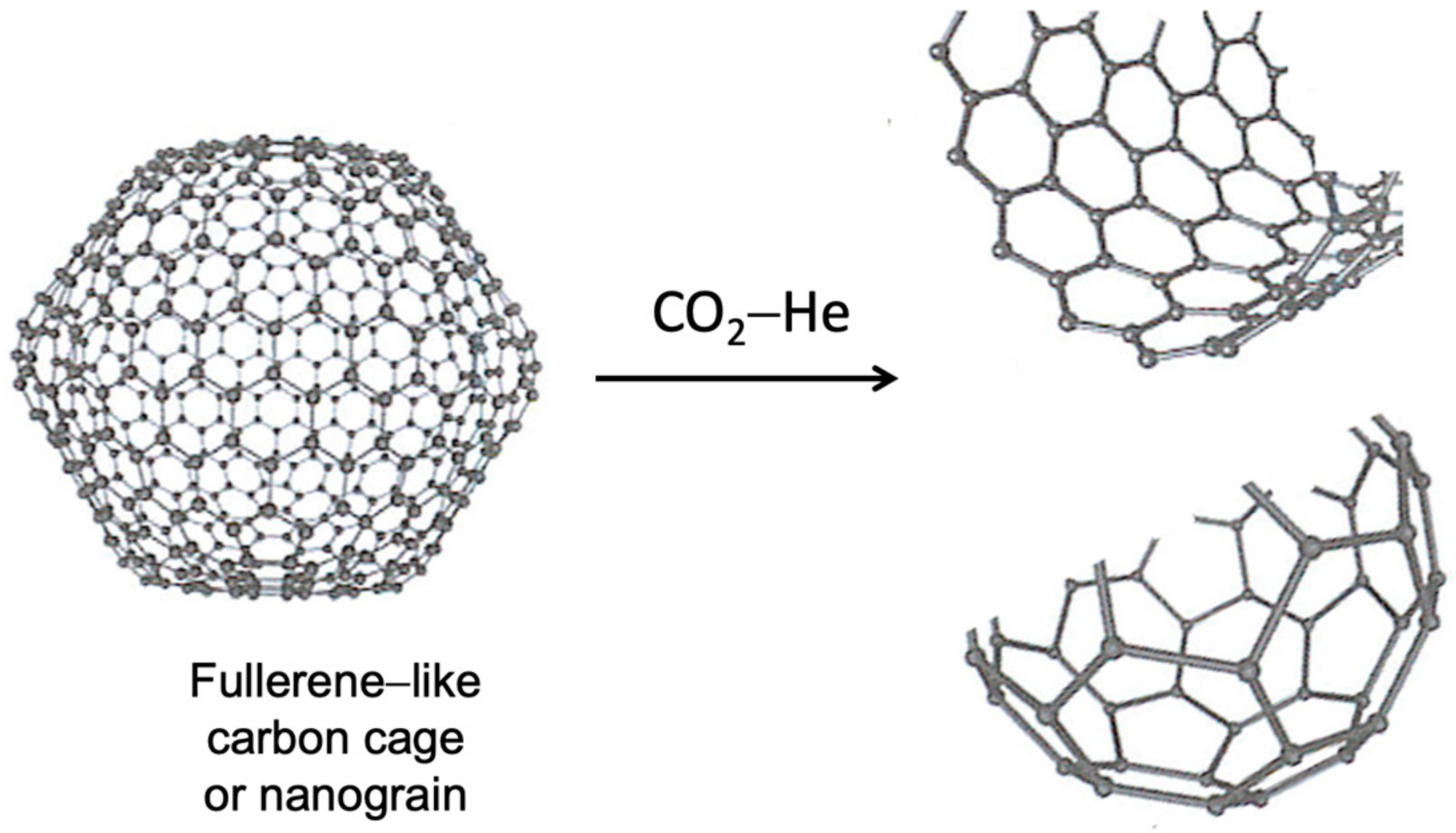
3.1. The Consecutive Conversion Steps from PET Plastic to MCR
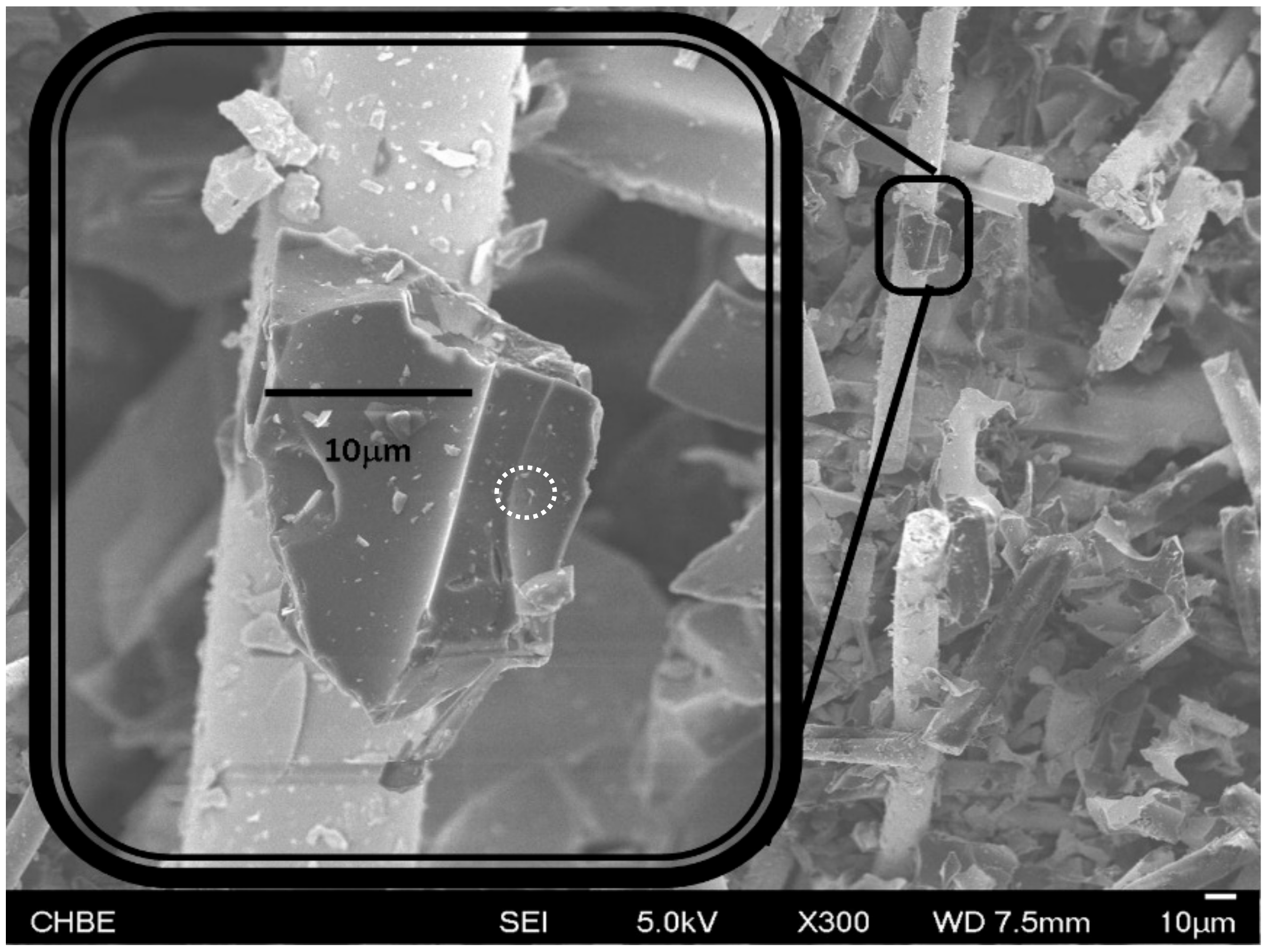
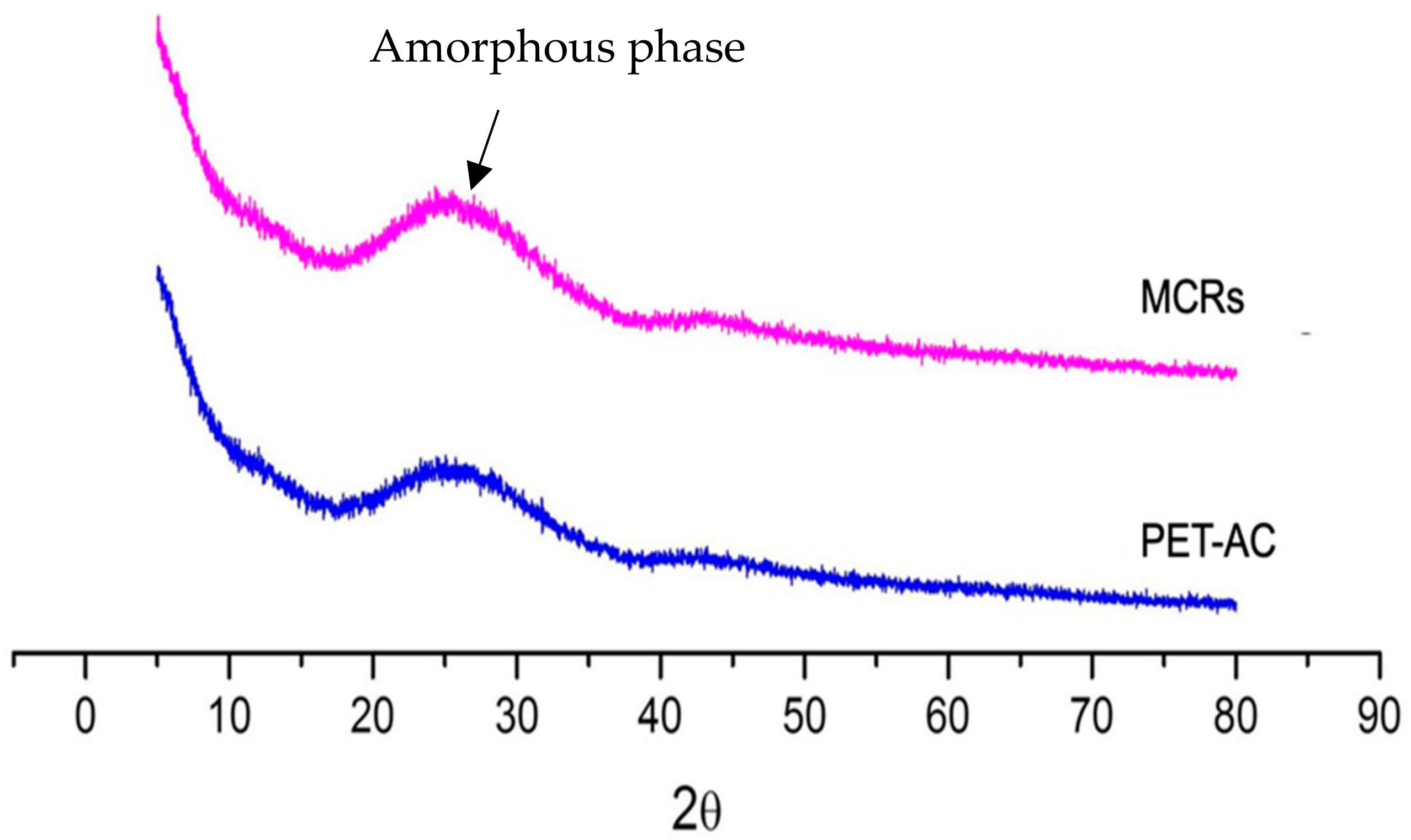
3.2. The Surface and Bulk Features of MCR
4. Discussion
4.1. The Initial Stage That Leads to the Growth of Micro Carbon Rods (MCR)
4.2. How the P-Conjugated System Impacts Curvature Piling of PAHs
4.3. Future Improvement in the Technology to Prepare MCR from PET
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Lin, G.; Vaidya, U.; Wang, H. Past, present and future prospective of global carbon fibre composite developments and applications. Compos. Part B Eng. 2023, 250, 110463. [Google Scholar] [CrossRef]
- Agarwal, S.; Greiner, A.; Wendorff, J.H. Functional materials by electrospinning of polymers. Progr. Polym. Sci. 2013, 38, 963–991. [Google Scholar] [CrossRef]
- Maitra, T.; Sharma, S.; Srivastava, A.; Cho, Y.-K.; Madou, M.; Sharma, A. Improved graphitization and electrical conductivity of suspended carbon nanofibers derived from carbon nanotube/polyacrylonitrile composites by directed electrospinning. Carbon 2012, 50, 1753–1761. [Google Scholar] [CrossRef]
- Qiu, Y.; Yu, J.; Shi, T.; Zhou, X.; Bai, X.; Huang, J.Y. Nitrogen-doped ultrathin carbon nanofibers derived from electrospinning: Large-scale production, unique structure, and application as electrocatalysts for oxygen reduction. J. Power Sources 2011, 196, 9862–9867. [Google Scholar] [CrossRef]
- Song, X.; Wang, Z.; Li, Z.; Wang, C. Ultrafine porous carbon fibers for SO2 adsorption via electrospinning of polyacrylonitrile solution. J. Colloid Interface Sci. 2008, 327, 388–392. [Google Scholar] [CrossRef]
- Greiner, A.; Wendorff, J.H. Electrospinning: A fascinating method for the preparation of ultrathin fibers. Angew. Chem. Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef]
- Zussman, E.; Chen, X.; Ding, W.; Calabri, L.; Dikin, D.A.; Quintana, J.P. Ruoff, Mechanical and structural characterization of electrospun PAN-derived carbon nanofibers. Carbon 2005, 43, 2175–2185. [Google Scholar] [CrossRef]
- Peng, M.; Li, D.; Shen, L.; Chen, Y.; Zheng, Q.; Wang, H. Nanoporous structured submicrometer carbon fibers prepared via solution electrospinning of polymer blends. Langmuir 2006, 22, 9368–9374. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, M.; Yang, Y.; Kang, F. Carbon nanofibers prepared via electrospinning. Adv. Mater. 2012, 24, 2547–2566. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, S.; Jiang, Q.; Li, R.; Zhao, Y.; Huang, Y.; Wu, X.; Wang, B.; Zhang, R. Advanced functional carbon nanotube fibers from preparation to application. Cell Rep. Phys. Sci. 2022, 3, 100989. [Google Scholar] [CrossRef]
- Şahin, K.; Fasanella, N.A.; Chasiotis, I.; Lyons, K.M.; Newcomb, B.A.; Kamath, M.G.; Chae, H.G.; Kumar, S. High strength micron size carbon fibers from polyacrylonitrile–carbon nanotube precursors. Carbon 2014, 77, 442–453. [Google Scholar] [CrossRef]
- Zhu, J.T.; Jia, J.C.; Kwong, F.L.; Ng, D.H.L. Synthesis of bamboo-like carbon nanotubes on a copper foil by catalytic chemical vapor deposition from ethanol. Carbon 2012, 50, 2504–2512. [Google Scholar] [CrossRef]
- Chen, L.C.; Chang, S.W.; Chang, C.S.; Wen, C.Y.; Wu, J.J.; Chen, Y.F.; Huang, Y.S.; Chen, K.H. Catalyst-free and controllable growth of SiCxNy nanorods. J. Phys. Chem. Solids 2001, 62, 1567–1576. [Google Scholar] [CrossRef]
- Lee, D.C.; Mikulec, F.V.; Korgel, B.A. Carbon nanotube synthesis in supercritical toluene. J. Am. Chem. Soc. 2004, 126, 4951–4957. [Google Scholar] [CrossRef]
- Anthony, T.R.; Bradley, J.C.; Horoyski, P.J.; Thewalt, M.L.W. Graphite rod precursors for isotopically pure fullerenes and diamond. Carbon 1996, 34, 1323–1328. [Google Scholar] [CrossRef]
- Osman, A.I.; Farrell, C.; Al-Muhtaseb, A.H.; Al-Fatesh, A.S.; Harrison, J.; Rooney, D.W. Pyrolysis kinetic modelling of abundant plastic waste (PET) and in-situ emission monitoring. Environ. Sci. Eur. 2020, 32, 112. [Google Scholar] [CrossRef]
- Su, C.; Li, Z.; Mao, M.; Ye, W.; Zhong, J.; Ren, Q.; Cheng, H.; Huang, H.; Fu, M.; Wu, J.; et al. Unraveling specific role of carbon matrix over Pd/quasi-Ce-MOF facilitating toluene enhanced degradation. J. Rare Earths 2021, 40, 1751–1762. [Google Scholar] [CrossRef]
- Zhang, M.; Ling, H.; Wang, T.; Jiang, Y.; Song, G.; Zhao, W.; Zhao, L.; Cheng, T.; Xie, Y.; Guo, Y.; et al. An Equivalent Substitute Strategy for Constructing 3D Ordered Porous Carbon Foams and Their Electromagnetic Attenuation Mechanism. Nano-Micro Lett. 2022, 14, 157. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of pore volume and area distributions in porous substances. I. computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Rashid, M.; Mohammad, R.; Barakat, M.A.; Aburiazaiza, A.S.; Khan, H.; Ismail, I.M.I.; Jeya, D.; Jabbar, G.; Ali, H.; Abdul-Sattar, N. Catalytic Pyrolysis of Plastic Waste: Moving toward Pyrolysis Based Biorefineries. Front. Energy Res. 2019, 7, 1–27. Available online: https://www.frontiersin.org/articles/10.3389/fenrg.2019.00027 (accessed on 19 March 2019).
- Sandford, S.A.; Bernstein, M.P.; Materese, C.K. The infrared spectra of polycyclic aromatic hydrocarbons with excess peripheral H atoms (Hn-PAHs) and their relation to the 3.4 and 6.9 μm PAH emission features. Astrophys. J. Suppl. Ser. 2013, 205, 8. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.V.G.; Filho, E.S.; Uliana, F.; de Jesus, L.F.R.; de Melo, C.V.P.; Barthus, R.C.; Rodrigues, J.G.A.; Vanini, G. PET glycolysis optimization using ionic liquid [Bmin] ZnCl3 as catalyst and kinetic evaluation. Polímeros 2018, 28, 450–459. [Google Scholar] [CrossRef]
- Wiedemeier, D.B.; Abiven, S.; Hockaday, W.C.; Keiluweit, M.; Kleber, M.; Masiello, C.A.; McBeath, A.V.; Nico, P.S.; Pyle, L.A.; Schneider, M.P.; et al. Aromaticity and degree of aromatic condensation of char. Org. Geochem. 2015, 78, 135–143. [Google Scholar] [CrossRef]
- Chen, X.; Hong, L.; Chen, X.; Yeong, W.H.A.; Chan, W.K.I. Aliphatic chain grafted polypyrrole as a precursor of carbon membrane. J. Membr. Sci. 2011, 379, 353–360. [Google Scholar] [CrossRef]
- Ahmad, Z.; Choudhary, M.A.; Mehmood, A.; Wakeel, R.; Akhtar, T.; Rafiq, M.A. Synthesis of polypyrrole nano/microspheres using cobalt(III) as an oxidizing agent and its ammonia sensing behavior. Macromol. Res. 2016, 24, 596–601. [Google Scholar] [CrossRef]
- Jourdain, V.; Bichara, C. Current understanding of the growth of carbon nanotubes in catalytic chemical vapour deposition. Carbon 2013, 58, 2–39. [Google Scholar] [CrossRef]
- Tan, L.-L.; Ong, W.-J.; Chai, S.-P.; Mohamed, A.R. Growth of carbon nanotubes over non-metallic based catalysts: A review on the recent developments. Catal. Today 2013, 217, 1–12. [Google Scholar] [CrossRef]
- Takagi, D.; Hibino, H.; Suzuki, S.; Kobayashi, Y.; Homma, Y. Carbon nanotube growth from semiconductor nanoparticles. Nano Lett. 2007, 7, 2272–2275. [Google Scholar] [CrossRef]
- Liu, B.; Ren, W.; Gao, L.; Li, S.; Pei, S.; Liu, C.; Jiang, C.; Cheng, H.-M. Metal-catalyst-free growth of single-walled carbon nanotubes. J. Am. Chem. Soc. 2009, 131, 2082–2083. [Google Scholar] [CrossRef]
- Martin, J.W.; Slavchov, R.I.; Yapp, E.K.Y.; Akroyd, J.; Mosbach, S.; Kraft, M. The polarization of polycyclic aromatic hydrocarbons curved by pentagon incorporation: The role of the flexoelectric dipole. J. Phys. Chem. C 2017, 121, 27154–27163. [Google Scholar] [CrossRef]
- Mohamed, H.H.; Alsanea, A.A.; Alomair, N.A.; Akhtar, S.; Bahnemann, D.W. ZnO@ porous graphite nanocomposite from waste for superior photocatalytic activity. Environ. Sci. Pollut. Res. 2019, 26, 12288–12301. [Google Scholar] [CrossRef] [PubMed]
- Schwan, J.; Ulrich, S.; Batori, V.; Ehrhardt, H.; Silva, S.R.P. Raman spectroscopy on amorphous carbon films. J. Appl. Phys. 1996, 80, 440–447. [Google Scholar] [CrossRef]
- Havigh, R.S.; Chenari, H.M.A. Comprehensive study on the effect of carbonization temperature on the physical and chemical properties of carbon fibers. Sci. Rep. 2022, 12, 10704. [Google Scholar] [CrossRef]
- Mantelli, A.; Romani, A.; Suriano, R.; Diani, M.; Colledani, M.; Sarlin, E.; Turri, S.; Levi, M. UV-Assisted 3D printing of polymer composites from thermally and mechanically recycled carbon fibers. Polymers 2021, 13, 726. [Google Scholar] [CrossRef]
- Stejskal, J.; Trchová, M.; Lapčák, L.; Kolská, Z.; Kohl, M.; Pekárek, M.; Prokeš, J. Comparison of carbonized and activated polypyrrole globules, nanofibers, and nanotubes as conducting nanomaterials and adsorbents of organic dye. Carbon Trends 2021, 4, 100068. [Google Scholar] [CrossRef]






| Sample | Surface Area (m2/g) | Vt (cm3/g) | Vm (cm3/g) | Vm/Vt |
|---|---|---|---|---|
| PET-AC | 33.0 | 0.011 | 0.0100 | 0.91 |
| MCR | 28.4 | 0.010 | 0.0095 | 0.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Hong, L. Unusual Micro Carbon Rods Formed from PET Plastic via Pyrolysis and Annealing in CO2/He Co-Gas. J. Compos. Sci. 2023, 7, 205. https://doi.org/10.3390/jcs7050205
Zhou Y, Hong L. Unusual Micro Carbon Rods Formed from PET Plastic via Pyrolysis and Annealing in CO2/He Co-Gas. Journal of Composites Science. 2023; 7(5):205. https://doi.org/10.3390/jcs7050205
Chicago/Turabian StyleZhou, Yi’en, and Liang Hong. 2023. "Unusual Micro Carbon Rods Formed from PET Plastic via Pyrolysis and Annealing in CO2/He Co-Gas" Journal of Composites Science 7, no. 5: 205. https://doi.org/10.3390/jcs7050205