The Antioxidant Activity of Quercetin in Water Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Kinetic Measurements
2.3. Calculations
3. Results and Discussion
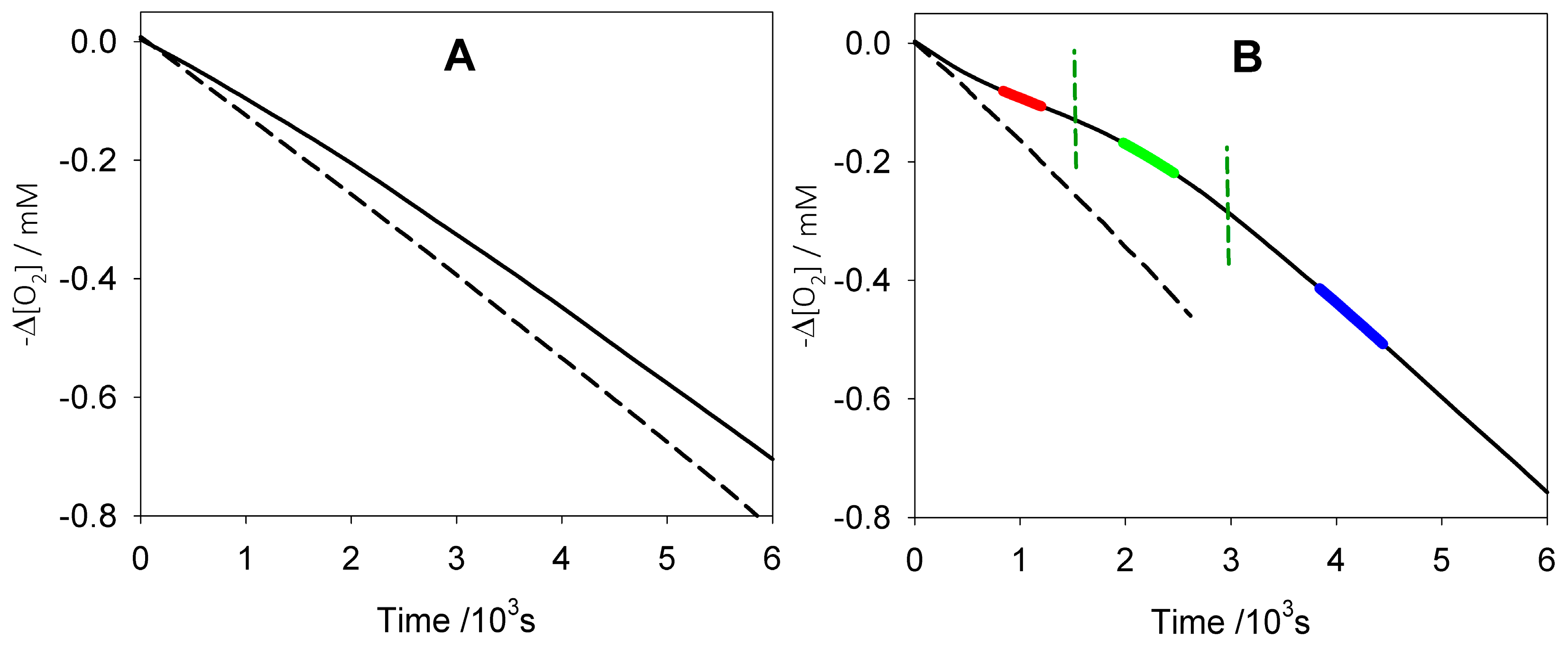
3.1. Kinetic Measurements with Peroxyl Radicals
3.2. Quanto-Mechanical Calculations on Quercetin
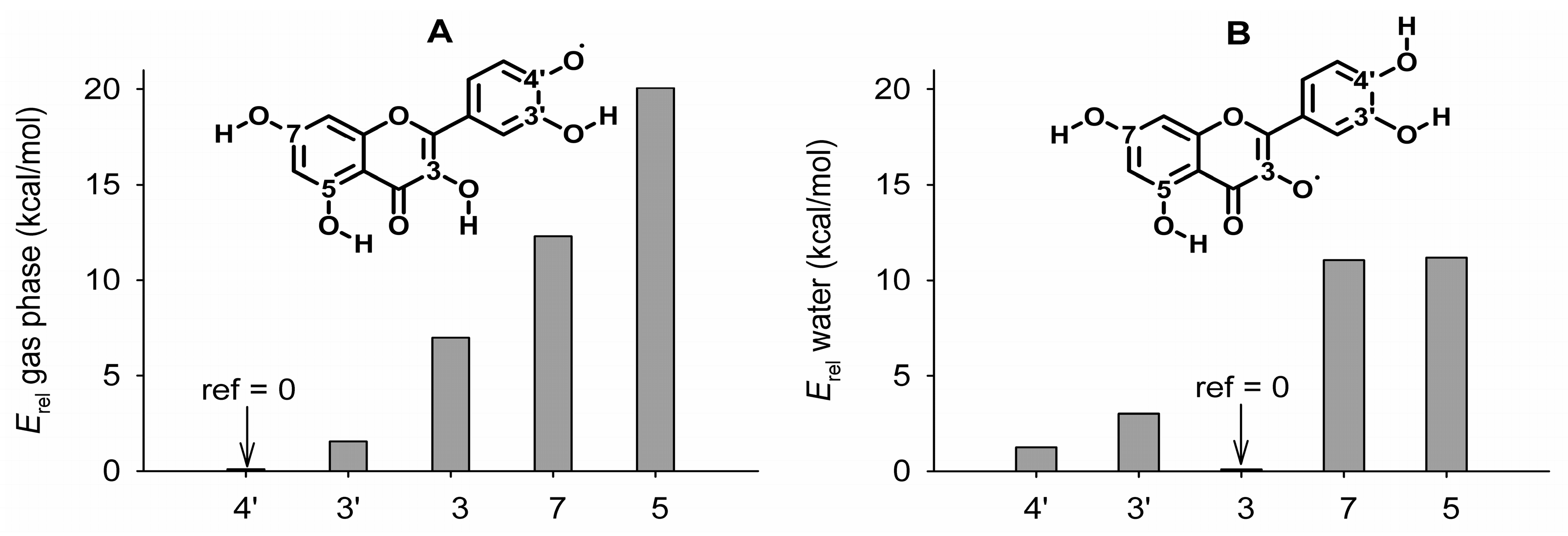
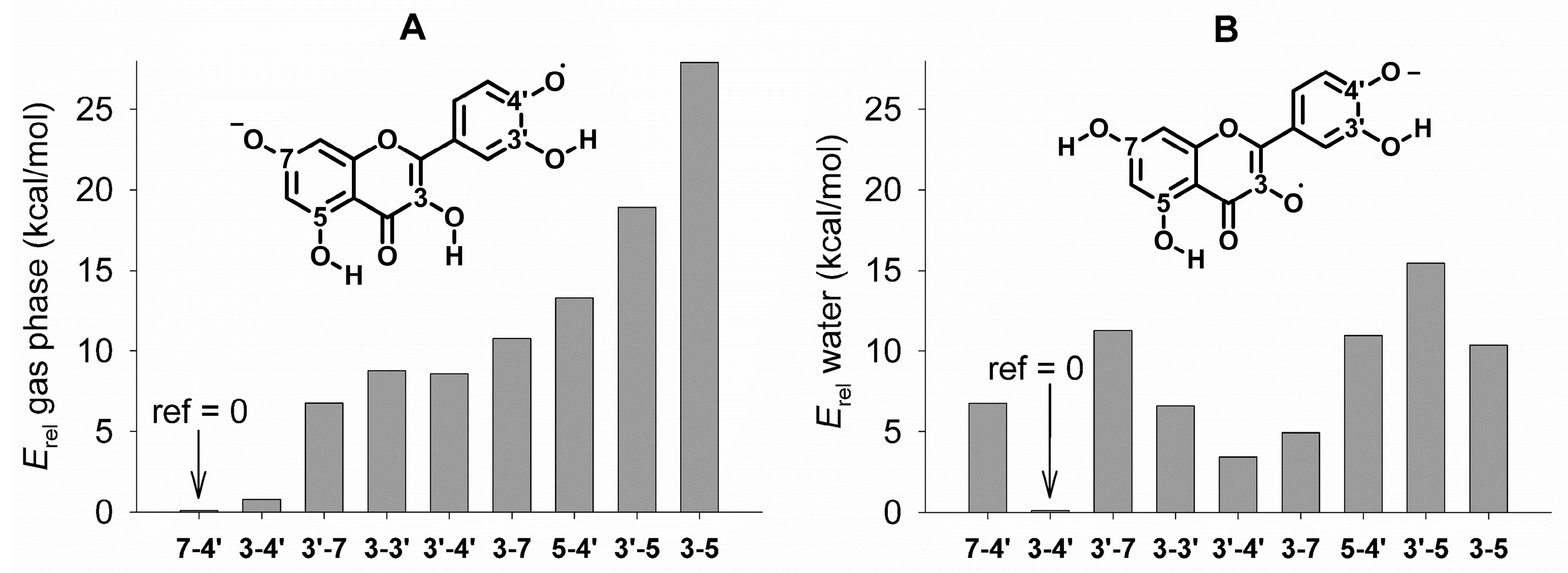
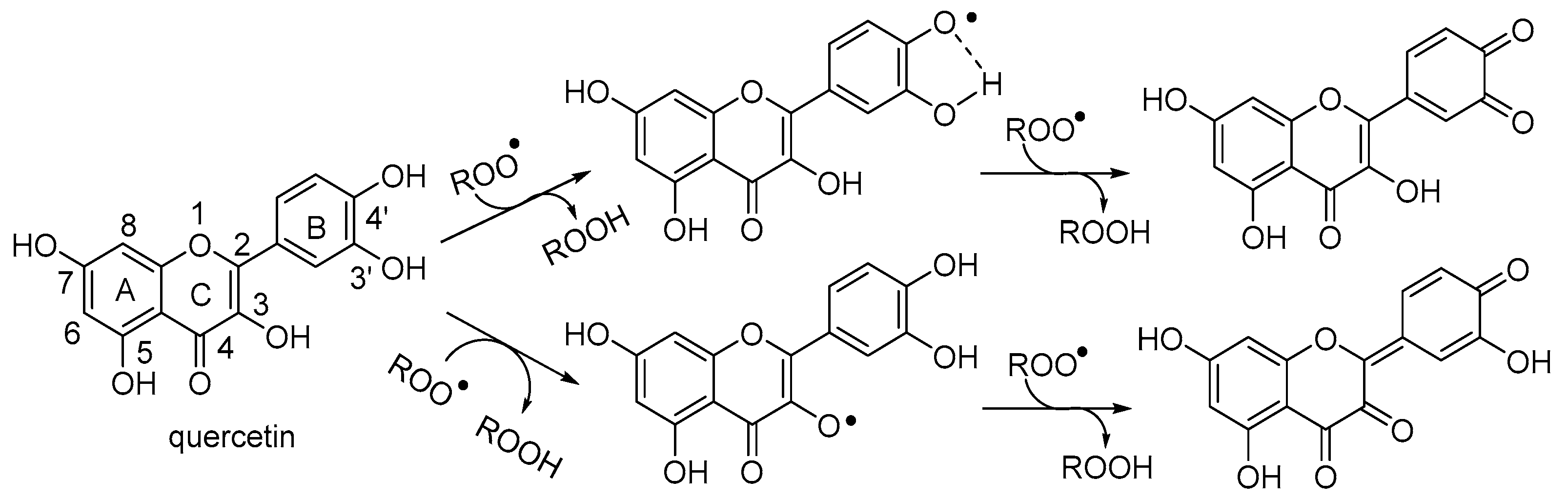
3.2.1. Neutral Radicals from Quercetin
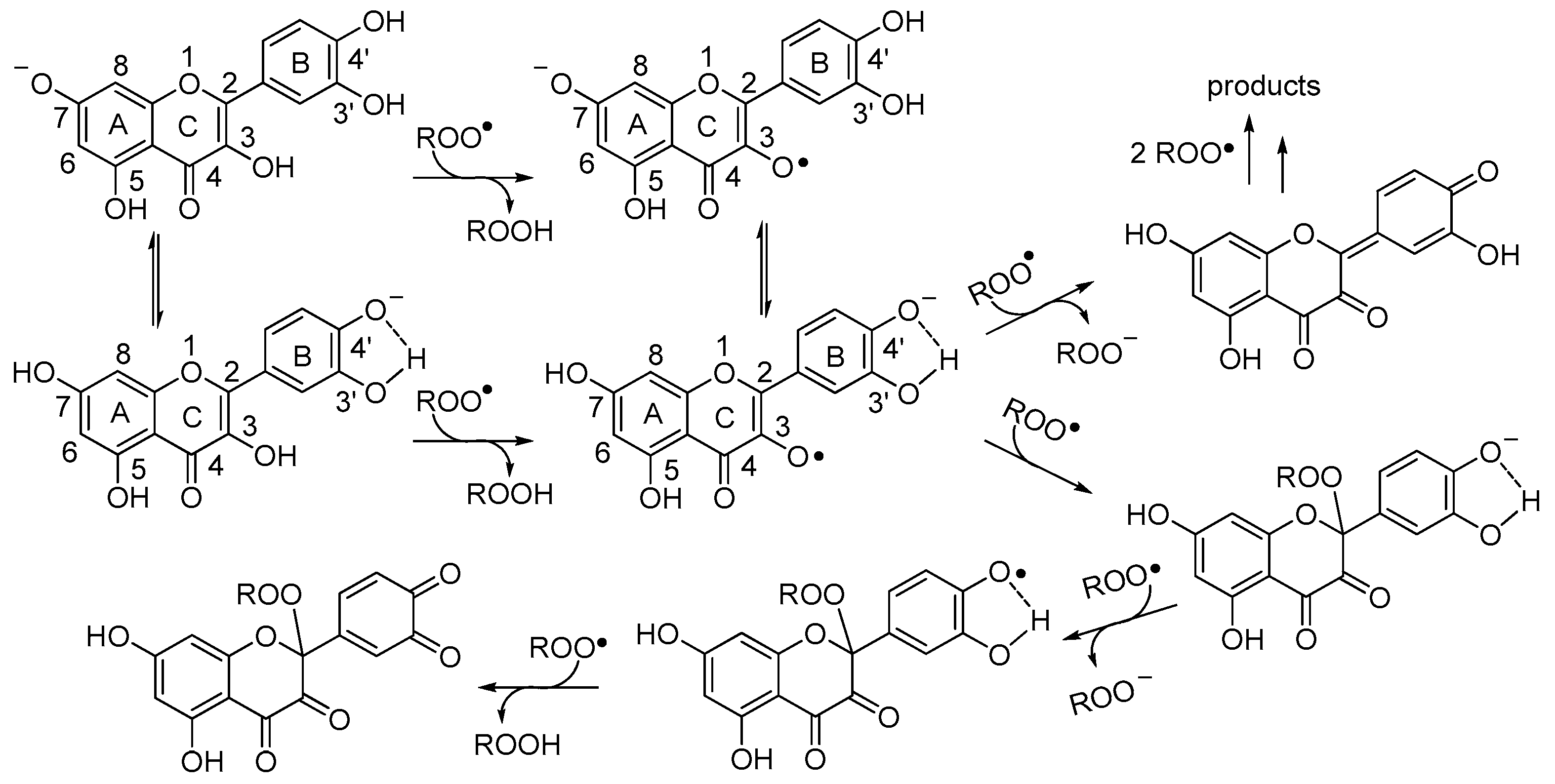
3.2.2. Radical Anions from Quercetin
3.3. Bond Dissociation Enthalpies
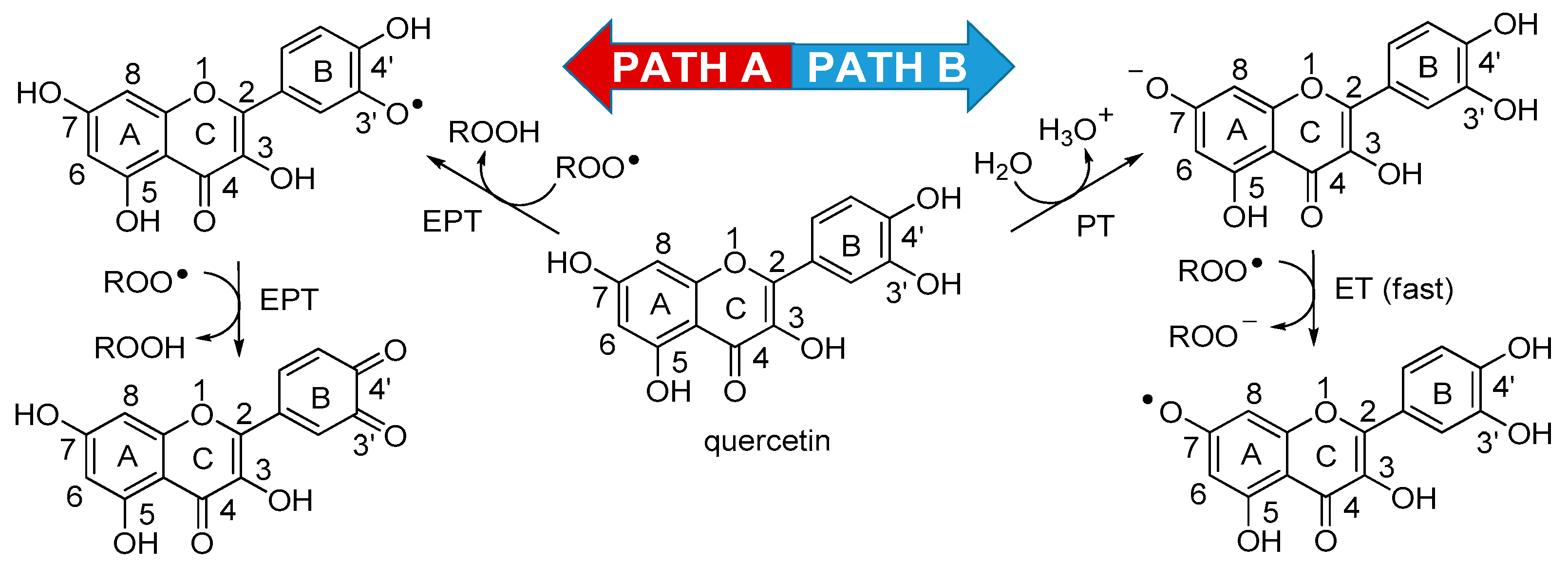
3.4. A Proposed Mechanism for the Antioxidant Activity of Quercetin in Water
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Crozier, A.; Jaganath, I.J.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef] [PubMed]
- Formica, J.V.; Regelson, W. Review of the biology of quercetin and related bioflavonoids. Food Chem. Toxicol. 1995, 33, 1061–1080. [Google Scholar] [CrossRef]
- Erlund, I. Review of the flavonoids quercetin, hesperetin, and naringenin. Dietary sources, bioactivities, bioavailability, and epidemiology. Nut. Res. 2004, 24, 851–874. [Google Scholar] [CrossRef]
- Bhagwat, S.; Haytowitz, D.B.; Holden, J.M. USDA Database for the Flavonoid Content of Selected Foods; Release 3; U.S. Department of Agriculture: Quilcene, WA, USA, 2011. Available online: https://www.ars.usda.gov/ARSUserFiles/80400525/Data/Flav/Flav_R03.pdf (accessed on 03 March 2017).
- Justesen, U.; Knuthsen, P. Composition of flavonoids in fresh herbs and calculation of flavonoid intake by use of herbs in traditional Danish dishes. Food Chem. 2001, 73, 245–250. [Google Scholar] [CrossRef]
- D’Andrea, G. Quercetin: A flavonol with multifaceted therapeutic applications? Fitoterapia 2015, 106, 256–271. [Google Scholar] [CrossRef] [PubMed]
- Sharmila, G.; Bhat, F.A.; Arunkumar, R.; Elumalai, P.; Raja Singh, P.; Senthilkumar, K.; Arunakaran, J. Chemopreventive effect of quercetin, a natural dietary flavonoid on prostate cancer in in vivo model. Clin. Nutr. 2014, 33, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Murakami, A.; Ashida, H.; Terao, J. Multitargeted cancer prevention by quercetin. Cancer Lett. 2008, 269, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Miles, S.I.; McFarland, M.; Niles, R.M. Molecular and physiological actions of quercetin: Need for clinical trials to assess its benefits in human disease. Nutr. Rev. 2014, 72, 720–734. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to quercetin and protection of DNA, proteins and lipids from oxidative damage (ID 1647), “cardiovascular system” (ID 1844), “mental state and performance” (ID 1845), and “liver, kidneys” (ID 1846) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2067. [Google Scholar] [CrossRef]
- Massaro, M.; Riela, S.; Guernelli, S.; Parisi, F.; Lazzara, G.; Baschieri, A.; Valgimigli, L.; Amorati, R. A synergic nanoantioxidant based on covalently modified halloysite-trolox nanotubes with intra-lumen loaded quercetin. J. Mater. Chem. B 2016, 4, 2229–2241. [Google Scholar] [CrossRef]
- Zhu, B.; Yu, L.; Yue, Q.C. Co-delivery of vincristine and quercetin by nanocarriers for lymphoma combination chemotherapy. Biomed. Pharmacother. 2017, 91, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Cao, Z.; Zhao, Y.; Zeng, R.; Tu, M.; Zhao, J. Novel self-assembled pH-responsive biomimetic nanocarriers for drug delivery. Mater. Sci. Eng. C 2016, 64, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Leopoldini, M.; Russo, N.; Toscano, M. The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem. 2011, 125, 288–306. [Google Scholar] [CrossRef]
- Galano, A.; Mazzone, G.; Alvarez-Diduk, R.; Marino, T.; Alvarez-Idaboy, J.R.; Russo, N. Food antioxidants: Chemical insights at the molecular level. Annu. Rev. Food Sci. Technol. 2016, 7, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, L.; Amorati, R.; Petrucci, S.; Pedulli, G.F.; Hu, D.; Hanthorn, J.J.; Pratt, D.A. Unexpected acid catalysis in reactions of peroxyl radicals with phenols. Angew. Chem. Int. Ed. 2009, 48, 8348–8351. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Engman, L.; Valgimigli, L.; Amorati, R.; Fumo, M.G.; Pedulli, G.F. Antioxidant profile of ethoxyquin and some of its S, Se, and Te analogues. J. Org. Chem. 2007, 72, 6046–6055. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Lynett, P.T.; Valgimigli, L.; Pratt, D.A. The reaction of sulfenic acids with peroxyl radicals: Insights into the radical-trapping antioxidant activity of plant-derived thiosulfinates. Chem. Eur. J. 2012, 18, 6370–6379. [Google Scholar] [CrossRef] [PubMed]
- Matera, R.; Gabbanini, S.; Berretti, S.; Amorati, R.; De Nicola, G.R.; Iori, R.; Valgimigli, L. Acylated anthocyanins from sprouts of Raphanus sativus cv. Sango: Isolation, structure elucidation and antioxidant activity. Food Chem. 2015, 166, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Valgimigli, L. Advantages and limitations of common testing methods for antioxidants. Free Radic. Res. 2015, 49, 633–649. [Google Scholar] [CrossRef] [PubMed]
- Pedrielli, P.; Pedulli, G.F.; Skibsted, L.H. Antioxidant mechanism of flavonoids. Solvent effect on rate constant for chain-breaking reaction of quercetin and epicatechin in autoxidation of methyl linoleate. J. Agric. Food Chem. 2001, 49, 3034–3040. [Google Scholar] [CrossRef] [PubMed]
- Tarozzi, A.; Bartolini, M.; Piazzi, L.; Valgimigli, L.; Amorati, R.; Bolondi, C.; Djemil, A.; Mancini, F.; Andrisano, V.; Rampa, A. From the dual function lead AP2238 to AP2469, a multi-target-directed ligand for the treatment of Alzheimer’s disease. Pharmacol. Res. Perspect. 2014, 2, e00023. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Valgimigli, L.; Panzella, L.; Napolitano, A.; d’Ischia, M. 5-S-lipoylhydroxytyrosol, a multidefense antioxidant featuring a solvent-tunable peroxyl radical-scavenging 3-thio-1,2-dihydroxybenzene motif. J. Org. Chem. 2013, 78, 9857–9864. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, Z.; Presseau, N.; Amorati, R.; Valgimigli, L.; Pratt, D.A. Redox chemistry of selenenic acids and the insight it brings on transition state geometry in the reactions of peroxyl radicals. J. Am. Chem. Soc. 2014, 136, 1570–1578. [Google Scholar] [CrossRef] [PubMed]
- Musialik, M.; Kuzmicz, R.; Pawłowski, T.S.; Litwinienko, G. Acidity of hydroxyl groups: An overlooked influence on antiradical properties of flavonoids. J. Org. Chem. 2009, 74, 2699–2709. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Baschieri, A.; Morroni, G.; Gambino, R.; Valgimigli, L. Peroxyl radical reactions in water solution: A gym for proton-coupled electron-transfer theories. Chem. Eur. J. 2016, 22, 7924–7934. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Baschieri, A.; Valgimigli, L. Measuring antioxidant activity in bioorganic samples by the differential oxygen uptake apparatus: Recent advances. J. Chem. 2017, 2017, 6369358. [Google Scholar] [CrossRef]
- Scott, A.P.; Radom, L. Harmonic vibrational frequencies: An evaluation of Hartree–Fock, Møller–Plesset, quadratic configuration interaction, density functional theory, and semiempirical scale factors. J. Phys. Chem. 1996, 100, 16502–16513. [Google Scholar] [CrossRef]
- Guerra, M.; Amorati, R.; Pedulli, G.F. Water effect on the O–H dissociation enthalpy of para-substituted phenols: A DFT study. J. Org. Chem. 2004, 69, 5460–5467. [Google Scholar] [CrossRef] [PubMed]
- Lind, J.; Shen, T.; Eriksen, E.; Merenyi, G. The one-electron reduction potential of 4-substituted phenoxyl radicals in water. J. Am. Chem. Soc. 1990, 112, 479–482. [Google Scholar] [CrossRef]
- Amat, A.; Clementi, C.; De Angelis, F.; Sgamellotti, A.; Fantacci, S. Absorption and emission of the apigenin and luteolin flavonoids: A TDDFT investigation. J. Phys. Chem. A 2009, 113, 15118–15126. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03, Revision D.02; Gaussian, Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
- Bremond, E.A.G.; Kieffer, J.; Adamo, C. A reliable method for fitting TD-DFT transitions to experimental UV–visible spectra. J. Mol. Struct. THEOCHEM 2010, 954, 52–56. [Google Scholar] [CrossRef]
- Amorati, R.; Valgimigli, L. Modulation of the antioxidant activity of phenols by non-covalent interactions. Org. Biomol. Chem. 2012, 10, 4147–4158. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, M.; Pedulli, G.F.; Valgimigli, L. Do peroxyl radicals obey the principle that kinetic solvent effects on H-Atom abstraction are independent of the nature of the abstracting radical? J. Org. Chem. 1998, 63, 4497–4499. [Google Scholar] [CrossRef]
- Foti, M.C.; Daquino, C.; Di Labio, G.A.; Ingold, K.U. Kinetics of the oxidation of quercetin by 1,1-diphenyl-2-picrylhydrazyl (DPPH). Org. Lett. 2011, 13, 4826–4829. [Google Scholar] [CrossRef] [PubMed]
- Mukai, K.; Oka, W.; Watanabe, K.; Egawa, Y.; Nagaoka, S.; Terao, J. Kinetic study of free-radical-scavenging action of flavonoids in homogeneous and aqueous Triton X-100 micellar solutions. J. Phys. Chem. A 1997, 101, 3746–3753. [Google Scholar] [CrossRef]
- Jovanovic, S.V.; Steenken, S.; Hara, Y.; Simic, M.G. Reduction potentials of flavonoid and model phenoxyl radicals. Which ring in flavonoids is responsible for antioxidant activity? J. Chem. Soc. Perkin Trans. 2 1996, 2497–2504. [Google Scholar] [CrossRef]
- Shen, L.; Zhang, H.-Y.; Ji, H.-F. Successful application of TD-DFT in transient absorption spectra assignment. Org. Lett. 2005, 7, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Lalevee, J.; Allonas, X.; Fouassier, J.-P.; Ingold, K.U. Absolute rate constants for some intermolec ular reactions of aminoalkylperoxyl radicals. Comparison with alkylperoxyls. J. Org. Chem. 2008, 73, 6489–6496. [Google Scholar] [CrossRef] [PubMed]
- Chatgilialoglu, C.; D’Angelantonio, M.; Guerra, M.; Kaloudis, P.; Mulazzani, Q.G. A Reevaluation of the ambident reactivity of the guanine moiety towards hydroxyl radicals. Angew. Chem. Int. Ed. 2009, 48, 2214–2217. [Google Scholar] [CrossRef] [PubMed]
- Kaloudis, P.; D’Angelantonio, M.; Guerra, M.; Spadafora, M.; Cismas, C.; Gimisis, T.; Mulazzani, Q.G.; Chatgilialoglu, C. Comparison of isoelectronic 8-HO-G and 8-NH2-G derivatives in redox processes. J. Am. Chem. Soc. 2009, 131, 15895–15902. [Google Scholar] [CrossRef] [PubMed]
- Leopoldini, M.; Marino, T.; Russo, N.; Toscano, M. Density functional computations of the energetic and spectroscopic parameters of quercetin and its radicals in the gas phase and in solvent. Theor. Chem. Acc. 2004, 111, 210–216. [Google Scholar] [CrossRef]
- Fiorucci, S.; Golebiowski, J.; Cabrol-Bass, D.; Antonczak, S. DFT study of quercetin activated forms involved in antiradical, antioxidant, and prooxidant biological processes. J. Agric. Food Chem. 2007, 55, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Milane, H.A.; Ubeaud, G.; Vandamme, T.F.; Jung, L. Isolation of quercetin’s salts and studies of their physicochemical properties and antioxidant relationships. Bioorg. Med. Chem. 2004, 12, 3627–3635. [Google Scholar] [CrossRef] [PubMed]



| Water | MeCN | PhCl | ||||||
|---|---|---|---|---|---|---|---|---|
| pH = 2.1 | pH = 7.4 | |||||||
| kinh (M−1 s−1) | n | kinh (M−1 s−1) | n | kinh (M−1 s−1) | n | kinh (M−1 s−1) | n | |
| Quercetin | (4.0 ± 0.5) × 103 | - | (1.6 ± 0.3) × 105 | 2.1 ± 0.2 | 1.2 × 104 a | 2.1 | 5.6 × 105 b | 2.1 |
| (7.0 ± 0.5) × 104 | 2.0 ± 0.2 | |||||||
| Catechol c | 3.0 × 103 | - | 7.0 × 103 | - | 2.5 × 104 | 2 | 5.5 × 105 b | 2.0 |
| PMHC c | 1.9 × 105 | 1.8 | 2.0 × 105 | 1.8 | 6.8 × 105 | 2 | 3.2 × 106 | 2.0 |
| Starting Compound | Abstracted OH | BDEOH (kcal/mol) |
|---|---|---|
| Neutral Quercetin | 4′ | 83.3 |
| 3′ | 86.0 | |
| 3 | 83.1 | |
| 7 | 94.0 | |
| 5 | 94.7 | |
| Anion 4′ | 7 | 81.2 |
| 3′ | 79.1 | |
| 3 | 75.0 | |
| 5 | 86.4 | |
| Anion 7 | 4′ | 79.5 |
| 3′ | 84.8 | |
| 3 | 78.7 | |
| 5 | 95.7 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amorati, R.; Baschieri, A.; Cowden, A.; Valgimigli, L. The Antioxidant Activity of Quercetin in Water Solution. Biomimetics 2017, 2, 9. https://doi.org/10.3390/biomimetics2030009
Amorati R, Baschieri A, Cowden A, Valgimigli L. The Antioxidant Activity of Quercetin in Water Solution. Biomimetics. 2017; 2(3):9. https://doi.org/10.3390/biomimetics2030009
Chicago/Turabian StyleAmorati, Riccardo, Andrea Baschieri, Adam Cowden, and Luca Valgimigli. 2017. "The Antioxidant Activity of Quercetin in Water Solution" Biomimetics 2, no. 3: 9. https://doi.org/10.3390/biomimetics2030009
APA StyleAmorati, R., Baschieri, A., Cowden, A., & Valgimigli, L. (2017). The Antioxidant Activity of Quercetin in Water Solution. Biomimetics, 2(3), 9. https://doi.org/10.3390/biomimetics2030009









