Catechol-Containing Hydroxylated Biomimetic 4-Thiaflavanes as Inhibitors of Amyloid Aggregation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
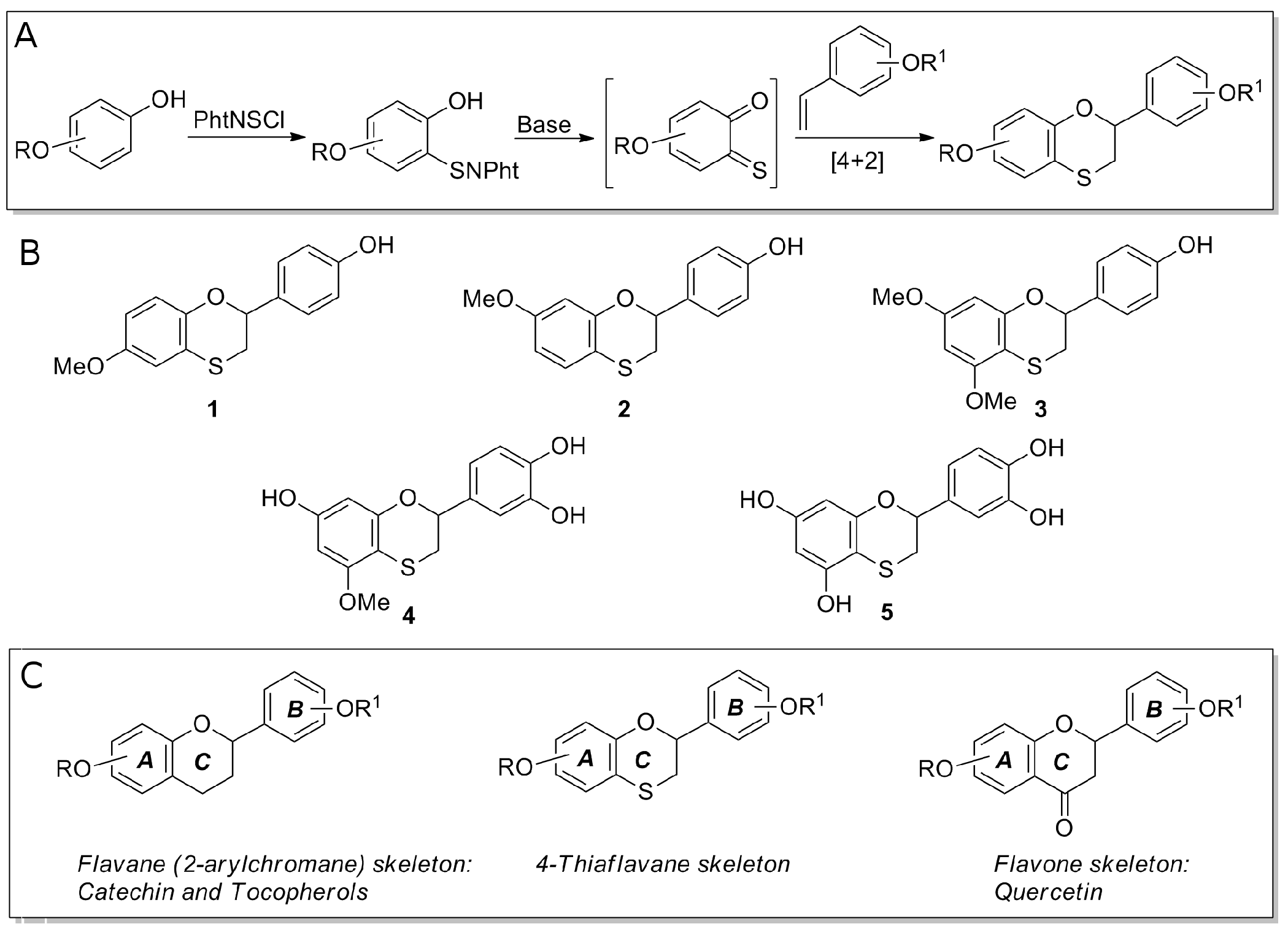
2.2. Preparation of 4-Thiaflavane Derivatives
2.3. Preparation of Hen-Egg White Lysozyme for Aggregation
2.4. Aggregation Conditions
2.5. Thioflavin T Assay
2.6. Atomic Force Microscopy
2.7. Cell Growth and Citotoxicity Assay
3. Results
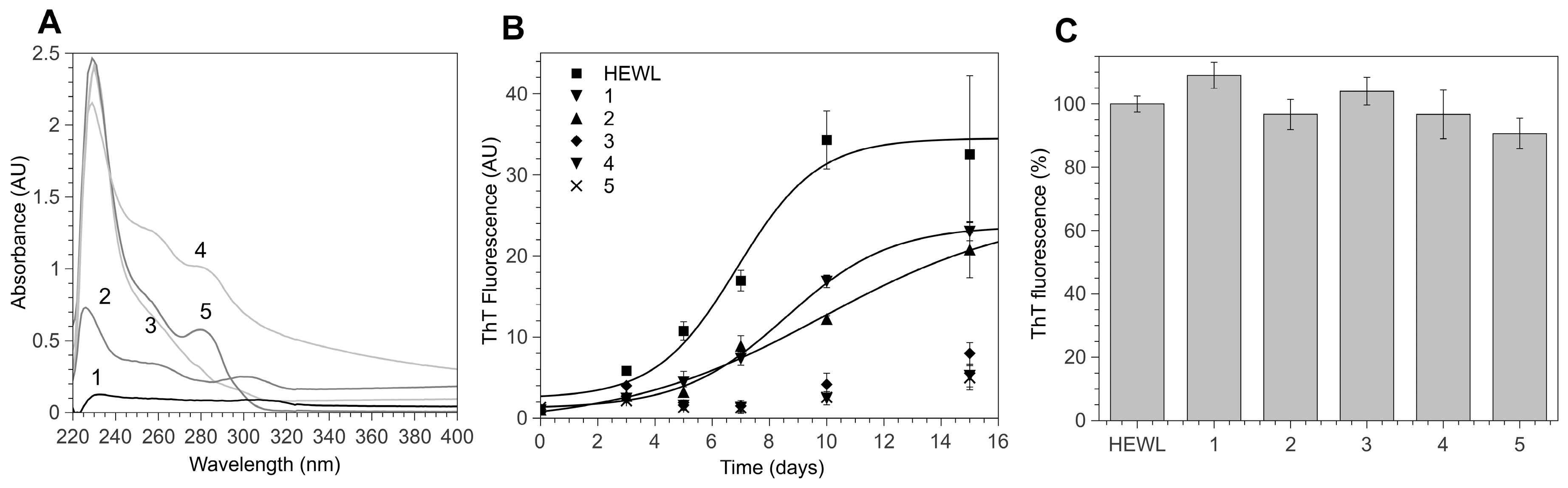
3.1. 4-Thiaflavane Derivatives Obstacle/Impair Amyloid Aggregation Kinetics
3.2. 4-Thiaflavane Derivatives Do Not Compete with Thioflavin T
3.3. Polyhydroxylated 4-Thiaflavanes Inhibit the Formation of Amyloid Fibrils
3.4. 4-Thiaflavane Derivatives Do Not Prevent Toxicity Induced by Early Amyloid Aggregates
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sideras, K.; Gertz, M.A. Amyloidosis. Adv. Clin. Chem. 2009, 47, 1–44. [Google Scholar] [PubMed]
- Chiti, F.; Dobson, C.M. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 2006, 75, 333–366. [Google Scholar] [CrossRef] [PubMed]
- Gertz, M.A.; Benson, M.D.; Dyck, P.J.; Grogan, M.; Coelho, T.; Cruz, M.; Berk, J.L.; Plante-Bordeneuve, V.; Schmidt, H.H.; Merlini, G. Diagnosis, prognosis, and therapy of transthyretin amyloidosis. J. Am. Coll. Cardiol. 2015, 66, 2451–2466. [Google Scholar] [CrossRef] [PubMed]
- Bemporad, F.; Calloni, G.; Campioni, S.; Plakoutsi, G.; Taddei, N.; Chiti, F. Sequence and structural determinants of amyloid fibril formation. Acc. Chem. Res. 2006, 39, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.W. Amyloid fibril formation and protein misassembly: A structural quest for insights into amyloid and prion diseases. Structure 1997, 5, 595–600. [Google Scholar] [CrossRef]
- Stefani, M.; Dobson, C.M. Protein aggregation and aggregate toxicity: New insights into protein folding, misfolding diseases and biological evolution. J. Mol. Med. (Berl) 2003, 81, 678–699. [Google Scholar] [CrossRef] [PubMed]
- Dobson, C.M. Principles of protein folding, misfolding and aggregation. Semin. Cell Dev. Biol. 2004, 15, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Merlini, G.; Bellotti, V. Lysozyme: A paradigmatic molecule for the investigation of protein structure, function and misfolding. Clin. Chim. Acta 2005, 357, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Dumoulin, M.; Kumita, J.R.; Dobson, C.M. Normal and aberrant biological self-assembly: Insights from studies of human lysozyme and its amyloidogenic variants. Acc. Chem. Res. 2006, 39, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Malisauskas, M.; Ostman, J.; Darinskas, A.; Zamotin, V.; Liutkevicius, E.; Lundgren, E.; Morozova-Roche, L.A. Does the cytotoxic effect of transient amyloid oligomers from common equine lysozyme in vitro imply innate amyloid toxicity? J. Biol. Chem. 2004, 280, 6269–6275. [Google Scholar] [CrossRef] [PubMed]
- Frare, E.; Polverino De Laureto, P.; Zurdo, J.; Dobson, C.M.; Fontana, A. A highly amyloidogenic region of hen lysozyme. J. Mol. Biol. 2004, 340, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- Arnaudov, L.N.; de Vries, R. Thermally induced fibrillar aggregation of hen egg white lysozyme. Biophys. J. 2004, 88, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Sethuraman, A.; Belfort, G. Protein structural perturbation and aggregation on homogeneous surfaces. Biophys. J. 2004, 88, 1322–1333. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.N.; Forny-Germano, L.; Saraiva, L.M.; Sebollela, A.; Martinez, A.M.; Houzel, J.C.; De Felice, F.G.; Ferreira, S.T. Soluble oligomers from a non-disease related protein mimic Aβ-induced tau hyperphosphorylation and neurodegeneration. J. Neurochem. 2007, 103, 736–748. [Google Scholar] [CrossRef] [PubMed]
- Walzem, R.L. Wine and health: State of proofs and research needs. Inflammopharmacology 2008, 16, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Higdon, J.V.; Frei, B. Tea catechins and polyphenols: Health effects, metabolism, and antioxidant functions. Crit. Rev. Food Sci. Nutr. 2003, 43, 89–143. [Google Scholar] [CrossRef] [PubMed]
- Virgili, F.; Marino, M. Regulation of cellular signals from nutritional molecules: A specific role for phytochemicals, beyond antioxidant activity. Free Radic. Biol. Med. 2008, 45, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, B.S.; Lee, K.G.; Choi, C.Y.; Jang, S.S.; Kim, Y.H.; Lee, S.E. Effects of naturally occurring compounds on fibril formation and oxidative stress of beta-amyloid. J. Agric. Food Chem. 2005, 53, 8537–8541. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [PubMed]
- Leri, M.; Nosi, D.; Natalello, A.; Porcari, R.; Ramazzotti, M.; Chiti, F.; Bellotti, V.; Doglia, S.M.; Stefani, M.; Bucciantini, M. The polyphenol Oleuropein aglycone hinders the growth of toxic transthyretin amyloid assemblies. J. Nutr. Biochem. 2016, 30, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Leri, M.; Bemporad, F.; Oropesa-Nuñez, R.; Canale, C.; Calamai, M.; Nosi, D.; Ramazzotti, M.; Giorgetti, S.; Pavone, F.S.; Bellotti, V.; et al. Molecular insights into cell toxicity of a novel familial amyloidogenic variant of β2-microglobulin. J. Cell. Mol. Med. 2016, 20, 1443–1456. [Google Scholar] [CrossRef] [PubMed]
- Porat, Y.; Abramowitz, A.; Gazit, E. Inhibition of amyloid fibril formation by polyphenols: Structural similarity and aromatic interactions as a common inhibition mechanism. Chem. Biol. Drug Des. 2006, 67, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Yamada, M. Antioxidant compounds have potent anti-fibrillogenic and fibril-destabilizing effects for α-synuclein fibrils in vitro. J. Neurochem. 2006, 97, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Ramazzotti, M.; Melani, F.; Marchi, L.; Mulinacci, N.; Gestri, S.; Tiribilli, B.; Degl'Innocenti, D. Mechanisms for the inhibition of amyloid aggregation by small ligands. Biosci. Rep. 2016, 36, e00385. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Song, X.H.; Zeng, C.M. Inhibition of amyloid fibrillation of lysozyme by phenolic compounds involves quinoprotein formation. FEBS Lett. 2012, 586, 3951–3955. [Google Scholar] [CrossRef] [PubMed]
- Viglianisi, C.; Menichetti, S. Dihydrobenzo[1,4]oxathiine: A multi-potent pharmacophoric heterocyclic nucleus. Curr. Med. Chem. 2010, 17, 915–928. [Google Scholar] [CrossRef] [PubMed]
- Menichetti, S.; Aversa, M.C.; Cimino, F.; Contini, A.; Viglianisi, C.; Tomaino, A. Synthesis and “double-faced” antioxidant activity of polyhydroxylated 4-thiaflavans. Org. Biomol. Chem. 2005, 3, 3066–3072. [Google Scholar] [CrossRef] [PubMed]
- Menichetti, S.; Amorati, R.; Bartolozzi, M.G.; Pedulli, G.F.; Salvini, A.; Viglianisi, C. A straightforward hetero-Diels–Alder approach to (2-ambo,4’R,8’R)-α/β/γ/δ-4-thiatocopherol. Eur. J. Org. Chem. 2010, 10, 2218–2225. [Google Scholar] [CrossRef]
- Viglianisi, C.; Bartolozzi, M.G.; Pedulli, G.F.; Amorati, R.; Menichetti, S. Optimization of the antioxidant activity of hydroxy-substituted 4-thiaflavanes: A proof-of-concept study. Chemistry 2011, 17, 12396–12404. [Google Scholar] [CrossRef] [PubMed]
- Krebs, M.R.; Wilkins, D.K.; Chung, E.W.; Pitkeathly, M.C.; Chamberlain, A.K.; Zurdo, J.; Robinson, C.V.; Dobson, C.M. Formation and seeding of amyloid fibrils from wild-type hen lysozyme and a peptide fragment from the β-domain. J. Mol. Biol. 2000, 300, 541–549. [Google Scholar] [CrossRef] [PubMed]
- LeVine, H. Thioflavine T interaction with synthetic Alzheimer's disease β-amyloid peptides: Detection of amyloid aggregation in solution. Protein Sci. 1993, 2, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Hudson, S.A.; Ecroyd, H.; Kee, T.W.; Carver, J.A. The thioflavin T fluorescence assay for amyloid fibril detection can be biased by the presence of exogenous compounds. FEBS J. 2009, 276, 5960–5972. [Google Scholar] [CrossRef] [PubMed]
- Lodovici, M.; Menichetti, S.; Viglianisi, C.; Caldini, S.; Giuliani, E. Polyhydroxylated 4-thiaflavans as multipotent antioxidants: Protective effect on oxidative DNA damage in vitro. Bioorg. Med. Chem. Lett. 2006, 16, 1957–1960. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Fumo, M.G.; Pedulli, G.F.; Menichetti, S.; Pagliuca, C.; Viglianisi, C. Antioxidant and antiradical activity of hydroxy-substituted 4-thiaflavanes. Helv. Chim. Acta 2006, 89, 2462–2472. [Google Scholar] [CrossRef]
- Stefani, M. Biochemical and biophysical features of both oligomer/fibril and cell membrane in amyloid cytotoxicity. FEBS J. 2010, 277, 4602–4613. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramazzotti, M.; Paoli, P.; Tiribilli, B.; Viglianisi, C.; Menichetti, S.; Degl’innocenti, D. Catechol-Containing Hydroxylated Biomimetic 4-Thiaflavanes as Inhibitors of Amyloid Aggregation. Biomimetics 2017, 2, 6. https://doi.org/10.3390/biomimetics2020006
Ramazzotti M, Paoli P, Tiribilli B, Viglianisi C, Menichetti S, Degl’innocenti D. Catechol-Containing Hydroxylated Biomimetic 4-Thiaflavanes as Inhibitors of Amyloid Aggregation. Biomimetics. 2017; 2(2):6. https://doi.org/10.3390/biomimetics2020006
Chicago/Turabian StyleRamazzotti, Matteo, Paolo Paoli, Bruno Tiribilli, Caterina Viglianisi, Stefano Menichetti, and Donatella Degl’innocenti. 2017. "Catechol-Containing Hydroxylated Biomimetic 4-Thiaflavanes as Inhibitors of Amyloid Aggregation" Biomimetics 2, no. 2: 6. https://doi.org/10.3390/biomimetics2020006









