Doping LiMnPO4 with Cobalt and Nickel: A First Principle Study
Abstract
:1. Introduction
2. Computational Details
3. Crystal Structures
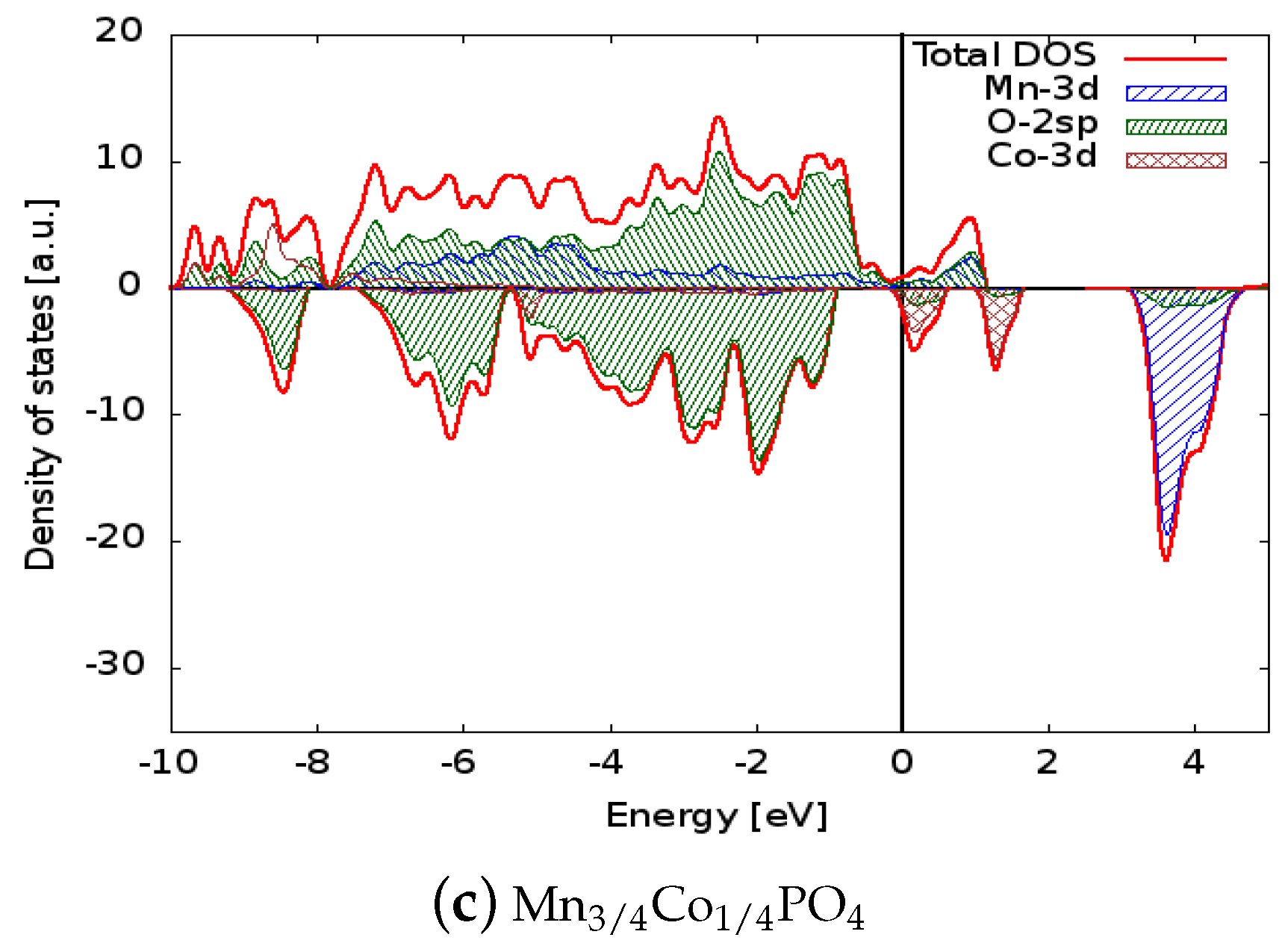
4. Structural and Electronic Properties
5. Lithium Ion Diffusion
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DFT | Density functional theory |
| DFT+U | Hubbard U density functional theory |
| GGA | Generalized gradient approximation |
References
- Van Schalkwijk, W.; Scrosati, B. Advances in Lithium-Ion Batteries; Springer: Boston, MA, USA, 2002. [Google Scholar]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Afyon, S.; Krumeich, F.; Mensing, C.; Borgschulte, A.; Nesper, R. New high capacity cathode materials for rechargeable Li-ion batteries: Vanadate-borate glasses. Sci. Rep. 2014, 4, 7113. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Meng, F.; Jin, S. High-capacity lithium-ion battery conversion cathodes cased on iron fluoride nanowires and insights into the conversion mechanism. Nano Lett. 2012, 12, 6030–6037. [Google Scholar] [CrossRef] [PubMed]
- Eglitis, R.; Borstel, G. Towards a practical rechargeable 5 v Li ion battery. Phys. Status Solidi (A) 2005, 202, R13–R15. [Google Scholar] [CrossRef]
- Eglitis, R.I. Theoretical prediction of the 5 V rechargeable Li ion battery using Li2CoMn3O8 as a cathode. Phys. Scr. 2015, 90, 094012. [Google Scholar] [CrossRef]
- Yi, T.F.; Zhu, Y.; Zhu, R. Density functional theory study of lithium intercalation for 5 V LiNi0.5Mn1.5O4 cathode materials. Solid State Ionics 2008, 179, 2132–2136. [Google Scholar] [CrossRef]
- Linden, D.; Reddy, T. Handbook of Batteries, 3rd ed.; McGraw-Hill: New York, NY, USA, 2002; Chapter 35. [Google Scholar]
- Padhi, A.; Nanjundaswamy, K.; Goodenough, J. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. J. Electrochem. Soc. 1997, 144, 1188–1194. [Google Scholar] [CrossRef]
- Li, G.; Azuma, H.; Tohda, M. LiMnPO4 as the cathode for lithium batteries. Electrochem. Solid-State Lett. 2002, 5, A135–A137. [Google Scholar] [CrossRef]
- Yamada, A.; Yonemura, M.; Takei, Y.; Sonoyama, N. Comparative kinetic study of olivine LixMPO4 (M = Fe, Mn). J. Electrochem. Soc. 2004, 151, A1352–A1356. [Google Scholar]
- Wolfenstine, J.; Allen, J. Ni3+/Ni2+ redox potential in LiNiPO4. J. Power Sources 2005, 142, 389–390. [Google Scholar] [CrossRef]
- Bramnik, N.N.; Bramnik, K.G.; Baehtz, C.; Ehrenberg, H. Study of the effect of different synthesis routes on Li extraction-insertion from LiCoPO4. J. Power Sources 2005, 145, 74–81. [Google Scholar] [CrossRef]
- Minakshi, M.; Kandhasamy, S. Utilizing active multiple dopants (Co and Ni) in olivine LiMnPO4. Curr. Opin. Solid State Mater. Sci. 2012, 16, 163–167. [Google Scholar] [CrossRef]
- Li, J.; Garlea, V.O.; Zarestky, J.L.; Vaknin, D. Spin-waves in antiferromagnetic single-crystal LiFePO4. Phys. Rev. B 2006, 73, 024410. [Google Scholar] [CrossRef]
- Zhou, F.; Kang, K.; Maxisch, T.; Ceder, G.; Morgan, D. The electronic structure and band gap of LiFePO4 and LiMnPO4. Solid State Commun. 2004, 132, 181–186. [Google Scholar] [CrossRef]
- Zhou, F.; Cococcioni, M.; Kang, K.; Ceder, G. The Li intercalation potential of LiMPO4 and LiMSiO4 olivines with M = Fe, Mn, Co, Ni. Electrochem. Commun. 2004, 6, 1144–1148. [Google Scholar] [CrossRef]
- Zhou, F.; Marinetti, C.; Cococcioni, M.; Morgan, D.; Ceder, G. Phase separation in LixFePO4 induced by correlation effects. Phys. Rev. B 2004, 69, 201101. [Google Scholar] [CrossRef]
- Zhou, F.; Marinetti, C.; Cococcioni, M.; Morgan, D.; Ceder, G. First-principles prediction of redox potentials in transition-metal compounds with LDA + U. Phys. Rev. B 2004, 70, 235121. [Google Scholar] [CrossRef]
- Anisimov, V.; Izyumov, Y. Electronic Structure of Strongly Correlated Materials; Springer-Verlag Berlin Heidelberg: Heidelberg, German, 2010. [Google Scholar]
- Lin, H.; Wen, Y.; Zhang, C.; Zhang, L.; Huang, Y.; Shan, B.; Chen, R. A GGA + U study of lithium diffusion in vanadium doped LiFePO4. Solid State Commun. 2012, 152, 999–1003. [Google Scholar] [CrossRef]
- Inseok, S.; Senthilkumar, B.; Kim, K.H.; Kim, J.K.; Kim, Y.; Ahn, J.H. Atomic structural and electrochemical impact of Fe substitution on nano porous LiMnPO4. J. Power Sources 2016, 320, 59–67. [Google Scholar]
- Ni, J.; Gao, L. Effect of copper doping on LiMnPO4 prepared via hydrothermal route. J. Power Sources 2011, 196, 6498–6501. [Google Scholar] [CrossRef]
- Oh, S.; Jung, H.G.; Yoon, C.S.; Myung, S.; Chen, Z.; Amine, K.; Sun, Y.K. Enhanced electrochemical performance of carbon-LiMn1-xFexPO4 nanocomposite cathode for lithium-ion batteries. J. Power Sources 2011, 196, 6924–6928. [Google Scholar] [CrossRef]
- Fang, H.; Dai, E.; Yang, B.; Yao, Y.; Ma, W. LiMn0.8Fe0.19Mg0.01PO4/C as a high performance cathode material for lithium ion batteries. J. Power Sources 2012, 204, 193–196. [Google Scholar] [CrossRef]
- Wang, K.; Maljuk, A.; Blum, C.; Kolb, T.; Jähne, C.; Neef, C.; Grafe, H.; Giebeler, L.; Wadepohl, H.; Meyer, H.; et al. Growth, characterization, and magnetic properties of a Li(Mn,Ni)PO4 single crystal. J. Cryst. Growth 2014, 386, 16–21. [Google Scholar] [CrossRef]
- Yang, G.; Ni, H.; Liu, H.; Gao, P.; Ji, H.; Roy, S.; Pinto, J.; Jiang, X. The doping effect on the crystal structure and electrochemical properties of LiMnxM1-xPO4 (M = Mg, V, Fe, Co, Gd). J. Power Sources 2011, 196, 4747–4755. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B Rapid Commun. 1990, 41, 7892. [Google Scholar] [CrossRef]
- Materialscloud. Available online: http://materialscloud.org (accessed on 15 March 2014).
- Lejaeghere, K.; Bihlmayer, G.; Björkman, T.; Blaha, P.; Blügel, S.; Blum, V.; Caliste, D.; Castelli, I.E.; Clark, S.J.; Dal Corso, A.; et al. Reproducibility in density functional theory calculations of solids. Science 2016, 351, aad3000. [Google Scholar] [CrossRef] [PubMed]
- Dudarev, S.L.; Botton, G.A.; Savrasov, S.Y.; Humphreys, C.J.; Sutton, A.P. Electron energy loss spectra and the structural stability of nickel oxide: An LDA+U study. Phys. Rev. B 1998, 57, 1505–1509. [Google Scholar] [CrossRef]
- Cococcioni, M.; de Gironcoli, S. Linear response approach to the calculation of the effective interaction parameters in the LDA+U method. Phys. Rev. B 2005, 71, 035105. [Google Scholar] [CrossRef]
- Park, S.G.; Magyari-Köpe, B.; Nishi, Y. Electronic correlation effects in reduced rutile TiO2 within the LDA+U method. Phys. Rev. B 2010, 82, 115109. [Google Scholar] [CrossRef]
- Dathar, G.K.P.; Sheppard, D.; Stevenson, K.J.; Henkelman, G. Calculations of Li-ion diffusion in olivine phosphates. Chem. Mater. 2011, 23, 4032–4037. [Google Scholar] [CrossRef]
- Xin, X.-G.; Shen, J.-Q.; Shi, S.-Q. Structural and magnetic properties of LiNi0.5Mn1.5O4 and LiNi0.5Mn1.5O4-δ spinels: A first-principles study. Chin. Phys. B 2012, 21, 128202. [Google Scholar] [CrossRef]
- Santoro, R.P.; Newnham, R.E. Antiferromagnetism in LiFePO4. Acta Crystallogr. 1967, 22, 344–347. [Google Scholar] [CrossRef]
- Tang, P.; Holzwarth, N.A.W. Electronic structure of FePO4 , LiFePO4, and related materials. Phys. Rev. B 2003, 68, 165107. [Google Scholar] [CrossRef]
- Broyden, C.G. The convergence of a class of double-rank minimization algorithms. J. Inst. Math. Appl. 1970, 6, 76–90. [Google Scholar] [CrossRef]
- Fletcher, R. A new approach to variable metric algorithms. Computer J. 1970, 13, 317–322. [Google Scholar] [CrossRef]
- Goldfarb, D. A family of variable metric updates derived by variational means. Math. Comput. 1970, 24, 23–26. [Google Scholar] [CrossRef]
- Shanno, D.F. Conditioning of quasi-Newton methods for function minimization. Math. Comput. 1970, 24, 647–656. [Google Scholar] [CrossRef]
- Aydinol, M.K.; Kohan, A.F.; Ceder, G.; Cho, K.; Joannopoulos, J. Ab initio study of lithium intercalation in metal oxides and metal dichalcogenides. Phys. Rev. B 1997, 56, 1354–1365. [Google Scholar] [CrossRef]
- Henkelman, G.; Uberuaga, B.; Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef]
- Hahn, T. International Tables for Crystallography, Volume A: Space Group, 5th ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2002. [Google Scholar]
- Morgan, D.; der Ven, A.V.; Ceder, G. Li Conductivity in LixMPO4 (M = Mn, Fe, Co, Ni Olivine Materials). Electrochem. Solid-State Lett. 2004, 7, A30–A32. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Li, J.; Gao, J.; Jiang, C.; He, X. First-principles study of doping in LiMnPO4. Int. J. Electrochem. Sci. 2012, 7, 3362–3370. [Google Scholar]
- Haas, P.; Tran, F.; Blaha, P. Calculation of the lattice constant of solids with semilocal functionals. Phys. Rev. B 2009, 79, 085104. [Google Scholar] [CrossRef]
- Kwon, N.H.; Fromm, K.M. Enhanced electrochemical performance of <30 nm thin LiMnPO4 nanorods with a reduced amount of carbon as a cathode for lithium ion batteries. Electrochim. Acta 2012, 69, 38–44. [Google Scholar]
- García-Moreno, O.; Alvarez-Vega, M.; García-Alvarado, F.; García-Jaca, J.; Gallardo-Amores, J.M.; Sanjuán, M.L.; Amador, U. Influence of the structure on the electrochemical performance of lithium transition metal phosphates as cathodic materials in rechargeable lithium batteries: A new high-pressure form of LiMPO4 (M = Fe and Ni). Chem. Mater. 2001, 13, 1570–1576. [Google Scholar] [CrossRef]
- Wang, C.; Hong, J. Ionic/electronic conducting characteristics of LiFePO4 cathode materials. Electrochem. Solid-State Lett. 2007, 10, A65–A69. [Google Scholar] [CrossRef]
- Sun, C.; Zhou, Z.; Xu, Z.; Wang, D.; Wei, J.; Bian, X.; Yan, J. Improved high-rate charge/discharge performances of LiFePO4/C via V-doping. J. Power Sources 2009, 193, 841–845. [Google Scholar] [CrossRef]
- Wang, K.; Cai, R.; Yuan, T.; Yu, X.; Ran, R.; Shao, Z. Process investigation, electrochemical characterization and optimization of LiFePO4/C composite from mechanical activation using sucrose as carbon source. Electrochim. Acta 2009, 54, 2861–2868. [Google Scholar] [CrossRef]
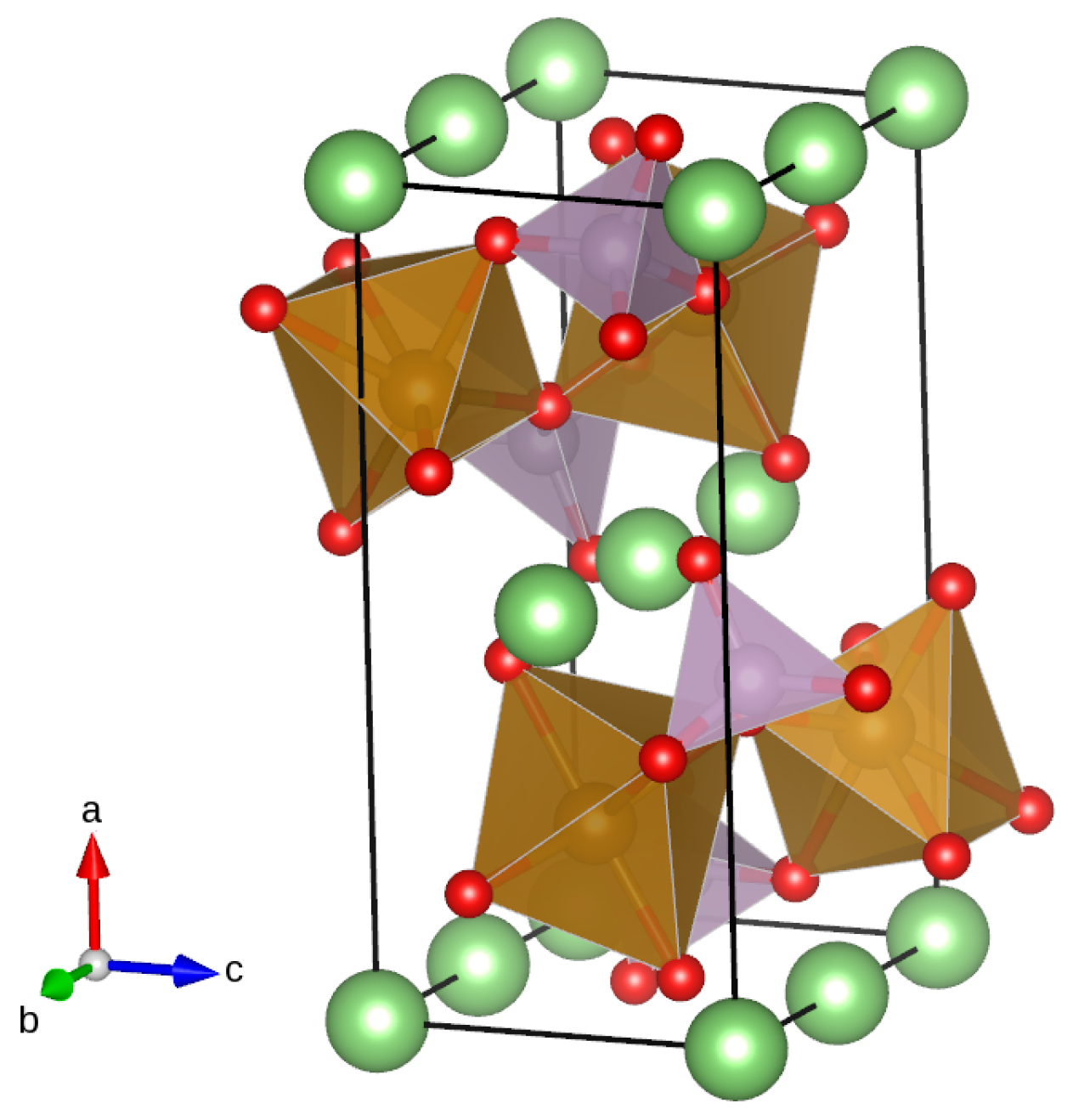
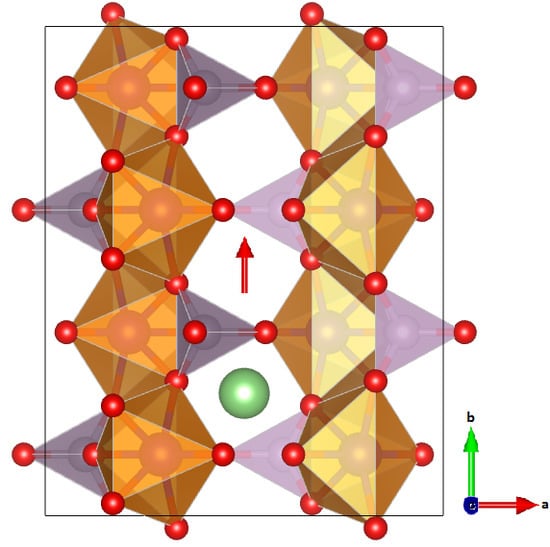
| Compound | a (Å) | b (Å) | c (Å) | V (Å) |
|---|---|---|---|---|
| LiMnPO (Exp.) | 10.44 | 6.09 | 4.75 | 302.00 |
| LiMnPO | 10.53 | 6.13 | 4.75 | 306.81 |
| LiMnNiPO | 10.42 | 6.07 | 4.73 | 299.23 |
| LiMnCoPO | 10.44 | 6.10 | 4.74 | 301.59 |
| MnPO (Exp.) | 9.69 | 5.79 | 4.78 | 274.67 |
| MnPO | 9.81 | 6.03 | 4.88 | 288.44 |
| MnNiPO | 9.79 | 5.98 | 4.88 | 285.33 |
| MnCoPO | 9.79 | 5.94 | 4.83 | 280.84 |
| Material | Band Gap (eV) | Insertion Voltage (V) | Cell Magnetization () |
|---|---|---|---|
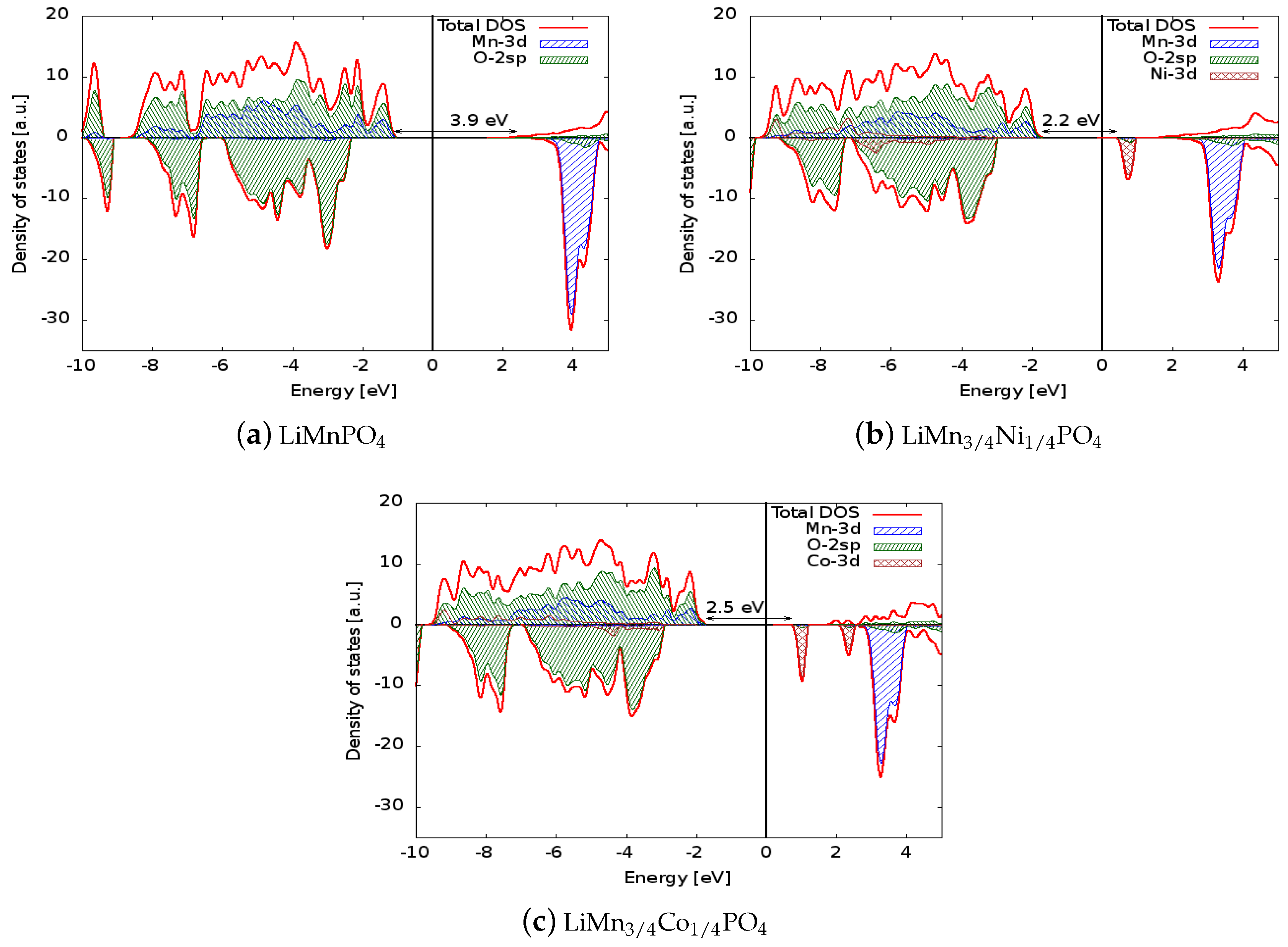
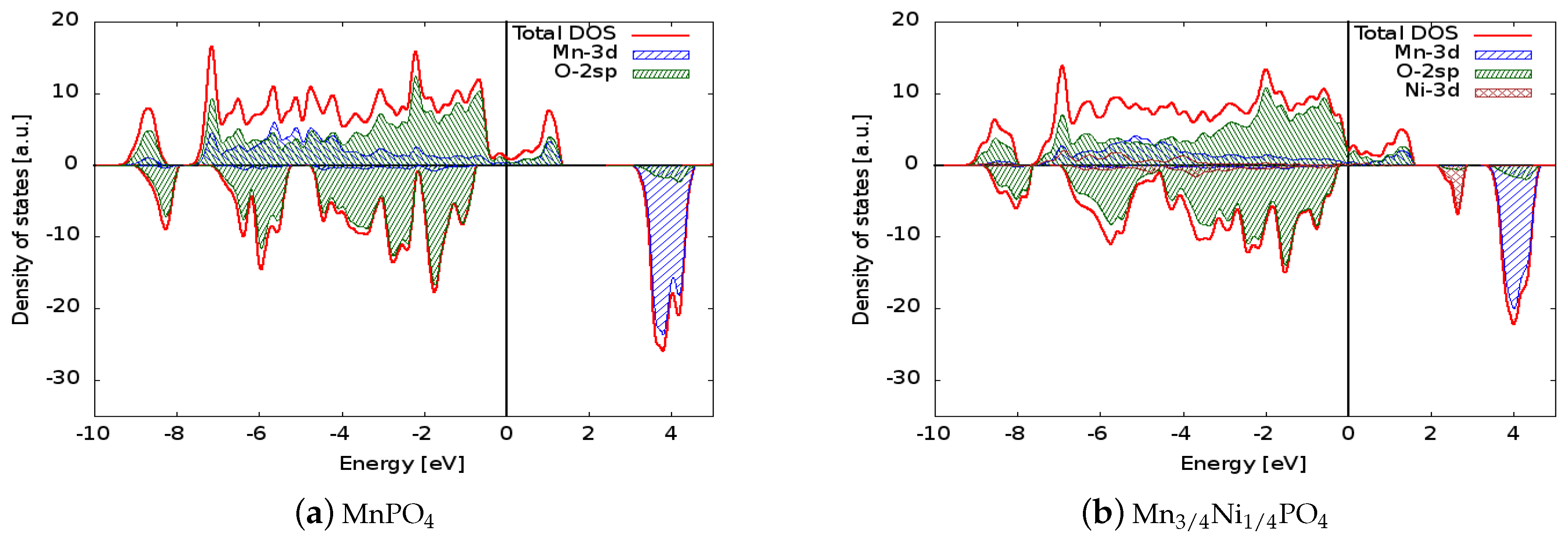
| LiMnPO | 3.9 | 4.07 | 5.0 |
| LiMnPO (Exp.) | 3.8 [16] | 4.1 [10] | - |
| LiMnNiPO | 2.2 | 4.35 | 4.25 |
| LiMnCoPO | 2.5 | 4.30 | 4.5 |
| Material | E (eV) |
|---|---|
| LiMnPO | 0.40 |
| MnPO | 0.44 |
| LiMnNiPO | 0.49 |
| MnNiPO | 0.44 |
| LiMnCoPO | 0.43 |
| MnCoPO | 0.43 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sgroi, M.F.; Lazzaroni, R.; Beljonne, D.; Pullini, D. Doping LiMnPO4 with Cobalt and Nickel: A First Principle Study. Batteries 2017, 3, 11. https://doi.org/10.3390/batteries3020011
Sgroi MF, Lazzaroni R, Beljonne D, Pullini D. Doping LiMnPO4 with Cobalt and Nickel: A First Principle Study. Batteries. 2017; 3(2):11. https://doi.org/10.3390/batteries3020011
Chicago/Turabian StyleSgroi, Mauro Francesco, Roberto Lazzaroni, David Beljonne, and Daniele Pullini. 2017. "Doping LiMnPO4 with Cobalt and Nickel: A First Principle Study" Batteries 3, no. 2: 11. https://doi.org/10.3390/batteries3020011
APA StyleSgroi, M. F., Lazzaroni, R., Beljonne, D., & Pullini, D. (2017). Doping LiMnPO4 with Cobalt and Nickel: A First Principle Study. Batteries, 3(2), 11. https://doi.org/10.3390/batteries3020011