3.1. Batch Process
Two different conditions (Ni-1 and Ni-2) were employed in the batch process [
15]. While Ni-1 has a composition that incorporates pure Ni as the cation source, which exhibits an exclusively β-Ni(OH)
2 structure, Ni-2 is doped with 14 at % Al and show a typical α-Ni(OH)
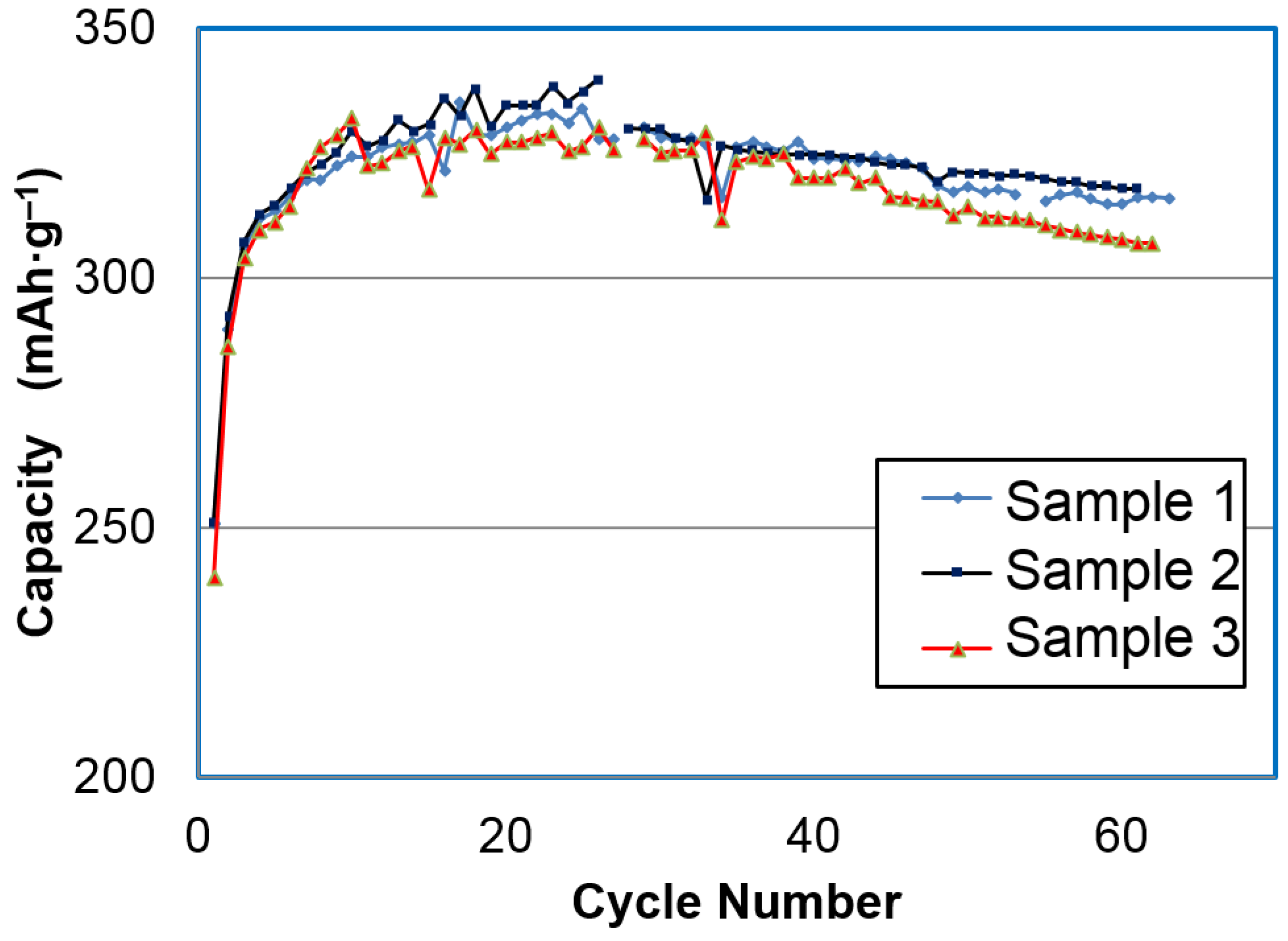
2 structure. The discharge capacities for the three samples from the Ni-2 process are shown in
Figure 3. It took approximately 15 cycles to stabilize the capacity. The highest discharge capacity obtained with a 25 mA·g
−1 rate was 346 mAh·g
−1 at the 25th cycle.
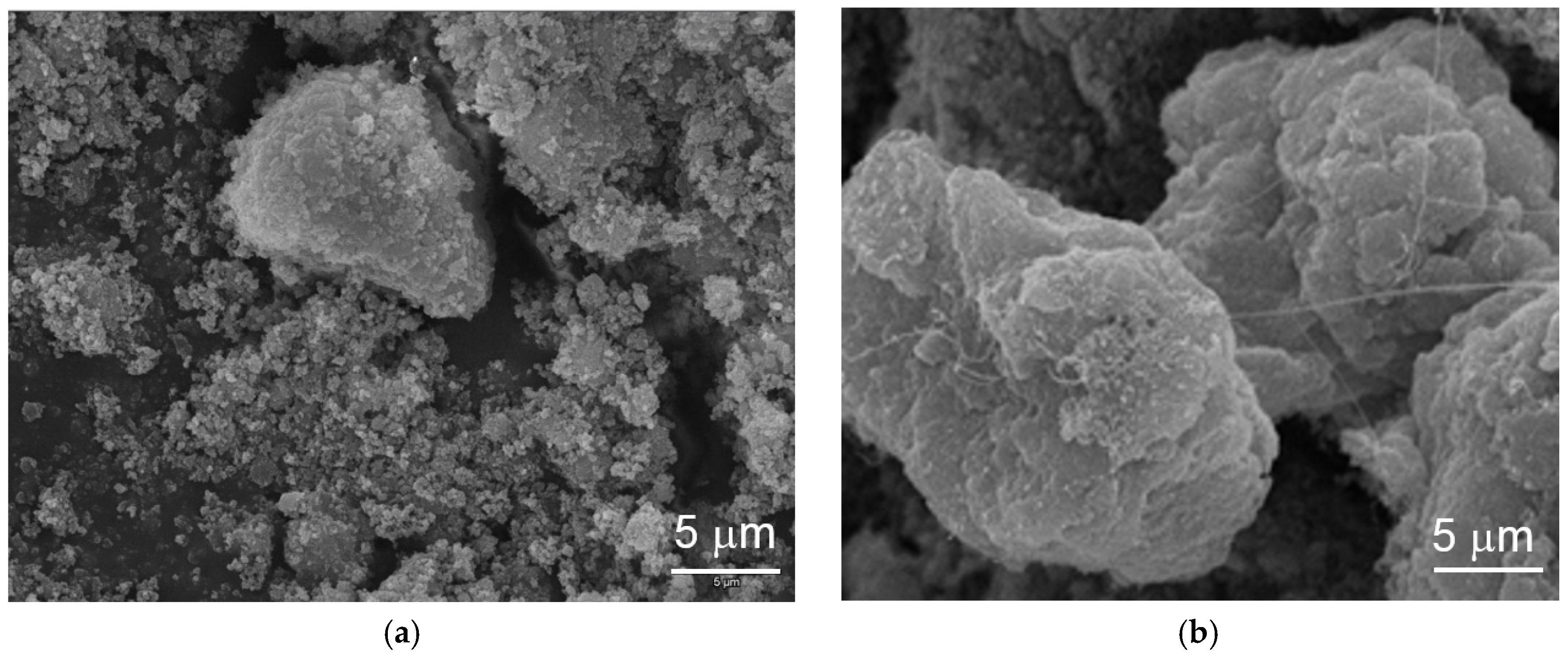
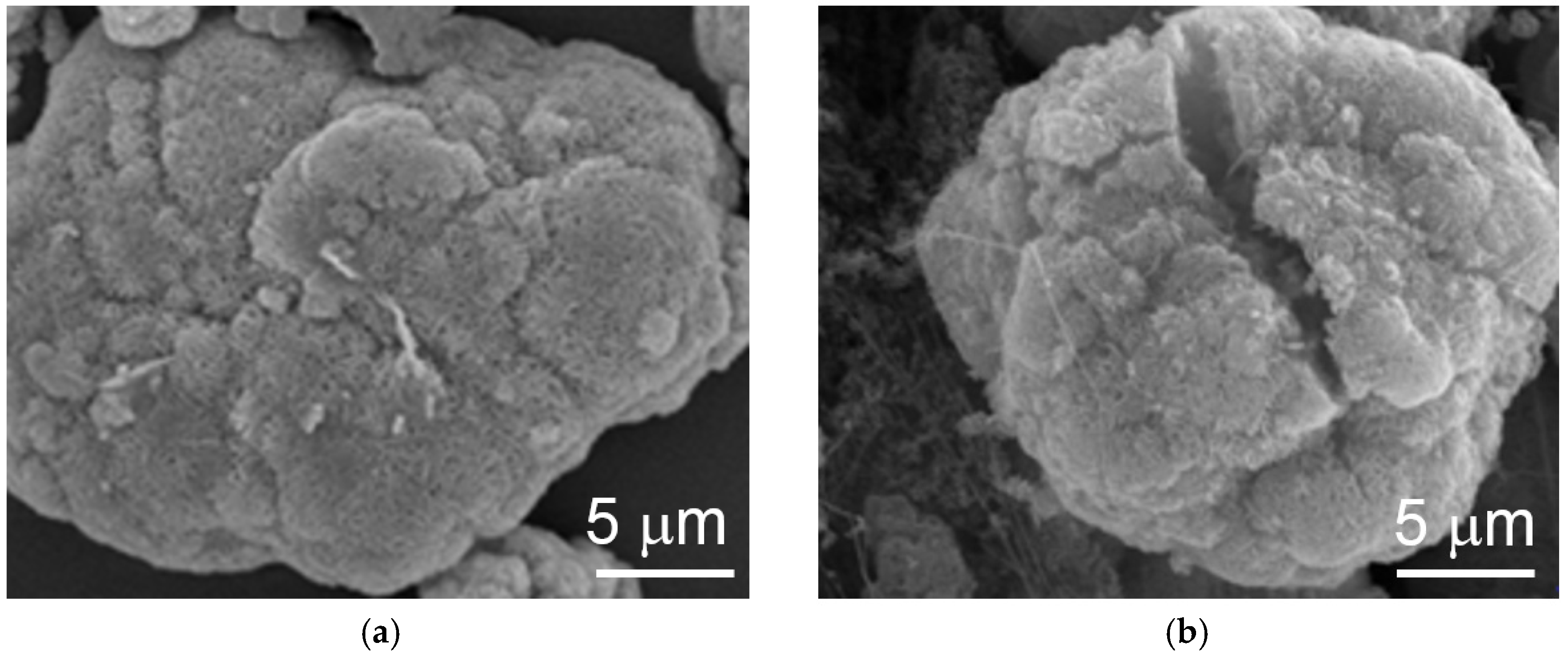
The topologies of the particles before and after electrochemical cycling were studied by SEM and the corresponding micrographs are shown in
Figure 4. The pristine material shows a granular structure and conglomerates into larger particles with cycling. The growth in size with cycling retards cracking of the particle, due to the lattice expansion from β-Ni(OH)
2 to α-Ni(OH)
2, which is a common failure mode for capacity degradation in α-Ni(OH)
2 [
12,
78]. Small amounts of capacity degradation can be seen after 25 cycles due to partial electrode disintegration in the flooded-configuration.
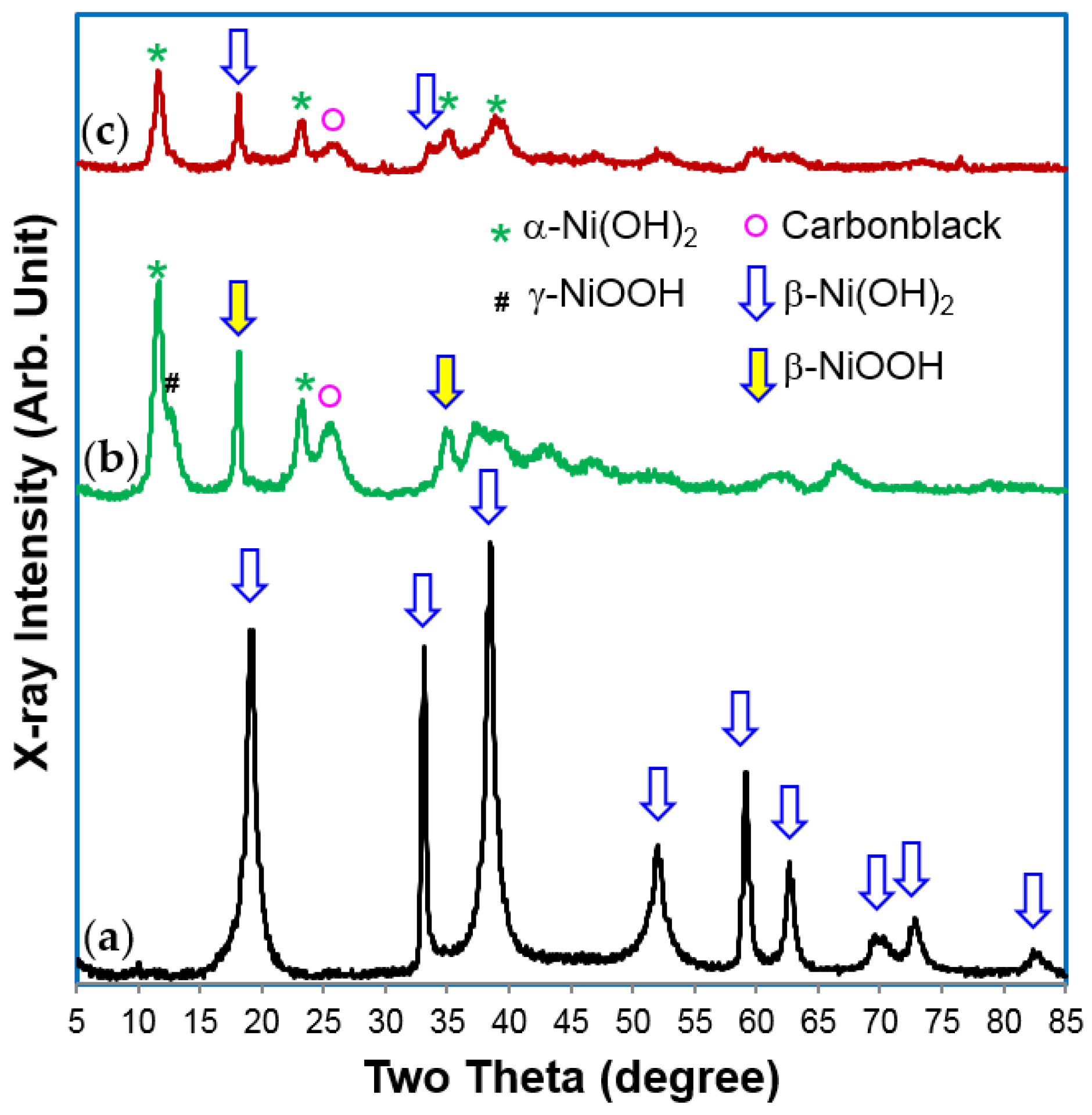
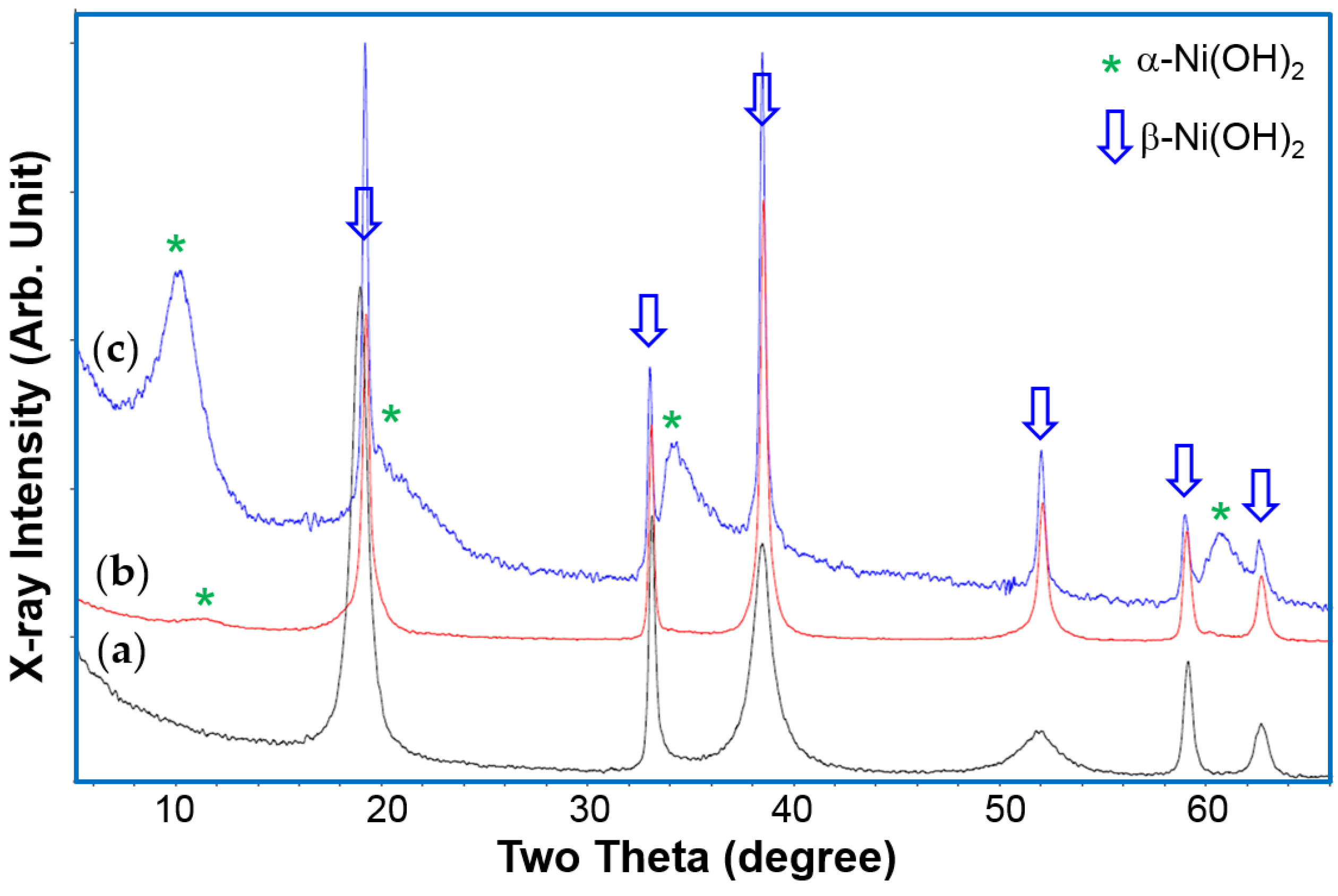
XRD analysis results indicate a β-Ni(OH)
2 structure for the material before cycling, which turned into an α-Ni(OH)
2–predominant structure after cycling (
Figure 5). The different peak widths of the XRD patterns (especially in
Figure 5a) are the products of preferential growth of Ni(OH)
2 flakes on the
ab-plane and various stacking faults [
79]. The product may be further improved (especially the cycle stability) for battery applications, but the process itself is complicated and currently limited to a laboratory scale. Mass production of the batch process will not be cost-effective.
3.2. Continuous Production
In the Robust Affordable Next Generation Energy Storage System (RANGE) program funded by the US Department of Energy, a series of research efforts were dedicated to the synthesis of a durable and high capacity nickel hydroxide using the CSTR method [
15]. We first confirmed that Al is effective in promoting nucleation of the α-Ni(OH)
2 phase. The XRD patterns from three samples with 0, 4, and 20 at % Al are shown in
Figure 6. While the pristine Al-free sample shows a pure β-Ni(OH)
2 structure (
Figure 6a), the sample with 4% Al shows a small hint of α-Ni(OH)
2, and the third sample, with 20% Al-content, is dominated by the α-Ni(OH)
2 structure even without electrochemical cycling. For the rest of the materials, two chemistries were chosen for comparison: WM02 and WM12 (compositions listed in
Table 3). Their preparation parameters and key performances are also listed in
Table 3.
The results from the capacity measurement of WM02, WM12, and AP50 (a control sample of β-Ni(OH)
2 with a cation composition of Ni
0.91Zn
0.045Co
0.045) are presented in
Figure 7. Both WM02 and WM12 show a higher discharge capacity than AP50. WM02 has a higher initial capacity, but also a more severe degradation in capacity. The XRD patterns of WM02 and WM12 in pristine, charged, and discharged states are compared in
Figure 8. According to the XRD results, both WM02 and WM12 started with a pure β-Ni(OH)
2 structure and are converted into α-Ni(OH)
2-predominated and α/β mixed Ni(OH)
2 state, respectively. The XRD peaks in the pristine WM12 are broader than those in the pristine WM02, indicating a smaller crystallite (platelet) in WM12, which is confirmed by the TEM work showed later in this session.
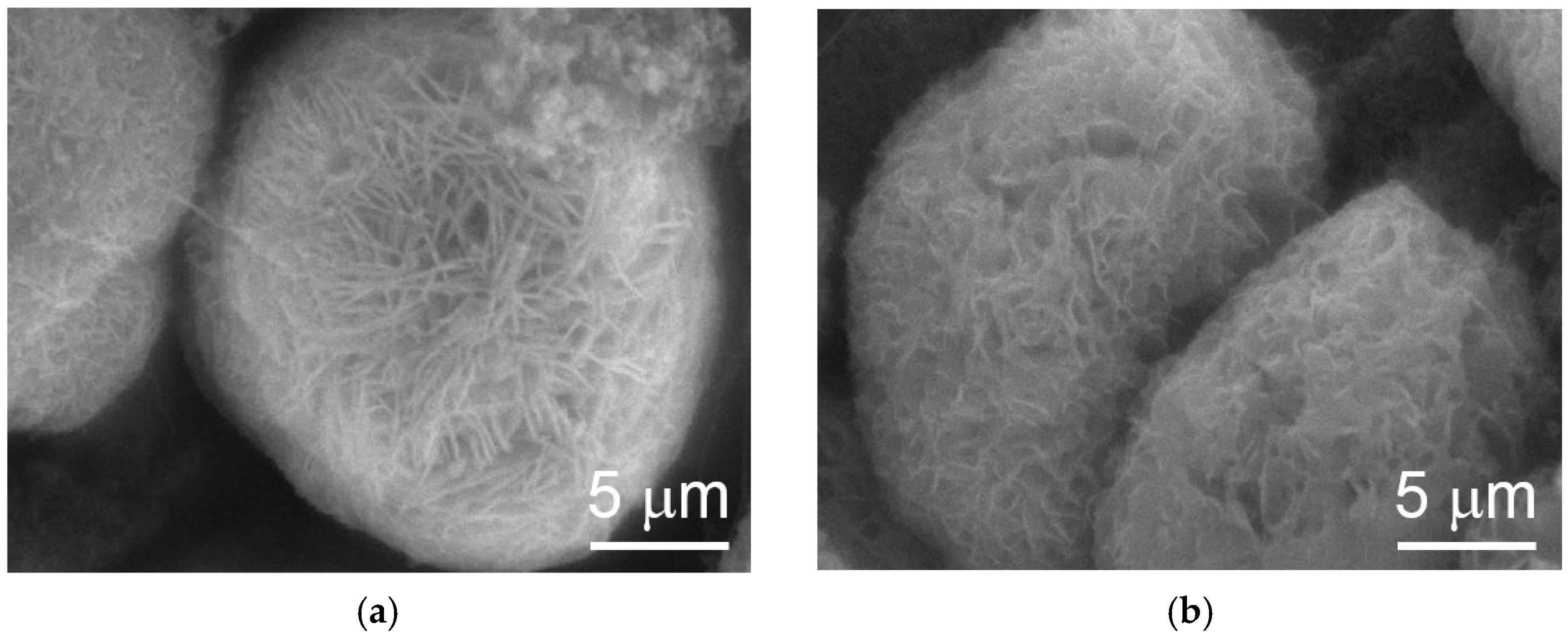
SEM analysis results show that cracking in the cycled WM02 spherical particles is due to swelling caused by the β-to-α transition (
Figure 9b) and that WM12 maintained the same shape after 20 cycles (
Figure 10). In addition, the surface morphologies of the two materials are different. While WM02 spherical particles have a more compact surface with a granular texture, WM12 spherical particles have a less dense surface with crystallite plates aligning perpendicular to the surface. The surface area and pore density of WM02 are smaller than those of WM12 with the same average pore diameter (
Table 3).
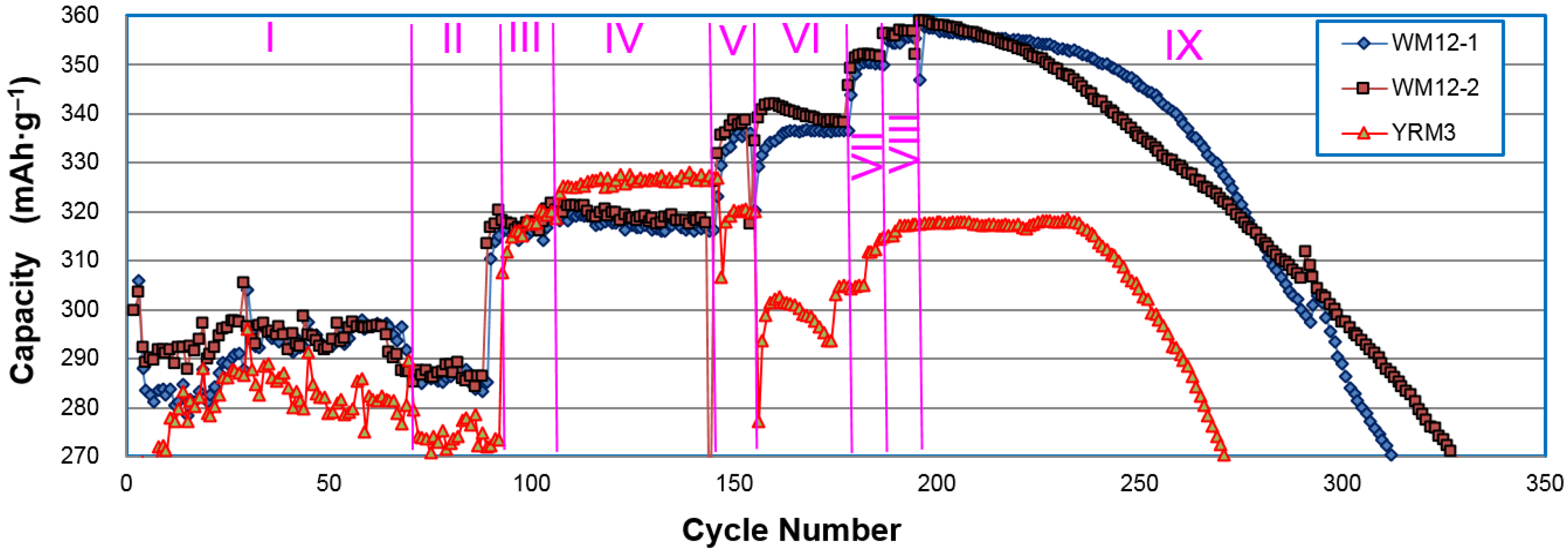
In another comparison test, three samples (two WM12 and one control β-Ni(OH)
2) (YRM3: Ni
0.93Co
0.02Al
0.05(OH)
2) underwent nine different charge/discharge conditions (
Table 4) and the obtained capacities are plotted in
Figure 11. While the increases in charge current density from 50 to 75 and 125 mA·g
‒1 in stages III and IV boosted capacities for both WM12 and β-Ni(OH)
2, further increase in the charge current density (stage V) improved the capacity of WM12, but not β-Ni(OH)
2. It seems that WM12 benefits, but β-Ni(OH)
2 deteriorates with faster charge. From this comparison, the superiorities in the discharge capacity and cycle stability of WM12, compared to the regular material, are validated. The final failure mechanisms for both materials (WM12 and the β-Ni(OH)
2) are the same: electrode disintegration due to particle pulverization.
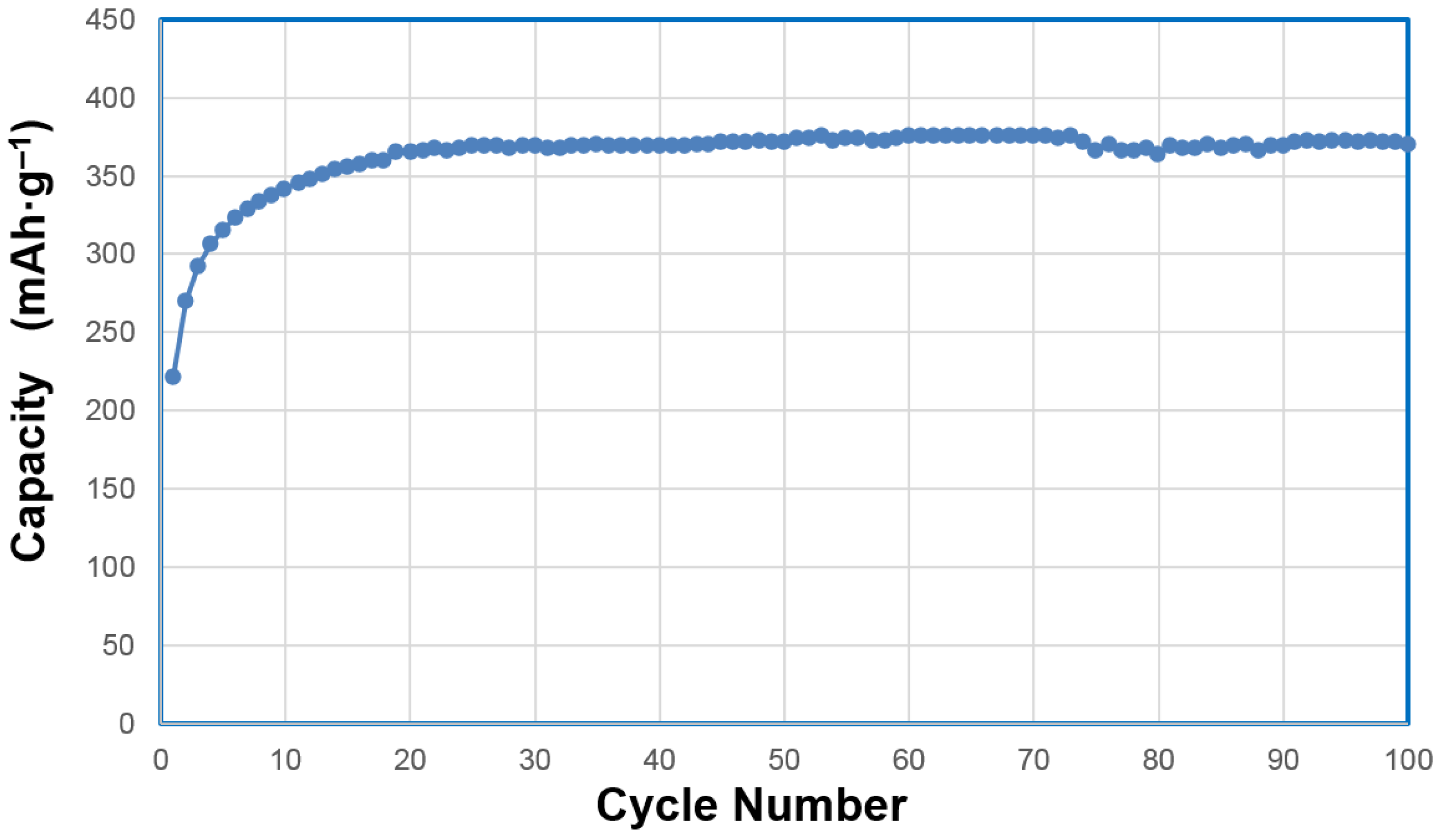
In a separate cycle life experiment, we performed a 100 mA·g
‒1 rate charge for 5.5 h and discharged at the same rate. Results are shown in
Figure 12. The highest capacity of 376 mAh·g
‒1 was obtained during the 61th cycle and the capacity at the 100th cycle was 371 mAh·g
‒1. With the success in the half-cell experiment (with 50% additional binder and electrical conduction enhancer), we began the full-cell measurement of WM12 and WM12 in the newly developed pouch-type Ni/MH battery [
15]. Only 10% binder and electrical conduction enhancer were added in the positive electrode and the capacity results, based on the total material weight (active plus additives), are plotted in
Figure 13. Discharge capacities of 329 mAh·g
‒1 and 311 mAh·g
‒1 were obtained for the positive electrodes of the WM12 and WM02 full cells, respectively. From this point, further tests on the full cells are planned and more results will be reported in the future, especially for the phase stability under storage condition since α-Ni(OH)
2 is known to have an aging issue when stored in an alkaline solution [
30]. It is obvious that the same WM12 material required less activation cycles and achieved a lower capacity in the sealed-cell configuration than that in the half-cell (6 versus 20). This is due to the reliance of the β–NiOOH to γ–NiOOH transition on the amount of over-charge. In the half-cell operated in the open atmosphere, the over-charging process has to compete with oxygen gas evolution and thus is less effective, compared to reactions operated in the sealed-cell configuration (more activation cycle is needed). The difference in the maximum capacities between two measurements is related to the expansion of unit cell that occurs in the β–NiOOH to γ–NiOOH transition. In the half-cell testing, the electrode was allowed to expand and, therefore, more NiOOH was converted, which differs from the limited space available in the sealed-cell.
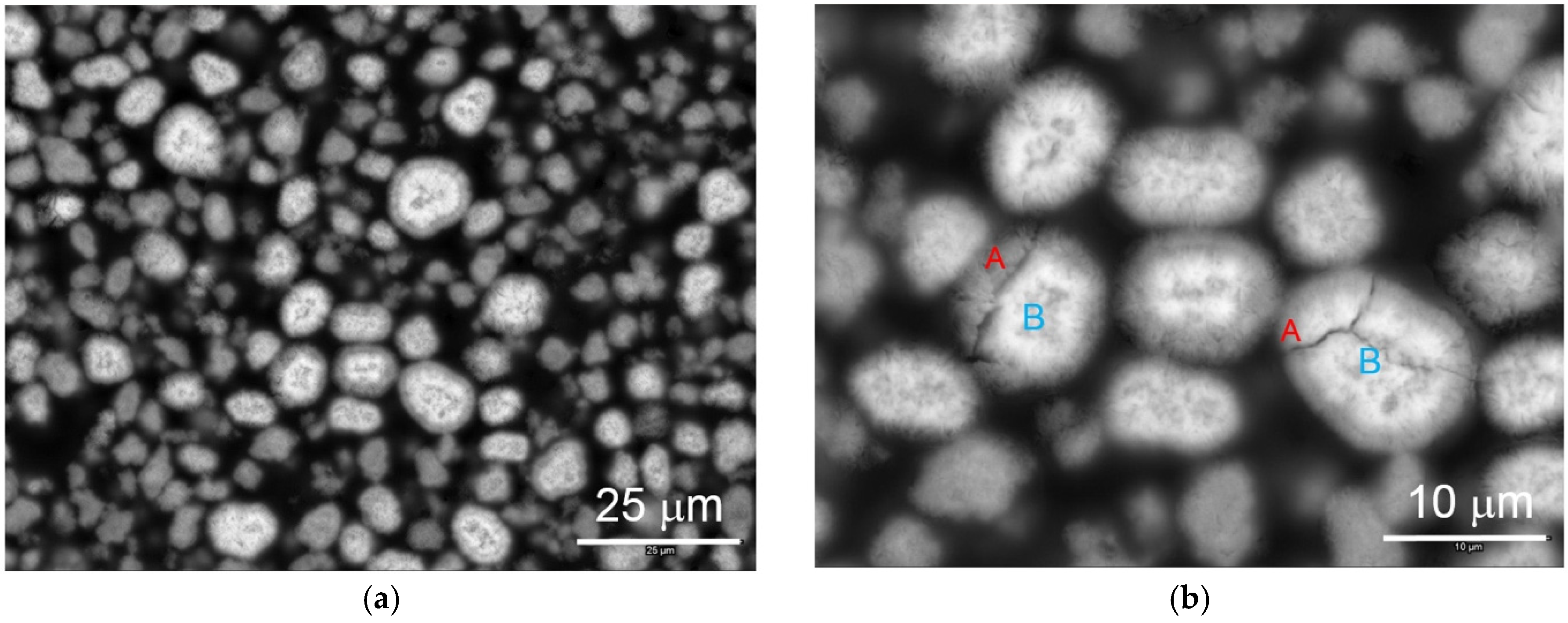
The microstructure of WM12 was studied by both SEM and TEM. Cross-section SEM backscattering electron images of activated WM12 at different magnifications are shown in
Figure 14. Different contrasts can be observed in the shell (A, darker) and core (B, lighter). EDS analysis results show that the surface region has a higher Al-content (ca. 4 at %) than the core region (ca. 1.5 at %) [
15]. The core–shell structure was produced in the CSTR with a differential precipitation of Al in the tank (
Figure 2b). In the nucleation stage, near the bottom of the tank, less Al becomes crystallite due to its relatively large size and, thus, an Al-lean core forms. As the particles are moved into the upper portion of the reactor by the stirring blade, they grow in size with a higher Al-content in the shell part of the particle. Materials produced at this stage are still β-Ni(OH)
2. Later, during electrochemical formation, the shell, with a relatively higher Al-content, develops a β-Ni(OH)
2 structure.
The microstructure of WM12 particle’s shell was further investigated by TEM. A representative TEM micrograph is shown in
Figure 15a. The high-resolution TEM images taken from areas ⓑ (brighter) and ⓒ (darker) are shown in
Figure 15b,c, respectively. Area ⓑ has a smaller inter-planar distance is β-Ni(OH)
2, while area ⓒ, which has a larger inter-planar discharge, is α-Ni(OH)
2. The electron diffraction from a representative selective 10-μm region, which covers the most of the particle shown in
Figure 15a, is shown in
Figure 15d. The integrated electron diffraction intensities were collected from the diffraction pattern for areas ⓐ‒ⓔ. For the convenience of comparison between the TEM and XRD results, the distance in reciprocal space obtained from TEM electron diffraction has been converted to a degree based system on the wave length of a Cu K
α X-ray and the results are shown in
Figure 15e with the conversion to the standard XRD suing Cu K
α as the radiation source. Although the electron density plot is not identical to the XRD pattern, due to different scattering factors between X-rays and electron beams, the main features from a and b can still be distinguished and areas ⓐ‒ⓔ have been identified as β, β, β, β, and mixed α/β structures, respectively. Therefore, we conclude that the shell region of WM12 is composed of nano-sized α-Ni(OH)
2 imbedded in a β-Ni(OH)
2 matrix, which helps to distribute the stress from the lattice expansion during the α-β transition. The broader XRD peaks in the pristine WM12 indicate a small crystallite form, even before the α-β transition occurs.
3.3. Low-Temperature Formation
It is well known that applying overcharge to a positive-limited Ni/MH battery will result in oxygen gas evolution. During normal operation of Ni/MH batteries, the produced oxygen gas will recombine with the hydrogen stored in the negative electrode, forming water and generating heat. A charging process in the Ni(OH)
2 based rechargeable alkaline battery is always a competition between oxidizing Ni(II) into Ni(III) and oxygen gas evolution. At room temperature, the oxygen evolution potential is raised by the co-precipitation of Co and Zn into spherical particles and, therefore, the charging process can be completed without any oxygen gas generation. At higher temperatures, where the potential of oxygen evolution is significantly reduced, the regular Ni-electrode cannot be charged fully and special high temperature Ni-electrodes have been developed to lower the oxidation potential of the Ni(II)/Ni(III) reaction [
80]. When the cell is overcharged, part of its β-NiOOH will be converted into γ-NiOOH (see Bode’s diagram in
Figure 1), but the conversion efficiency is low because most of the overcharge is consumed during oxygen gas evolution. If the overcharge is performed at a relative low temperature, then the β–γ conversion happens at a higher rate. This concept initiated the following experiment. Ten AA-cells were made from regular AB
5 and YRMS (Ni
0.93Co
0.02Al
0.05(OH)
2) as the active materials in the negative and positive electrodes, respectively. These cells were exposed to a low-temperature overcharge process (C/10 rate at 10 °C for 30 days). Their discharge capacities before and after the treatment are listed in
Table 5. More than a 15% increase in the discharge capacity was observed. The XRD patterns before and after the low-temperature treatment show a transition from 89% β-NiOOH/11% γ-NiOOH to 11% β-NiOOH/89% γ-NiOOH (
Figure 16). This validates the effectiveness of low-temperature formation for α/γ-NiOOH. After disassembling the cell, we found swelling in the positive electrode due to the formation of a α/γ-NiOOH phase with an enlarged unit cell (about 74% [
5]) from the insertion of a water layer between the NiOOH layers (
Figure 1). The swelling of the positive electrode causes an approximate 8% increase in the internal resistance (
Table 5) due to the breakdown in the Co- conductive network [
12]. Future work will be focused on a solution that addresses this electrode swelling. For example, swelling could be alleviated through a coating of CoOOH, Yb(OH)
3, or both on the spherical particles before electrode fabrication [
12].