Ni(II) Dimers of NNO Donor Tridentate Reduced Schiff Base Ligands as Alkali Metal Ion Capturing Agents: Syntheses, Crystal Structures and Magnetic Properties
Abstract
:1. Introduction
2. Results and Discussion
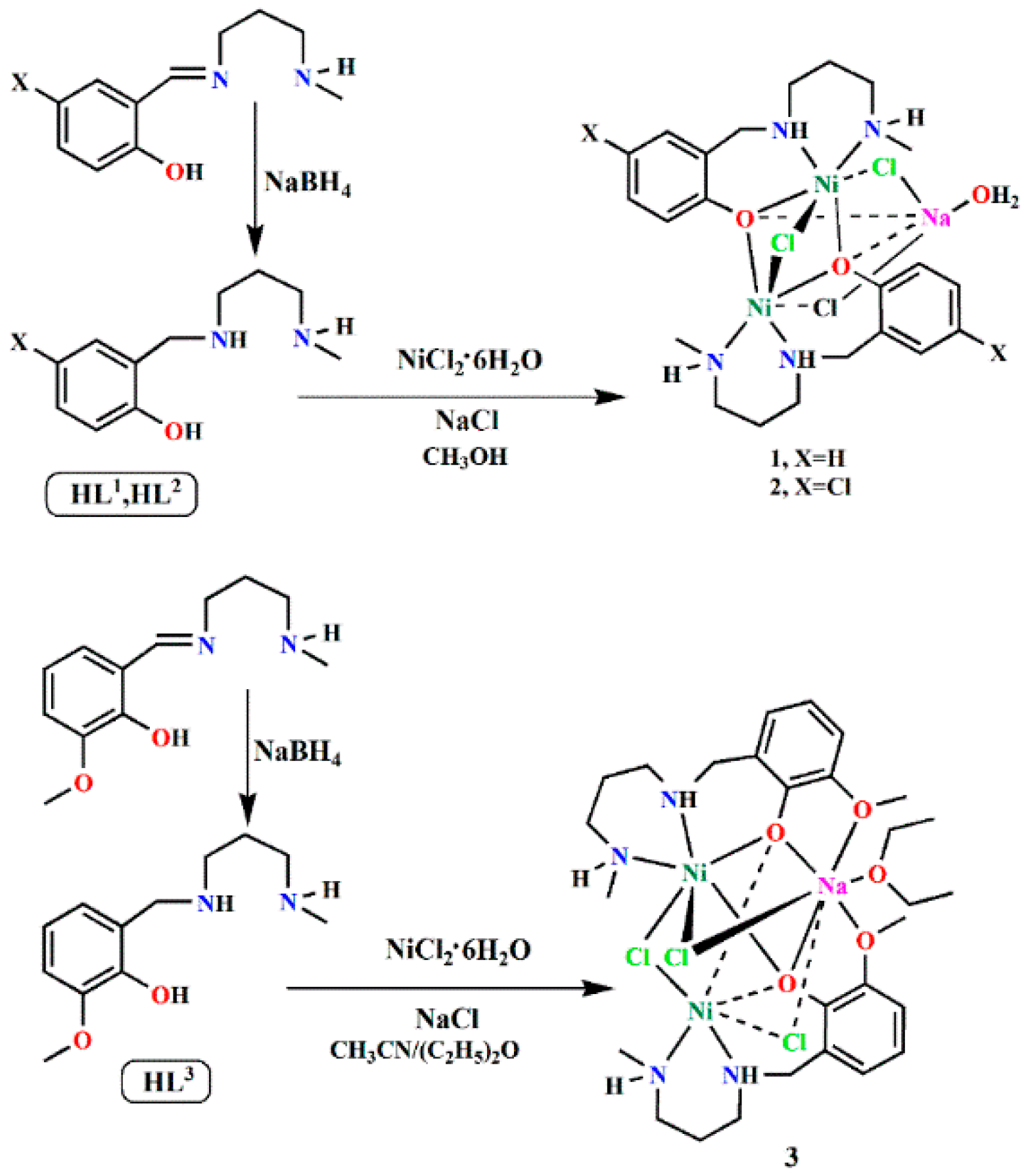
2.1. Syntheses of the Complexes
2.2. IR and UV–Vis Spectra of the Complexes
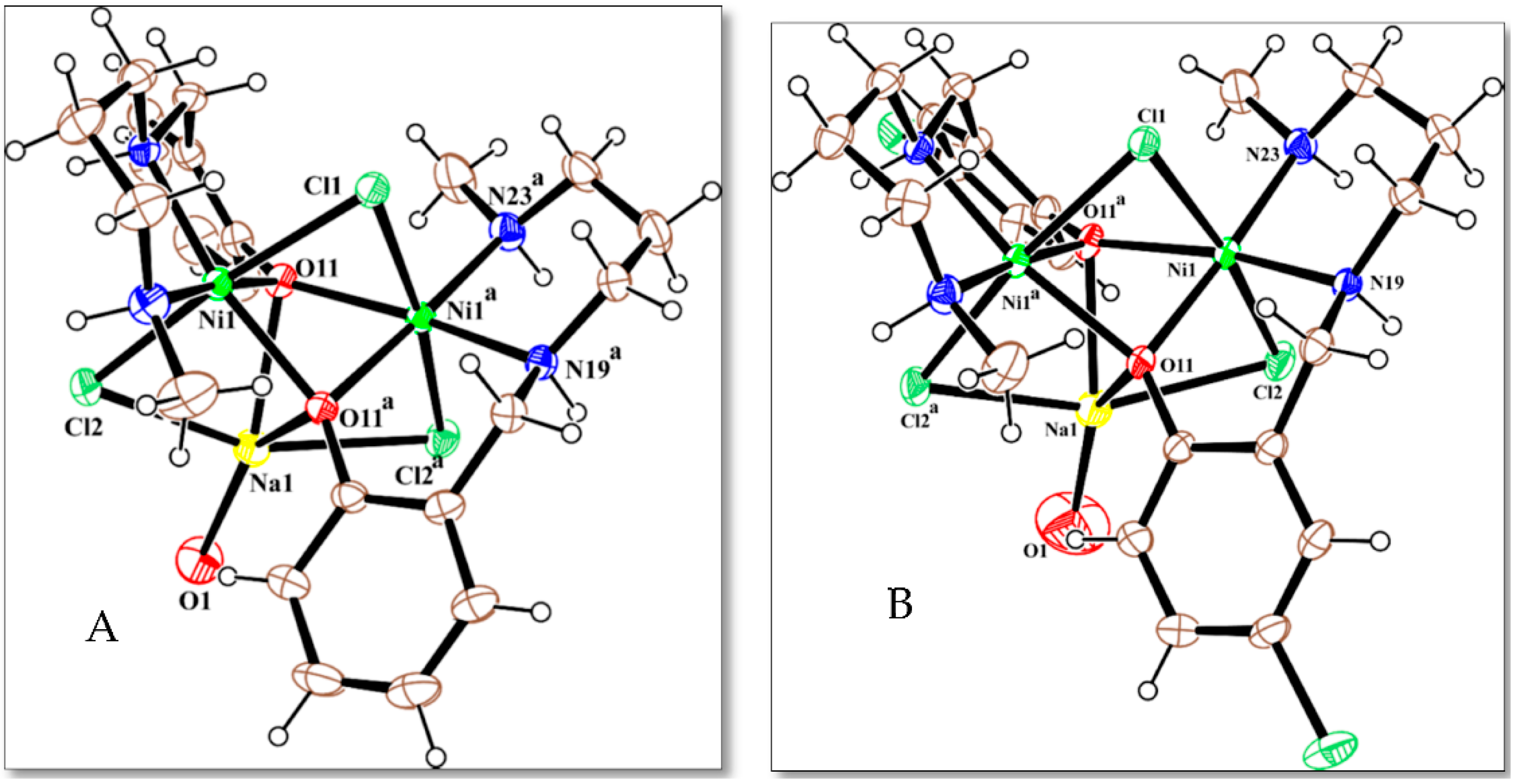
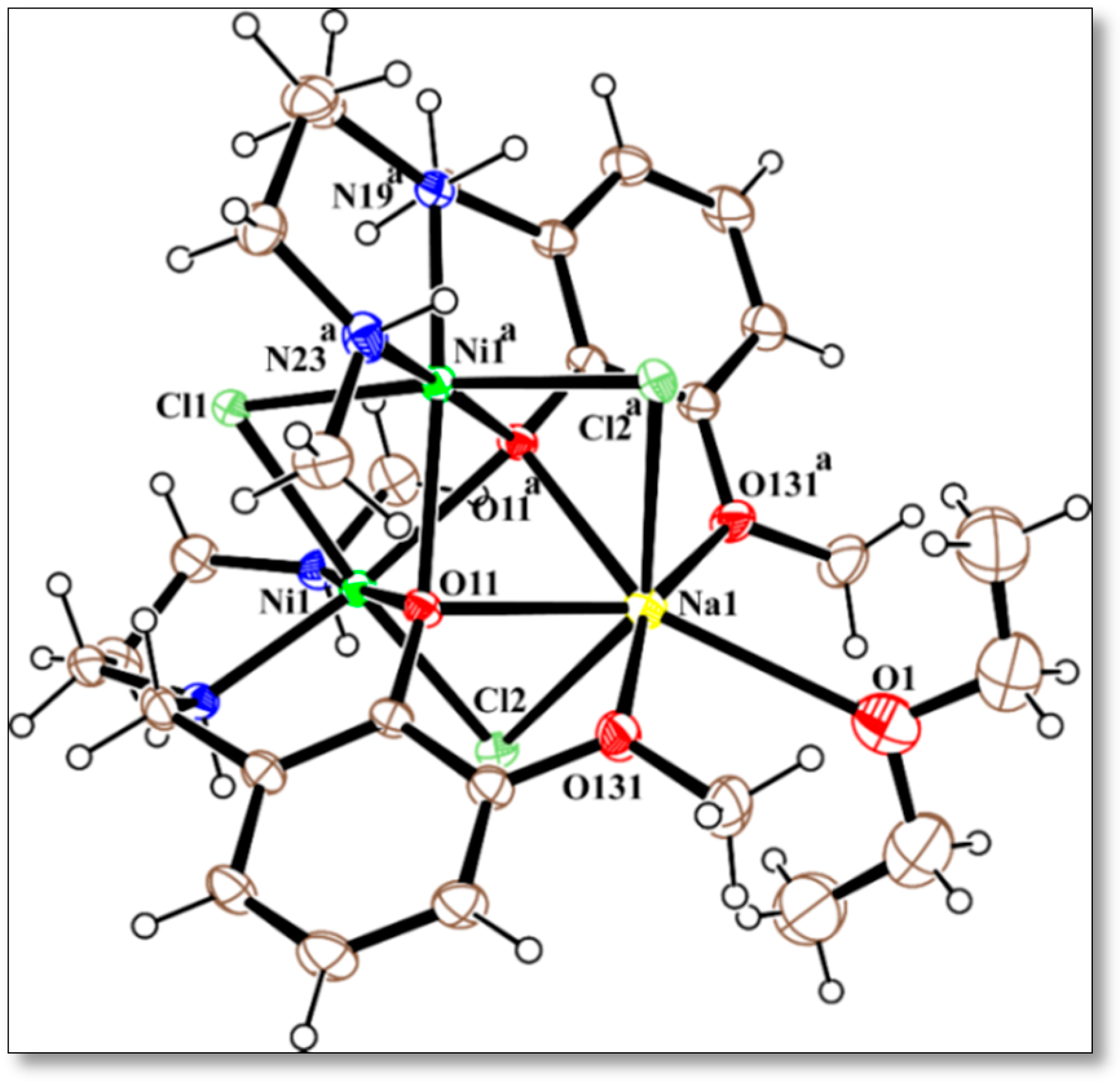
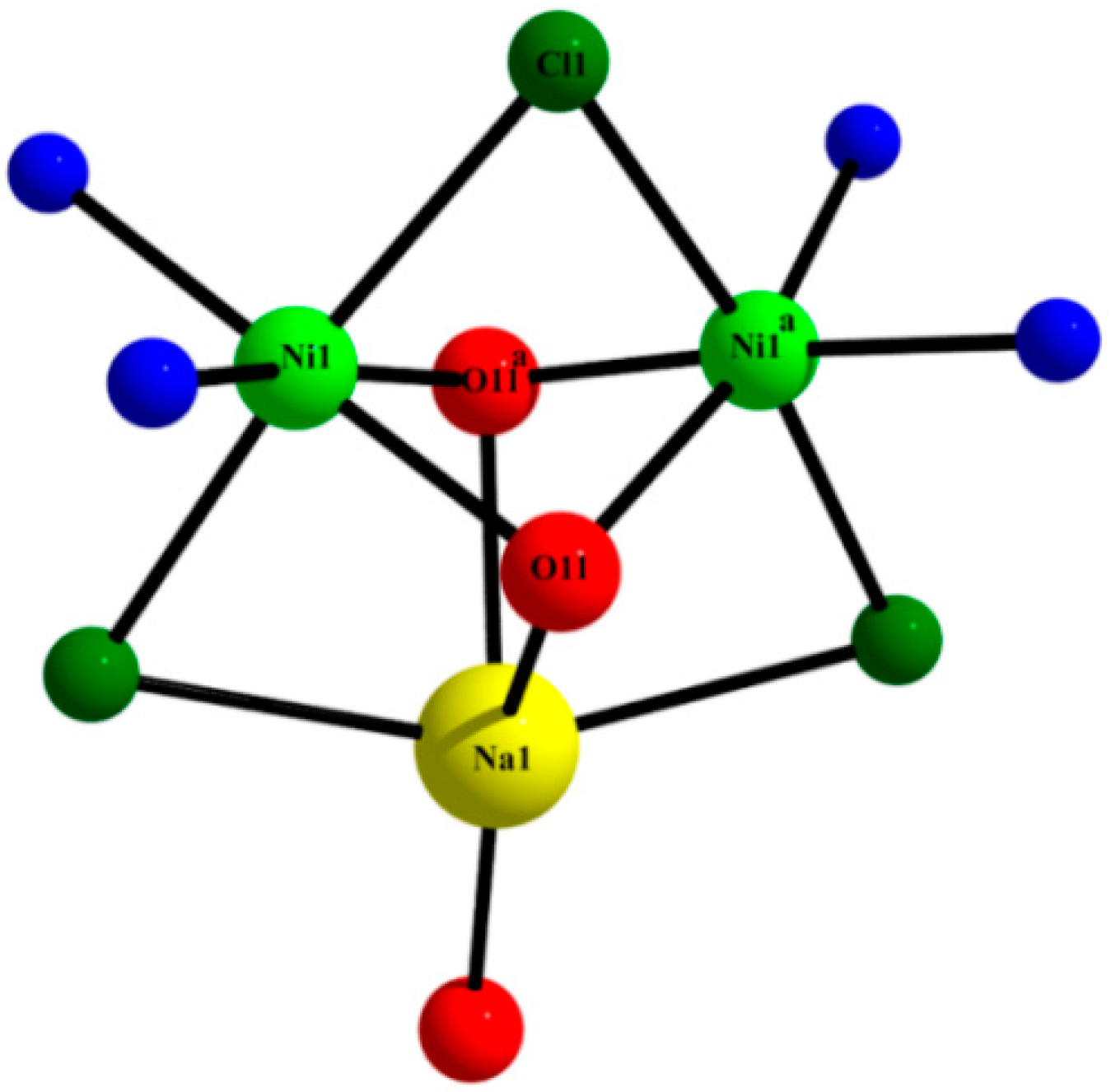
2.3. Description of the Crystal Structures
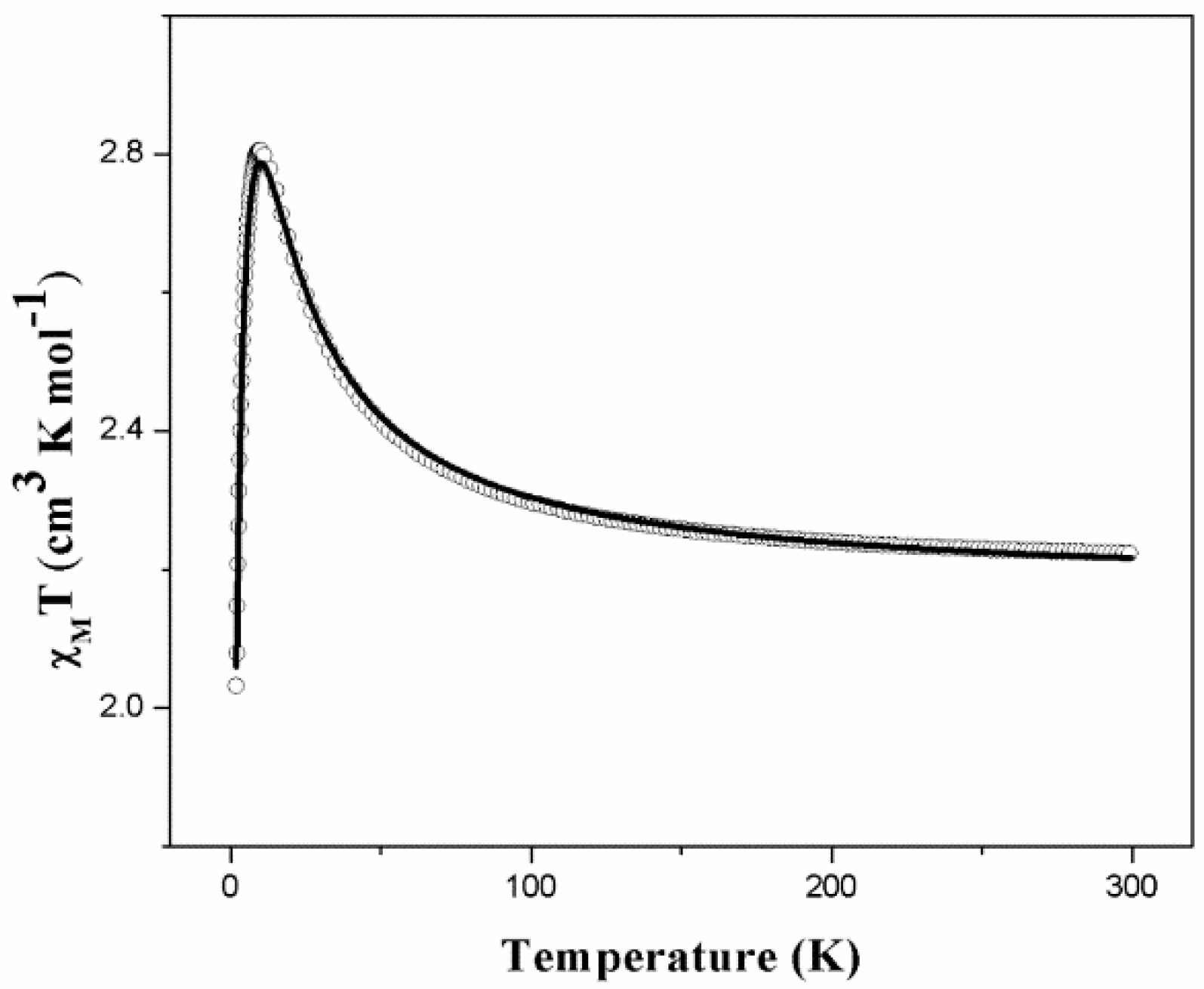
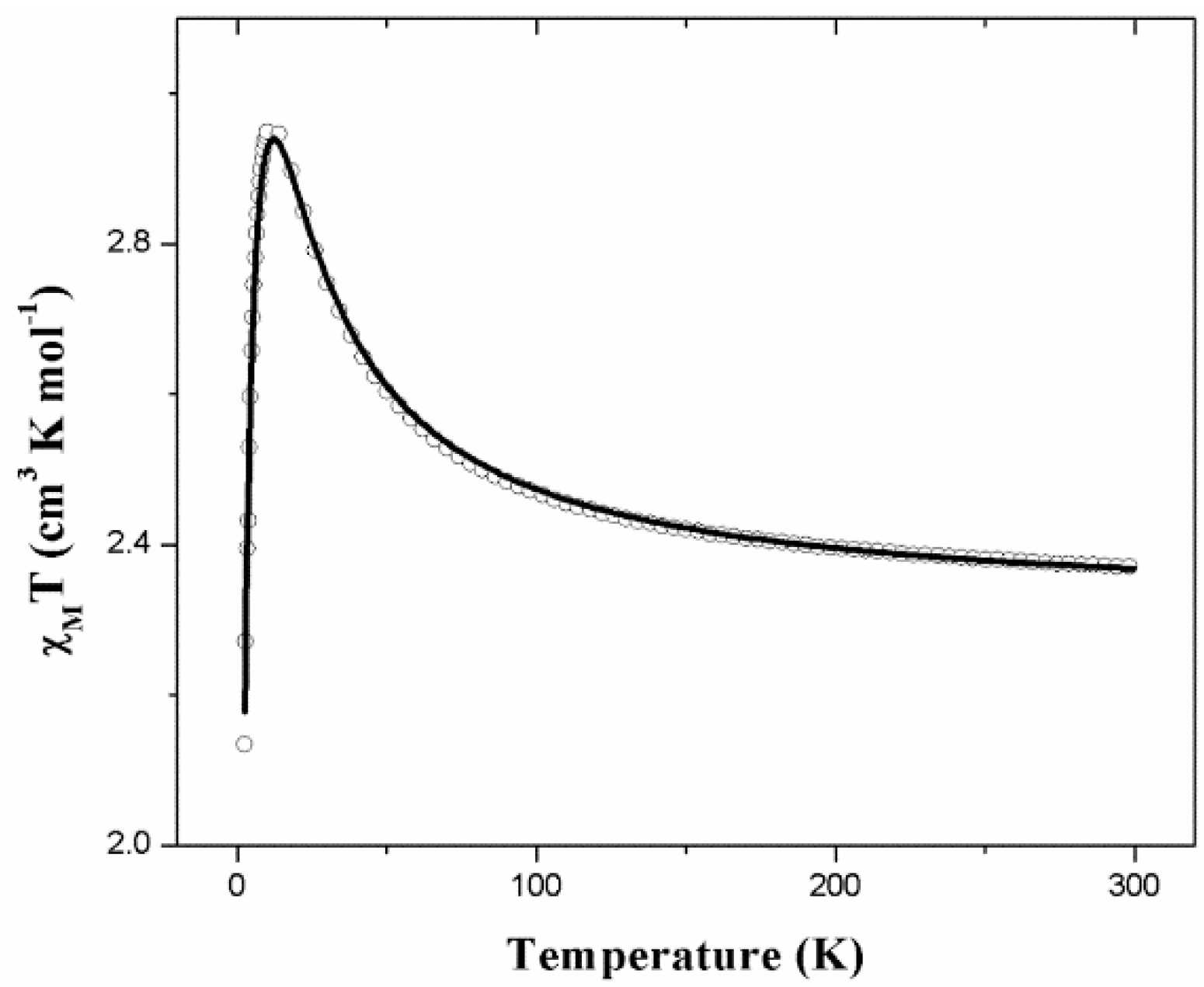
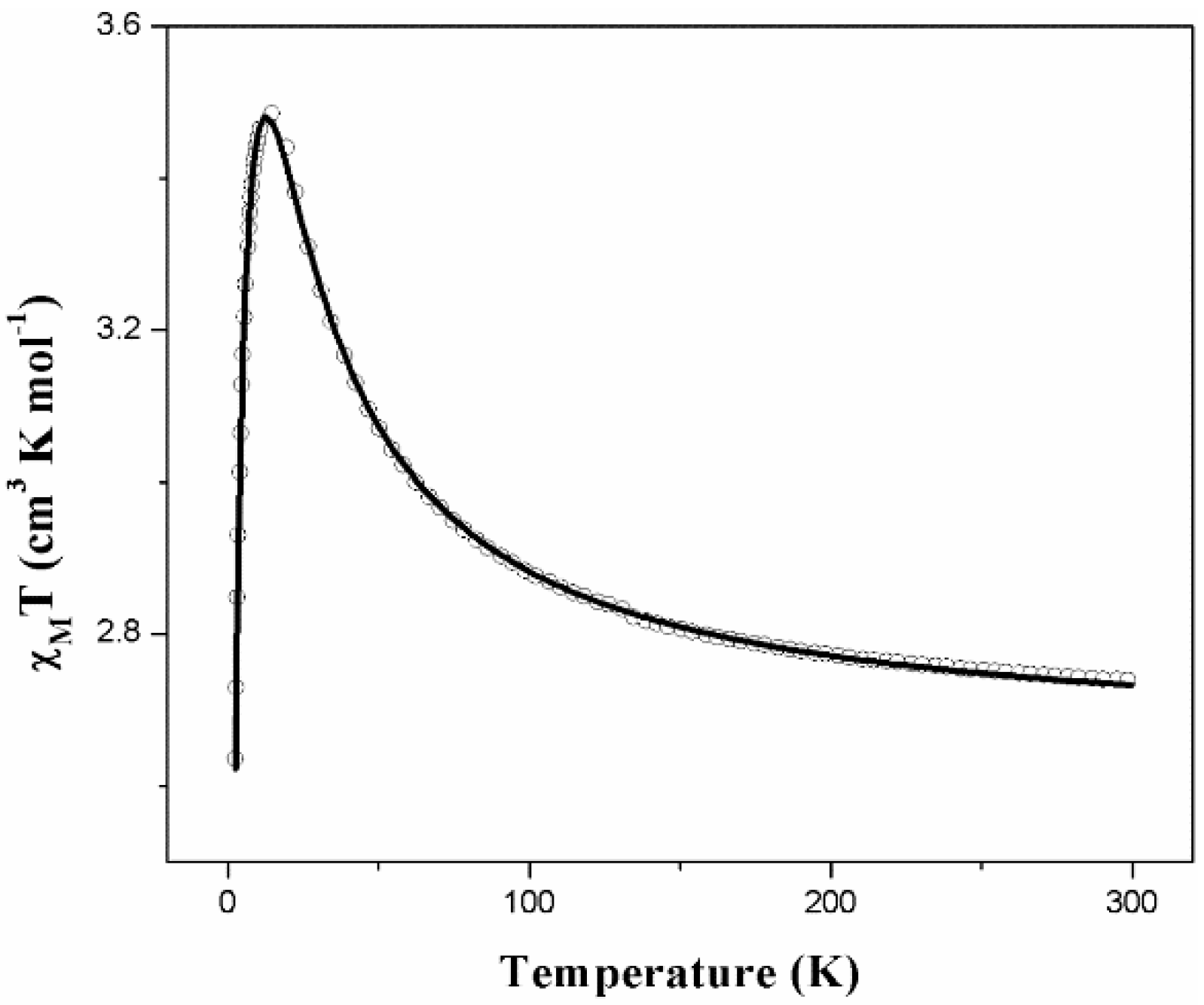
2.4. Magnetic Properties
2.5. Theoretical Magnetic Calculation
2.6. Comparison of Structural and Magnetic Parameters
3. Experimental Section
3.1. Starting Materials
3.2. Synthesis of the Ligands 2-[(3-Methylamino-propylamino)-methyl]-phenol (HL1), 2-[(3-Methylamino-propylamino)-methyl]-4-chloro-phenol (HL2), 2-[(3-Methylamino-propylamino)-methyl]-6-methoxy-phenol (HL3)
3.3. Synthesis of the Complexes [Ni2(L1)2NaCl3(H2O)]·H2O (1), [Ni2(L2)2NaCl3(H2O)] (2), and [Ni2(L3)2NaCl3(OC4H10)] (3)
3.4. Physical Measurements
3.5. X-ray Crystallographic Data Collection and Refinement
3.6. Computational Methodology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Deuss, P.J.; Heeten, R.; Laan, W.; Kamer, P.C. Bioinspired catalyst design and artificial metalloenzymes. Chem. Eur. J. 2011, 17, 4680–4698. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Thorp, H.H. Bond valence sum analysis of metal-ligand bond lengths in metalloenzymes and model complexes. 2. Refined distances and other enzymes. Inorg. Chem. 1993, 32, 4102–4105. [Google Scholar] [CrossRef]
- Suh, J. Model studies of metalloenzymes involving metal ions as Lewis acid catalysts. Acc. Chem. Res. 1992, 25, 273–279. [Google Scholar] [CrossRef]
- Kahn, O. Molecular Magnetism; VCH Publishers, Inc.: New York, NY, USA, 1993. [Google Scholar]
- Barrios, A.M.; Lippard, S.J. Interaction of urea with a hydroxide-bridged dinuclear nickel center: An alternative model for the mechanism of urease. J. Am. Chem. Soc. 2000, 122, 9172–9177. [Google Scholar] [CrossRef]
- Lanznaster, M.; Neves, A.; Bortoluzzi, A.J.; Szpoganicz, B.; Schwingel, E. New FeIIIZnII Complex Containing a Single Terminal Fe-O(phenolate) Bond as a Structural and Functional Model for the Active Site of Red Kidney Bean Purple Acid Phosphatase. Inorg. Chem. 2002, 41, 5641–5643. [Google Scholar] [CrossRef] [PubMed]
- Borovik, A.S.; Papaefthymiou, V.; Taylor, L.F.; Anderson, O.P.; Que, L., Jr. Models for iron-oxo proteins. Structures and properties of FeIIFeIII, ZnIIFeIII, and FeIIGaIII complexes with (.mu.-phenoxo) bis (.mu.-carboxylato) dimetal cores. J. Am. Chem. Soc. 1989, 111, 6183–6195. [Google Scholar] [CrossRef]
- Belle, C.; Gautier-Luneau, I.; Karmazin, L.; Pierre, J.L.; Albedyhl, S.; Krebs, B.; Bonin, M. Regio-Directed Synthesis of a ZnIIFeIII Complex from an Unsymmetrical Ligand and Its Relevance to Purple Acid Phosphatases. Eur. J. Inorg. Chem. 2002, 2002, 3087–3090. [Google Scholar] [CrossRef]
- Mayans, J.; Font-Bardia, M.; Di Bari, L.; Arrico, L.; Zinna, F.; Pescitelli, G.; Escuer, A. From Mesocates to Helicates: Structural, Magnetic and Chiro-Optical Studies on Nickel (II) Supramolecular Assemblies Derived from Tetradentate Schiff Bases. Chem. Eur. J. 2018, 24, 7653–7663. [Google Scholar] [CrossRef] [PubMed]
- Gheorghe, R.; Cucos, P.; Andruh, M.; Costes, J.P.; Donnadieu, B.; Shova, S. Oligonuclear 3d–4f Complexes as Tectons in Designing Supramolecular Solid-State Architectures: Impact of the Nature of Linkers on the Structural Diversity. Chem. Eur. J. 2006, 12, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, P.; Harms, K.; Chattopadhyay, S. Formation of polynuclear copper (II)–sodium (I) heterometallic complexes derived from salen-type Schiff bases. Polyhedron 2013, 49, 113–120. [Google Scholar] [CrossRef]
- Biswas, S.; Naiya, S.; Drew, M.G.B.; Estarellas, C.; Frontera, A.; Ghosh, A. Trinuclear and tetranuclear adduct formation between sodium perchlorate and copper(II) complexes of salicylaldimine type ligands: Structural characterization and theoretical investigation. Inorg. Chim. Acta 2011, 366, 219–226. [Google Scholar] [CrossRef]
- Sakamoto, S.; Fujinami, T.; Nishi, K.; Matsumoto, N.; Mochida, N.; Ishida, T.; Sunatsuki, Y.; Re, N. Carbonato-Bridged NiII2LnIII2 (LnIII = GdIII, TbIII, DyIII) Complexes Generated by Atmospheric CO2 Fixation and Their Single-Molecule-Magnet Behavior: [(μ4-CO3)2{NiII (3-MeOsaltn)(MeOH or H2O)LnIII(NO3)}2] solvent [3-MeOsaltn = N,N′-Bis (3-methoxy-2-oxybenzylidene)-1, 3-propanediaminato]. Inorg. Chem. 2013, 52, 7218–7229. [Google Scholar] [PubMed]
- Ghosh, S.; Biswas, S.; Bauza, A.; Barceló-Oliver, M.; Frontera, A.; Ghosh, A. Use of metalloligands [CuL](H2L = salen type di-Schiff bases) in the formation of heterobimetallic copper (II)-uranyl complexes: Photophysical investigations, structural variations, and theoretical calculations. Inorg. Chem. 2013, 52, 7508–7523. [Google Scholar] [CrossRef] [PubMed]
- Thurston, J.H.; Tang, C.G.Z.; Trahan, D.W.; Whitmire, K.H. Toward Rational Control of Metal Stoichiometry in Heterobimetallic Coordination Complexes: Synthesis and Characterization of Pb(Hsal)2 (Cu(salen*))2, [Pb(NO3)(Cu(salen*)) 2](NO3),Pb(OAc)2(Cu(salen*)), and [Pb(OAc)(Ni(salen*))2](OAc). Inorg. Chem. 2004, 43, 2708–2713. [Google Scholar] [CrossRef] [PubMed]
- Kilic, A.; Tas, E.; Deveci, B.; Yilmaz, I. Synthesis, electrochemical and in situ spectroelectrochemical studies of new transition metal complexes with two new Schiff-bases containing N2O2/N2O4 donor groups. Polyhedron 2007, 26, 4009–4018. [Google Scholar] [CrossRef]
- Biswas, R.; Ida, Y.; Baker, M.L.; Biswas, S.; Kar, P.; Nojiri, H.; Ishida, T.; Ghosh, A. A New Family of Trinuclear Nickel (II) Complexes as Single-Molecule Magnets. Chem. Eur. J. 2013, 19, 3943–3953. [Google Scholar] [CrossRef] [PubMed]
- Hung, W.C.; Lin, C.C. Preparation, characterization, and catalytic studies of magnesium complexes supported by NNO-tridentate Schiff-base ligands. Inorg. Chem. 2008, 48, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lah, M.S.; Pecoraro, V.L. Vanadium complexes of the tridentate Schiff base ligand N-salicylidene-N′-(2-hydroxyethyl) ethylenediamine: Acid-base and redox conversion between vanadium (IV) and vanadium (V) iminophenolates. Inorg. Chem. 1988, 27, 4657–4664. [Google Scholar] [CrossRef]
- Mondal, M.; Guha, P.M.; Giri, S.; Ghosh, A. Deactivation of catecholase-like activity of a dinuclear Ni (II) complex by incorporation of an additional Ni (II). J. Mol. Catal. A Chem. 2016, 424, 54–64. [Google Scholar] [CrossRef]
- Mukherjee, P.; Drew, M.G.B.; Gómez-García, C.J.; Ghosh, A. (Ni2),(Ni3), and (Ni2+ Ni3): A Unique Example of Isolated and Cocrystallized Ni2 and Ni3 Complexes. Inorg. Chem. 2009, 48, 4817–4827. [Google Scholar] [CrossRef] [PubMed]
- Adhikary, J.; Chakraborty, P.; Das, S.; Chattopadhyay, T.; Bauza, A.; Chattopadhyay, S.K.; Ghosh, B.; Mautner, F.A.; Frontera, A.; Das, D. A Combined Experimental and Theoretical Investigation on the Role of Halide Ligands on the Catecholase-like Activity of Mononuclear Nickel (II) Complexes with a Phenol-Based Tridentate Ligand. Inorg. Chem. 2013, 52, 13442–13452. [Google Scholar] [CrossRef] [PubMed]
- Mondal, M.; Giri, S.; Guha, P.M.; Ghosh, A. Dependence of magnetic coupling on ligands at the axial positions of NiII in phenoxido bridged dimers: Experimental observations and DFT studies. Dalton Trans. 2017, 46, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Nanda, K.K.; Thompson, L.K.; Bridson, J.N.; Nag, K. Linear dependence of spin exchange coupling constant on bridge angle in phenoxy-bridged dinickel (II) complex. J. Chem. Soc. Chem. Commun. 1994, 11, 1337–1338. [Google Scholar] [CrossRef]
- Burkhardt, A.; Spielberg, E.T.; Simon, S.; Görls, H.; Buchholz, A.; Plass, W. Hydrogen Bonds as Structural Directive towards Unusual Polynuclear Complexes: Synthesis, Structure, and Magnetic Properties of Copper (II) and Nickel (II) Complexes with a 2-Aminoglucose Ligand. Chem. Eur. J. 2009, 15, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Biswas, R.; Giri, S.; Saha, S.K.; Ghosh, A. One Ferromagnetic and Two Antiferromagnetic Dinuclear Nickel (II) Complexes Derived from a Tridentate N, N, O-Donor Schiff Base Ligand: A Density Functional Study of Magnetic Coupling. Eur. J. Inorg. Chem. 2012, 2012, 2916–2927. [Google Scholar] [CrossRef]
- Banerjee, S.; Drew, M.G.B.; Lu, C.Z.; Tercero, J.; Diaz, C.; Ghosh, A. Dinuclear Complexes of MII Thiocyanate (M= Ni and Cu) Containing a Tridentate Schiff-Base Ligand: Synthesis, Structural Diversity and Magnetic Properties. Eur. J. Inorg. Chem. 2005, 2005, 2376–2383. [Google Scholar] [CrossRef]
- Rodríguez, L.; Labisbal, E.; Sousa-Pedrares, A.; García-Vázquez, J.A.; Romero, J.; Durán, M.L.; Real, J.A.; Sousa, A. Coordination chemistry of amine bis (phenolate) cobalt (II), nickel (II), and copper (II) complexes. Inorg. Chem. 2006, 45, 7903–7914. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Wang, X.T.; Cheng, G.Z.; Gao, S.; Ji, Z.P. Synthesis, structural characterization, and magnetism of a butterfly-shaped hexanuclear Ni (II) complex. Inorg. Chem. Commun. 2008, 11, 769–771. [Google Scholar] [CrossRef]
- Biswas, A.; Drew, M.G.B.; Gómez-García, C.J.; Ghosh, A. Formation of a dinuclear and a trinuclear Ni (II) complex on slight variation of experimental conditions: Structural analysis and magnetic properties. Polyhedron 2017, 121, 80–87. [Google Scholar] [CrossRef]
- Ruiz, E.; Cano, J.; Alvarez, S.; Alemany, P. Magnetic coupling in end-on azido-bridged transition metal complexes: A density functional study. J. Am. Chem. Soc. 1998, 120, 11122–11129. [Google Scholar] [CrossRef]
- Biswas, R.; Kar, P.; Song, Y.; Ghosh, A. The importance of an additional water bridge in making the exchange coupling of bis (μ-phenoxo) dinickel (II) complexes ferromagnetic. Dalton Trans. 2011, 40, 5324–5331. [Google Scholar] [CrossRef] [PubMed]
- Biswas, R.; Diaz, C.; Ghosh, A. Three nickel (II) complexes derived from a tridentate NNO donor Schiff base ligand: Syntheses, crystal structures and magnetic properties. Polyhedron 2013, 56, 172–179. [Google Scholar] [CrossRef]
- Mukherjee, P.; Drew, M.G.B.; Gomez-Garcia, C.J.; Ghosh, A. The crucial role of polyatomic anions in molecular architecture: Structural and magnetic versatility of five nickel (II) complexes derived from AN, N, O-donor schiff base ligand. Inorg. Chem. 2009, 48, 5848–5860. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Drew, M.G.; Tangoulis, V.; Estrader, M.; Diaz, C.; Ghosh, A. Facile strategies for the synthesis and crystallization of linear trinuclear nickel (II)-Schiff base complexes with carboxylate bridges: Tuning of coordination geometry and magnetic properties. Polyhedron 2009, 28, 2989–2996. [Google Scholar] [CrossRef]
- Romanović, M.Č.; Čobeljić, B.R.; Pevec, A.; Turel, I.; Spasojević, V.; Tsaturyan, A.A.; Shcherbakov, I.N.; Anđelković, K.K.; Milenković, M.; Radanović, D.; et al. Synthesis, crystal structure, magnetic properties and DFT study of dinuclear Ni(II) complex with the condensation product of 2-quinolinecarboxaldehyde and Girard’s T reagent. Polyhedron 2017, 128, 30–37. [Google Scholar] [CrossRef]
- Mahapatra, P.; Ghosh, S.; Giri, S.; Ghosh, A. The unusual intermediate species in the formation of Ni(II) complexes of unsymmetrical Schiff bases by Elder’s method: Structural, electrochemical and magnetic characterizations. Polyhedron 2016, 117, 427–436. [Google Scholar] [CrossRef]
- Blanchet-Boiteux, C.; Mouesca, J.M. End-on azido-bridged copper dimers: Spin population analysis and spin polarization effect as exhibited by valence-bond/broken symmetry, density functional methods. J. Am. Chem. Soc. 2000, 122, 861–869. [Google Scholar] [CrossRef]
- Biswas, A.; Drew, M.G.; Diaz, C.; Bauzá, A.; Frontera, A.; Ghosh, A. Cis–trans isomerism in diphenoxido bridged dicopper complexes: Role of crystallized water to stabilize the cis isomer, variation in magnetic properties and conversion of both into a trinuclear species. Dalton Trans. 2012, 41, 12200–12212. [Google Scholar] [CrossRef] [PubMed]
- Nanda, K.K.; Das, R.; Thompson, L.K.; Venkatsubramanian, K.; Paul, P.; Nag, K. Magneto-structural correlations in macrocyclic dinickel (II) complexes: Tuning of spin exchange by varying stereochemistry and auxiliary ligands. Inorg. Chem. 1994, 33, 1188–1193. [Google Scholar] [CrossRef]
- Wang, C.; Fink, K.; Staemmler, V. A quantum chemical ab initio study of the superexchange coupling in binuclear oxygen-bridged Ni (II) complexes. Chem. Phys. 1995, 192, 25–35. [Google Scholar] [CrossRef]
- Torić, F.; Pavlović, G.; Pajić, D.; Cindric, M.; Zadro, K. Tetranuclear Ni4 cubane complexes with high χT maxima: Magneto-structural analysis. CrystEngComm 2018, 20, 3917–3927. [Google Scholar] [CrossRef]
- Bu, X.H.; Du, M.; Zhang, L.; Liao, D.Z.; Tang, J.K.; Zhang, R.H.; Shionoya, M. Anion-directed assembly: Framework conversion in dimensionality and photoluminescence. J. Chem. Soc. Dalton Trans. 2001, 5, 593–598. [Google Scholar] [CrossRef]
- Biswas, A.; Das, L.K.; Drew, M.G.B.; Aromí, G.; Gamez, P.; Ghosh, A. Synthesis, crystal structures, magnetic properties and catecholase activity of double phenoxido-bridged penta-coordinated dinuclear nickel (II) complexes derived from reduced Schiff-base ligands: Mechanistic inference of catecholase activity. Inorg. Chem. 2012, 51, 7993–8001. [Google Scholar] [CrossRef] [PubMed]
- Bain, G.A.; Berry, J.F. Diamagnetic corrections and Pascal’s constants. J. Chem. Educ. 2008, 85, 532. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Shelxs97, Program for Crystallographic solution and refinement. Acta Cryst. 2008, A64, 112. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Shelxl16/6, Program for Crystallographic Refinement. Acta Cryst. 2015, C71, 3. [Google Scholar]
- Platon, A.L. Spek. Acta Cryst. 2009, D65, 148. [Google Scholar]
- Noodleman, L. Valence bond description of antiferromagnetic coupling in transition metal dimers. J. Chem. Phys. 1981, 74, 5737–5743. [Google Scholar] [CrossRef]
- Noodleman, L.; Case, D.A. Density-Functional Theory of Spin Polarization and Spin Coupling in Iron—Sulfur Clusters. Adv. Inorg. Chem. 1992, 38, 423–470. [Google Scholar]
- Ruiz, E.; Rodríguez-Fortea, A.; Cano, J.; Alvarez, S.; Alemany, P. About the calculation of exchange coupling constants in polynuclear transition metal complexes. J. Comput. Chem. 2003, 24, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Becke’s three parameter hybrid method using the LYP correlation functional. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098. [Google Scholar] [CrossRef]
- Schäfer, A.; Huber, C.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr. J. Chem. Phys. 1994, 100, 5829–5835. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Giri, S.; Biswas, S.; Drew, M.G.B.; Ghosh, A.; Saha, S.K. Structure and magnetic properties of a tetranuclear Cu (II) complex containing the 2-(pyridine-2-yliminomethyl)-phenol ligand. Inorg. Chim.Acta. 2011, 368, 152–156. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
| D-H…A | d(D-H) | d(D…A) | d(H…A) | <(D-H…A) | |
|---|---|---|---|---|---|
| Complex 1 | N(19)-H(19)…Cl(2)$1 | 0.98 | 3.331(3) | 2.61 | 130 |
| Complex 2 | N(19)-H(19)…Cl(2)$1 | 0.98 | 3.332(2) | 2.56 | 136 |
| Complex 3 | N(19)-H(19)…Cl(2)$1 | 0.98 | 3.345(1) | 2.62 | 131 |
| Complex Name | Ni–O Bond Lengths (Å) | Ni–Ni Distance (Å) | Ni–O–Ni Angle(°) | Coupling Constant (J) Theoretical (BS DFT) (cm−1) | Coupling Constant (J) Experimental (cm−1) |
|---|---|---|---|---|---|
| 1 | 2.164(2) 2.049(2) | 2.963(1) | 89.32(8) | 4.25 | 3.97 |
| 2 | 2.164(2) 2.053(2) | 2.967(1) | 89.39(6) | 3.56 | 4.66 |
| 3 | 2.034(1) 2.318(1) | 3.011(1) | 87.32(4) | 5.50 | 5.50 |
| Ni1 | −1.626 |
| Na1 | 0.000 |
| Cl1 | 0.000 |
| Cl2 | −0.080 |
| O11 | −0.026 |
| Ni1a | 1.626 |
| Cl2a | 0.080 |
| O11a | 0.026 |
| Ni1 | −1.627 |
| Na1 | 0.000 |
| Cl1 | 0.000 |
| Cl2 | −0.079 |
| O11 | −0.024 |
| Ni1a | 1.627 |
| Cl2a | 0.079 |
| O11a | 0.024 |
| Ni1 | −1.629 |
| Na1 | 0.000 |
| Cl1 | 0.000 |
| Cl2 | −0.082 |
| O11 | −0.033 |
| Ni1a | 1.629 |
| Cl2a | 0.082 |
| O11a | 0.033 |
| Compound | 1 | 2 | 3 |
|---|---|---|---|
| Chemical formula | C22H36Cl3N4NaNi2O3 | C22H34Cl5N4NaNi2O3 | C28H48Cl3N4Na Ni2O5 |
| Formula weight | 651.31 | 720.19 | 767.46 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic |
| Space group | C2/c | C2/c | C2/c |
| a(Å) | 22.2673(8) | 17.9326(17) | 21.7316(10) |
| b (Å) | 11.8915(4) | 17.1847(16) | 12.1574(5) |
| c (Å) | 15.7543(10) | 11.7063(11) | 15.8456(12) |
| β, deg | 129.106(1) | 112.796(2) | 126.9090(10) |
| V (Å3) | 3237.1(3) | 3325.7(5) | 3347.4(3) |
| Z | 4 | 4 | 4 |
| ρcalc (g cm−3) | 1.336 | 1.438 | 1.523 |
| µ (Mo Kα) (mm−1) | 1.451 | 1.575 | 1.420 |
| F(000) | 1352 | 1480 | 1608 |
| Reflections collected | 17,507 | 40,193 | 32,846 |
| Independent reflections | 3603 | 2956 | 3815 |
| Reflections with I > 2σ(I) | 3031 | 2808 | 3470 |
| R1a, wR2b | 0.0489, 0.1745 | 0.0316, 0.0988 | 0.0269, 0.0706 |
| R1],wR2 [all data] | 0.0572, 0.1834 | 0.0331, 0.1004 | 0.0308, 0.0705 |
| GOF c | 1.065 | 1.125 | 1.036 |
| Residual electron Density, e/Å−3 | 1.762, −0.301 | 0.826, −0.413 | 0.518, −0.378 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mondal, M.; Chakraborty, M.; Drew, M.G.B.; Ghosh, A. Ni(II) Dimers of NNO Donor Tridentate Reduced Schiff Base Ligands as Alkali Metal Ion Capturing Agents: Syntheses, Crystal Structures and Magnetic Properties. Magnetochemistry 2018, 4, 51. https://doi.org/10.3390/magnetochemistry4040051
Mondal M, Chakraborty M, Drew MGB, Ghosh A. Ni(II) Dimers of NNO Donor Tridentate Reduced Schiff Base Ligands as Alkali Metal Ion Capturing Agents: Syntheses, Crystal Structures and Magnetic Properties. Magnetochemistry. 2018; 4(4):51. https://doi.org/10.3390/magnetochemistry4040051
Chicago/Turabian StyleMondal, Monotosh, Maharudra Chakraborty, Michael G. B. Drew, and Ashutosh Ghosh. 2018. "Ni(II) Dimers of NNO Donor Tridentate Reduced Schiff Base Ligands as Alkali Metal Ion Capturing Agents: Syntheses, Crystal Structures and Magnetic Properties" Magnetochemistry 4, no. 4: 51. https://doi.org/10.3390/magnetochemistry4040051






