Synthesis, Structure and Magnetic Study of a Di-Iron Complex Containing N-N Bridges
Abstract
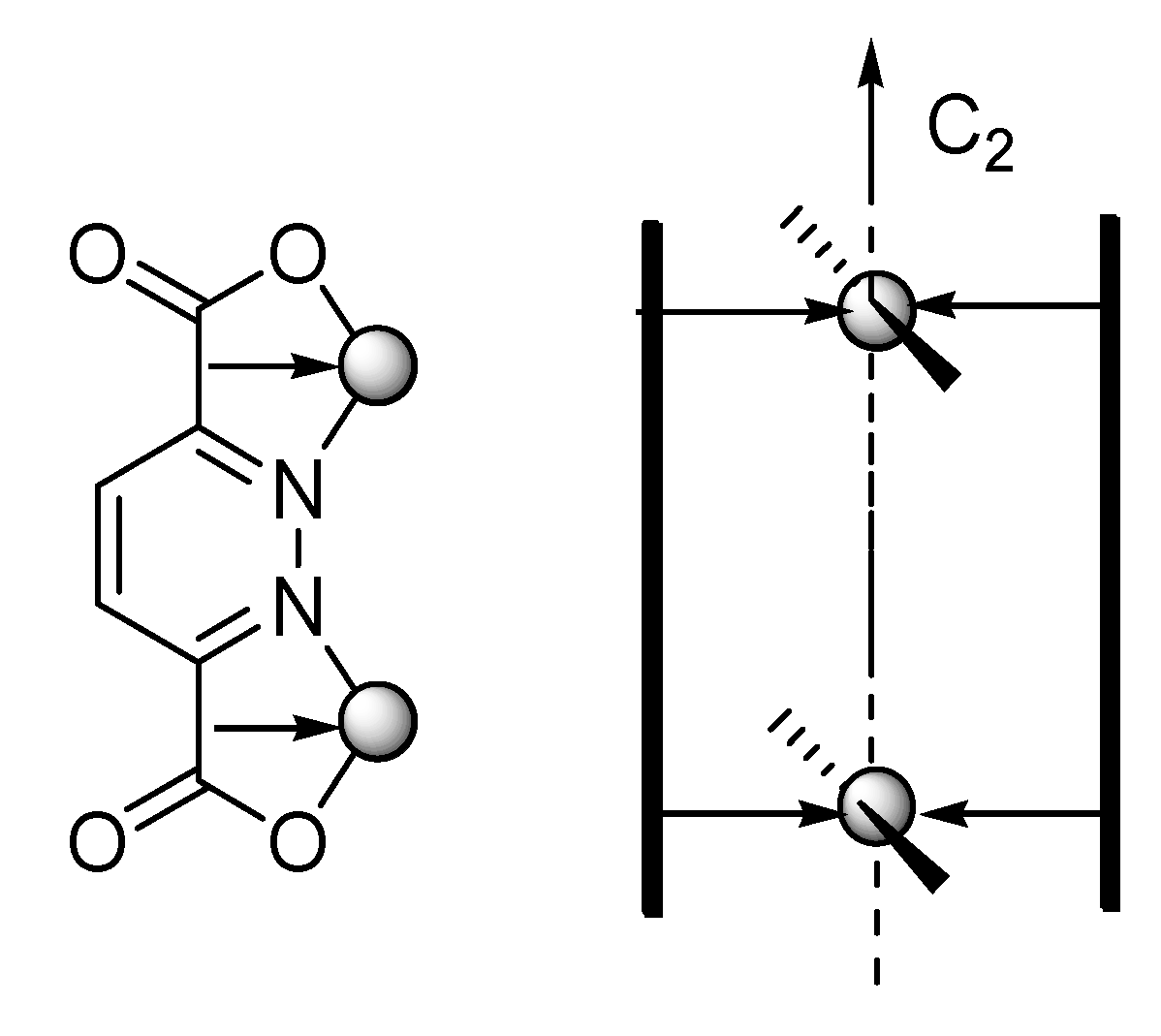
:1. Introduction
2. Experimental Results and Discussions
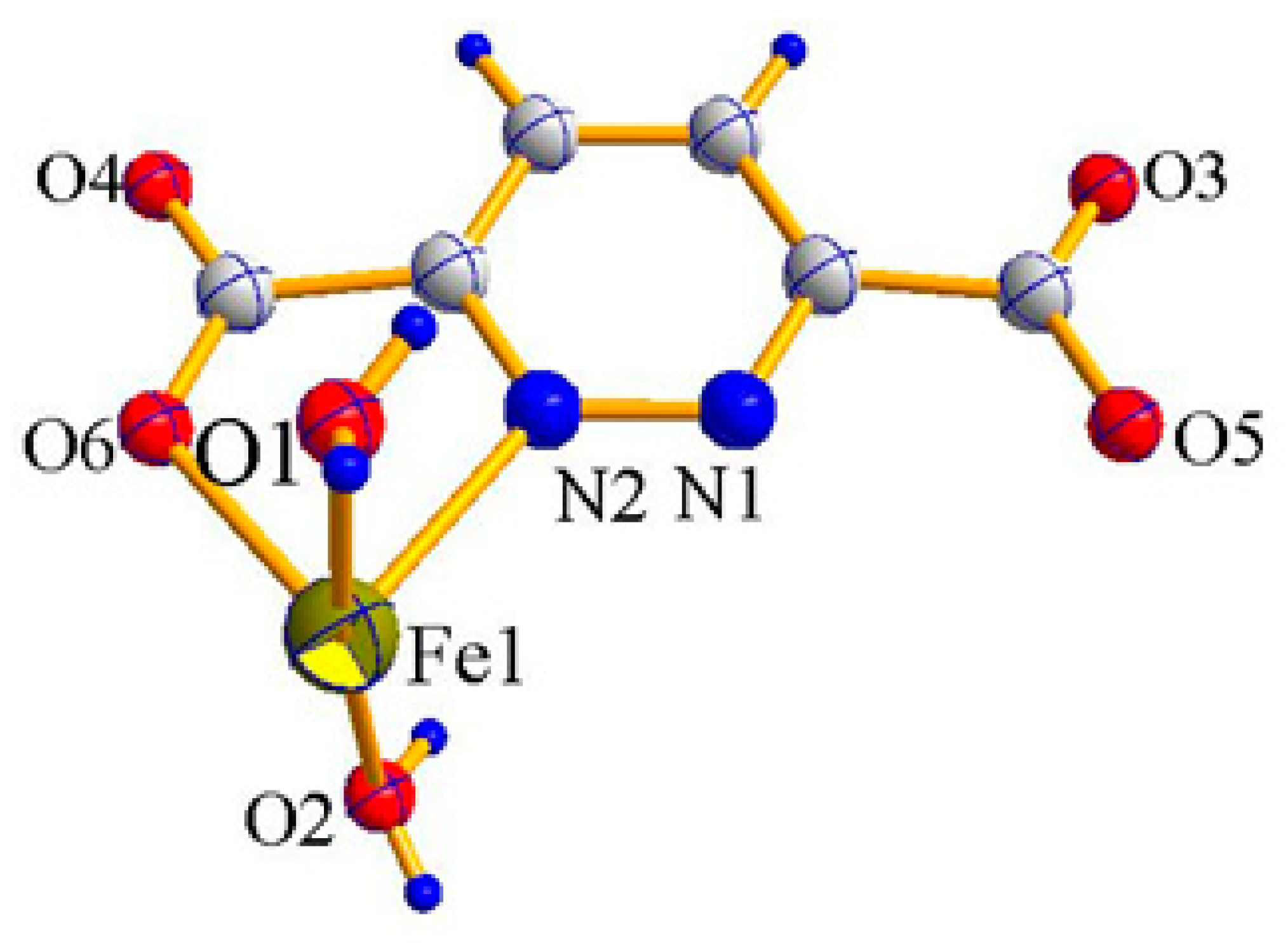
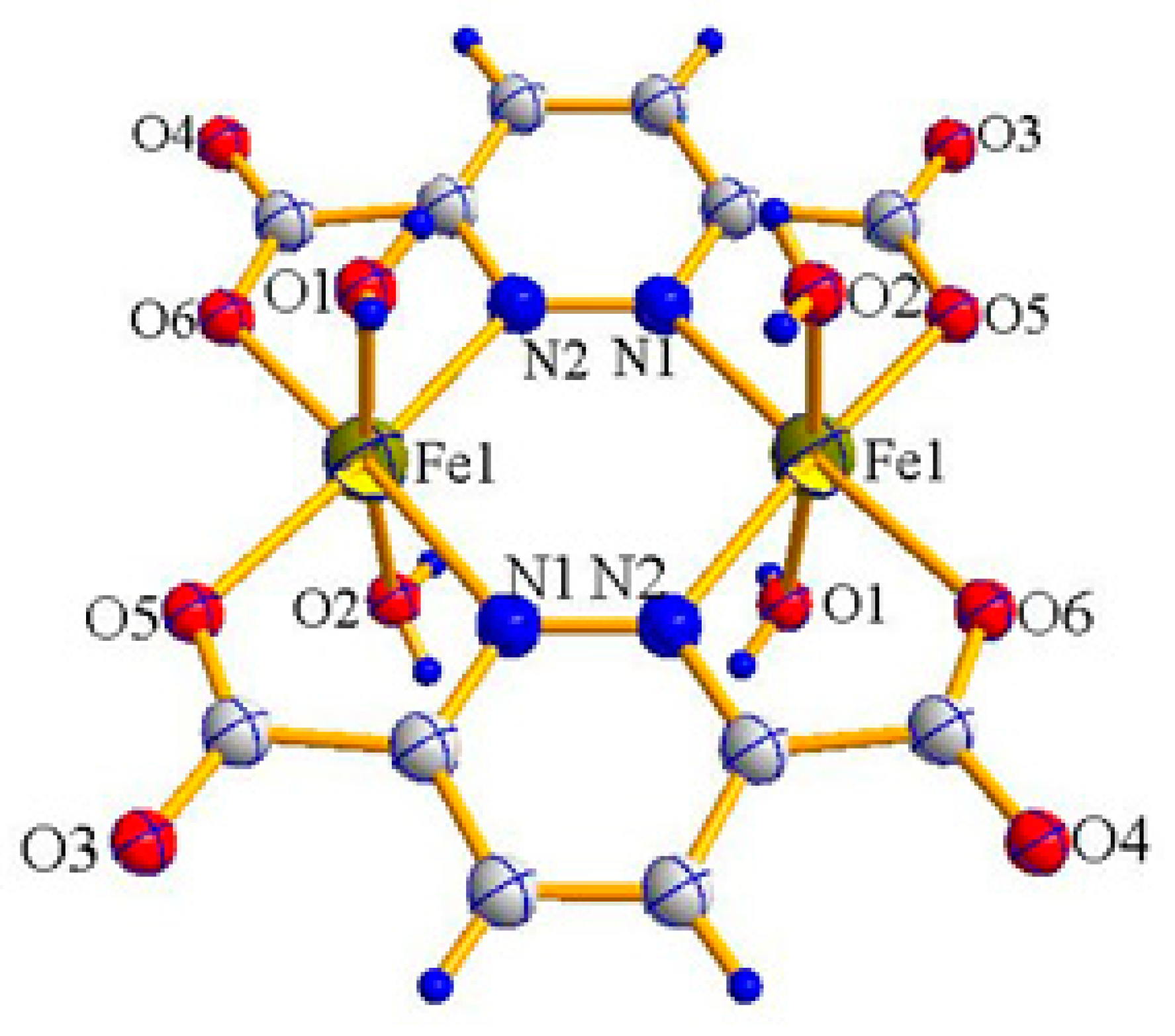
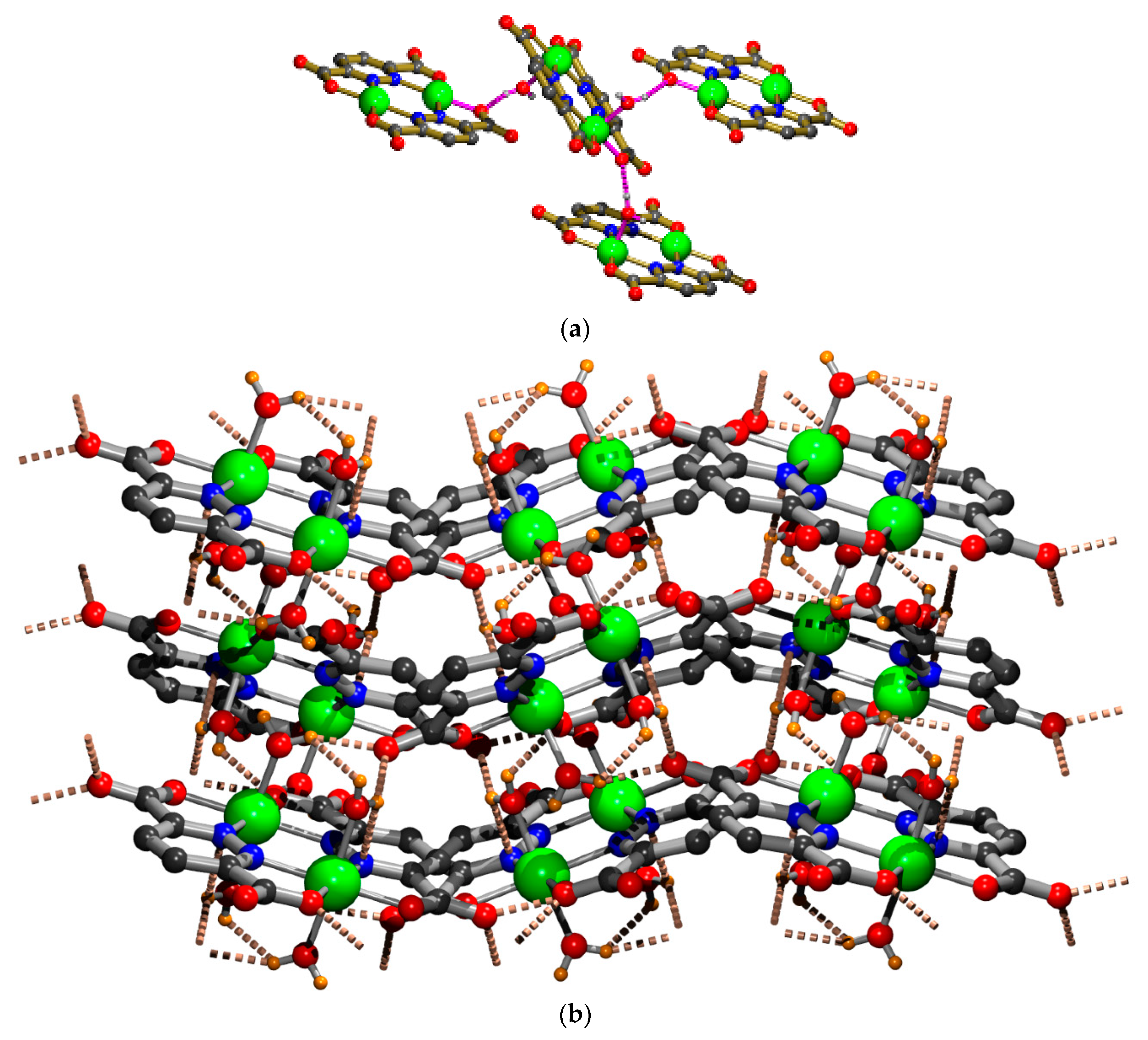
2.1. X-ray Crystal Structure Description
2.2. IR Spectroscopy Study
2.3. Thermal Study
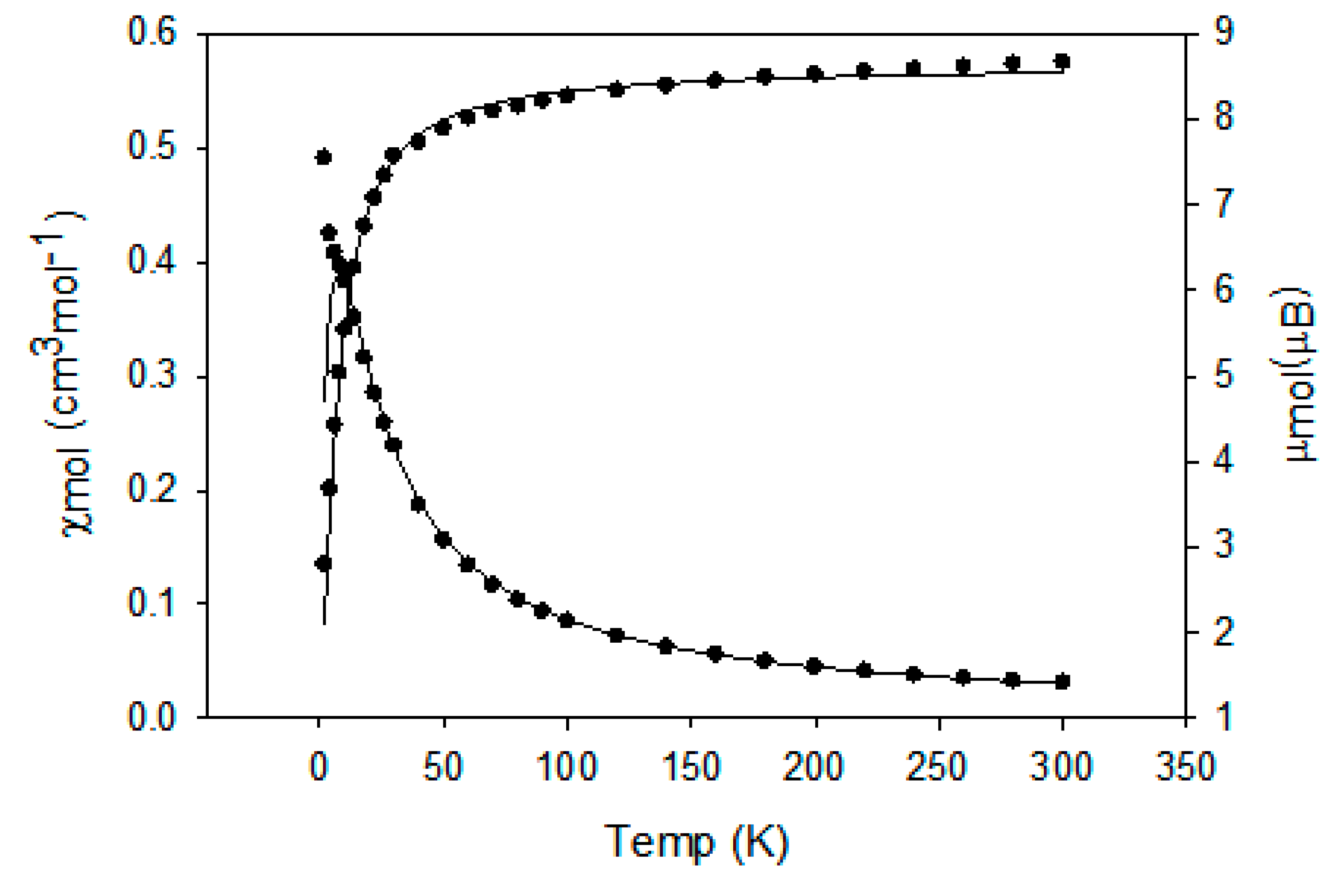
2.4. Magnetic Study
3. Materials and Methods
3.1. Materials
3.2. Synthesis of {[Fe (L)]. (H2O)2}2
3.3. Single-Crystal X-ray Diffraction Crystallography
3.4. Magnetic Measurement
3.5. Other Physical Measurements and Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grünwald, K.R.; Saischek, G.; Volpe, M.; Mösch-Zanetti, N.C. Pyridazine-based ligands and their coordinating ability towards first-row transition metals. Eur. J. Inorg. Chem. 2010, 15, 2297–2305. [Google Scholar] [CrossRef]
- Nuss, G.; Saischek, G.; Harum, B.N.; Volpe, M.; Belaj, F.; Mösch-Zanetti, N.C. Pyridazine based scorpionate ligand in a copper boratrane compound. Inorg. Chem. 2011, 50, 12632–12640. [Google Scholar] [CrossRef] [PubMed]
- Holler, S.; Tüchler, M.; Steller, B.G.; Belaj, F.; Veiros, L.F.; Kirchner, K.; Mösch-Zanetti, N.C. Thiopyridazine-based palladium and platinum boratrane complexes. Inorg. Chem. 2018, 57, 6921–6931. [Google Scholar] [CrossRef] [PubMed]
- Wasser, I.M.; Martens, C.F.; Verani, C.N.; Rentschler, E.; Huang, H.W.; Moenne-Loccoz, P.M.; Zakharov, L.N.; Rheingold, A.L.; Karlin, K.D. Synthesis and spectroscopy of μ-oxo (O2−)-bridged heme/non-hemediiron complexes: Models for the active site of nitric oxide reductase. Inorg. Chem. 2004, 43, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Berto, T.C.; Speelman, A.L.; Zheng, S.; Lehnert, N. Mono-and dinuclear non-heme iron–nitrosyl complexes:Models for key intermediates in bacterial nitric oxide reductases. Coord. Chem. Rev. 2013, 257, 244–259. [Google Scholar] [CrossRef]
- Solomon, E.I.; Brunold, T.C.; Davis, M.I.; Kemsley, J.N.; Lee, S.K.; Lehnert, N.; Neese, F.; Skulan, A.J.; Yang, Y.S.; Zhou, J. Geometric and electronic structure/function correlations in non-heme iron enzymes. Chem. Rev. 2000, 100, 235–350. [Google Scholar] [CrossRef] [PubMed]
- Bois, J.D.; Mizoguchi, T.J.; Lippard, S.J. Understanding the dioxygen reaction chemistry of diiron proteins through synthetic modeling studies. Coord. Chem. Rev. 2000, 443, 200–202. [Google Scholar]
- Domasevitch, K.V.; Gurałskiy, I.A.; Solntsev, P.V.; Rusanov, E.B.; Krautscheid, H.; Howard, J.A.; Chernega, A.N. Silver(I) ions bridged by pyridazine: doubling the ligand functionality for the design of unusual 3D coordination frameworks. Dalton Trans. 2007, 35, 3140–3148. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.K.; Woon, T.C.; Murphy, D.B.; Gabe, E.J.; Lee, F.L.; Page, Y.L. Binuclear copper(II) complexes of a series of tetradentatepyrazolyldiazines. Crystal and molecular structures of [.μ.-3,6-bis(3,5-dimethyl-1-pyrazolyl)pyridazine-N,.μ.-N3,.μ.-N3’,N](.μ.-hydroxo)dichlorodicopper(II) aquotrichlorocuprate hydrate, Cu3C14H21Cl5N6O3, and [.μ.-3,6-bis(3,5-dimethyl-1-pyrazolyl)pyridazine- N,.μ.-N6,.μ.-N7,N](.μ.-hydroxo)tris(nitrato) diaquo- dicopper(II) hydrate, Cu2C14H23N9O13. Inorg. Chem. 1985, 24, 4719–4725. [Google Scholar]
- Brooker, S. Some copper and cobalt complexes of Schiff base macrocycles containing pyridazine head units. Eur. J. Inorg. Chem. 2002, 10, 2535–2547. [Google Scholar] [CrossRef]
- Brooker, S.; Hay, S.J.; Plieger, P.G. A grid complex [CuL2]4+ and a mixed valent complex [CuIICuIL(MeCN)2]3+ of the pyridazine containing macrocycle ligand. Angew. Chem. Int. Ed. 2000, 39, 1968–1970. [Google Scholar] [CrossRef]
- Richardson, D.E.; Taube, H. Electronic interactions in mixed-valence molecules as mediated by organic bridging groups. J. Am. Chem. Soc. 1983, 105, 40–51. [Google Scholar] [CrossRef]
- Hiroki, O.; Norihisa, H.; Motohiro, N.; Franz, R.; Philipp, G. High spin wheel of a heptanuclear mixed valent FeII, III complex. Angew. Chem. Int. Ed. 2003, 115, 233–235. [Google Scholar]
- Escuer, A.; Vicente, R.; Mernari, B.; El Gueddi, A.; Pierrot, M. Syntheses, structure, and magnetic behavior of two new nickel(II) and cobalt(II) dinuclear complexes with 1,4-dicarboxylatopyridazine. MO Calculations of the superexchange pathway through the pyridazine bridge. Inorg. Chem. 1997, 36, 2511–2516. [Google Scholar] [CrossRef]
- Chisholm, M.H.; Patmore, N.J. Studies of electronic coupling and mixed valency in metal-metal quadruply bonded complexes linked by dicarboxylate and closely related ligands. Acc. Chem. Res. 2007, 40, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Hogue, R.W.; Feltham, H.L.C.; Miller, R.G.; Brooker, S. Spin crossover in dinuclear N4S2 iron (II) thioether-triazole complexes: Access to [HS-HS], [HS-LS], and [LS-LS] states. Inorg. Chem. 2016, 55, 4152–4165. [Google Scholar] [CrossRef] [PubMed]
- Lochenie, C.; Panzer, K.S.F.; Kurz, H.; Maier, B.; Puchtler, F.; Agarwal, S.; Köhler, A.; Weber, B. Spin crossover iron(II) coordination polymer with fluorescent properties: correlation between emission properties and spin state. J. Am. Chem. Soc. 2018, 140, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Shakir, M.; Begum, N.; Azim, Y.; Parveen, S. Synthesis of 14-membered pentaazabis(macrocyclic) complexes of Co(II), Ni(II), Cu(II) and Zn(II) derived from hydrazine and their physico-chemical studies. Synth. React. Inorg. Met.-Org. Chem. 2003, 33, 1367–1379. [Google Scholar] [CrossRef]
- Schneider, C.J.; Cashion, J.D.; Chilton, N.F.; Etrillard, C.; Fuentealba, M.; Howard, J.A.K.; Murray, K.S. Spin crossover in a 3,5-Bis(2-pyridyl)-1,2,4-triazolate-bridged dinuclear iron(II) complex [{Fe(NCBH3)(py)}2(μ-L1)2]—powder versus single crystal study. Eur. J. Inorg. Chem. 2013, 5–6, 850–864. [Google Scholar] [CrossRef]
- Kershaw, C.L.J.; Kulmaczewski, R.; Mohammed, R.; Dudley, S.; Barrett, S.A.; Little, M.A.; Deeth, R.; Halcrow, M.A. A unified treatment of the relationship between ligand substituent and spin state in a family of iron (II) complexes. Angew. Chem. Int. Ed. 2016, 55, 4327–4331. [Google Scholar] [CrossRef] [PubMed]
- Christer, B.A.; Alicia, M.B.; Brian, A.H. Total synthesis supramolecular style: Design and hydrogen-bond-directed assembly of ternary supermolecules. Angew. Chem. Int. Ed. 2001, 40, 3240–3242. [Google Scholar]
- Lehn, J.M. Supramolecular Chemistry-Concepts and Perspectives; VCH: Weinheim, Germany, 1995; ISBN 3-527-28330-7. [Google Scholar]
- Corey, E.J.; Nicolaou, K.C.; Sorensen, E.J. Classics in Total Synthesis: Targets, Strategies, Methods (Chemistry); Wiley-VCH: Weinheim, Germany, 1988; pp. 111–133. ISBN 978-3-527-29231-8. [Google Scholar]
- Kitaura, R.; Onoyama, G.; Sakamoto, H.; Matsuda, R.; Noro, S.; Kitagawa, S. Immobilization of a metalloschiff base into a microporous coordination polymer. Angew. Chem. Int. Ed. 2004, 43, 2684–2687. [Google Scholar] [CrossRef]
- Przychodzen, P.; Korzeniak, T.; Podgajny, R.; Sieklucka, B. Supramolecular coordination networks based on octacyanometalates: From structure to function. Coord. Chem. Rev. 2006, 250, 2234–2260. [Google Scholar] [CrossRef]
- Schneider, B.; Demeshko, S.; Neudeck, S.; Dechert, S.; Meyer, F. Mixed-spin [2 × 2] Fe4 grid complex optimized for quantum cellular automata. Inorg. Chem. 2013, 52, 13230–13237. [Google Scholar] [CrossRef] [PubMed]
- Jayabalakrishnan, C.; Natarajan, K. Ruthenium (II) carbonyl complexes with tridentate schiff bases and their antibacterial activity. Transition Met. Chem. 2002, 27, 75–79. [Google Scholar] [CrossRef]
- Wahab, Z.H.A.E.; Mashaly, M.M.; Salman, A.A.; El Shetary, B.A.; Faheim, A.A. Co(II), Ce(III) and UO2(VI) bis-salicylatothiosemicarbazide complexes: Binary and ternary complexes, thermal studies and antimicrobial activity. Spectrochim. Acta Part A 2004, 60, 2861–2873. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.K.; Thompson, L.K.; Dawe, L.N. A self-assembled hexadecanuclear 4 × [2 × 2] Mn (II) 16square grid from a pyridazinebis (hydrazone) ligand: synthesis, structure and magnetism. Chem. Commun. 2006, 4967–4969. [Google Scholar] [CrossRef] [PubMed]
- Sueur, S.; Lagrenee, M.; Abraham, F.; Bremard, C. New syntheses of polyaza derivatives. Crystal structure of pyridazine-3,6-dicarboxylic acid. J. Heterocyclic Chem. 1987, 24, 1285–1289. [Google Scholar] [CrossRef]
- Agilent Technologies. CrysAlisPro Software System; Version 1.171.33.31; Aglient Technologies: Oxford, UK, 2012. [Google Scholar]
- Agilent Technologies. Crysalis CCD and Crysalis RED Software System; Aglient Technologies: Oxford, UK, 2012. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. ORTEP-3 for Windows. J. Appl. Cryst. 1997, 30, 565. [Google Scholar] [CrossRef]
- Watkin, D.J.; Prout, C.K.; Pearce, L.J. CAMERON; Chemical Crystallography Laboratory, University of Oxford: Oxford, UK, 1996. [Google Scholar]
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Cryst. 1999, 32, 837–838. [Google Scholar] [CrossRef]
| Crystal Data | Complex 1 |
|---|---|
| Formula | C12H12Fe2N4O12 |
| Formula Weight | 515.96 |
| Crystal System | Orthorhombic |
| Space group | Pbca (No. 61) |
| a/Å | 13.458(5) |
| b/Å | 8.721(3) |
| c/Å | 14.760(6) |
| V/Å3 | 1732.3(11) |
| Z | 4 |
| D(calc)/ g cm−3 | 1.978 |
| μ(MoKα)/mm−1 | 1.753 |
| F(000) | 1040 |
| Crystal Size/mm | 0.22 × 0.18 × 0.15 |
| Temperature /K | 293 |
| Radiation/Å | MoKα 0.71073 |
| Theta Min-Max/° | 2.8, 29.2 |
| Data set | −18: 17; −11: 10; −19: 19 |
| Total | 10253 |
| Unique Data | 2072 |
| R(int) | 0.027 |
| Observed[I > 2.0 σ(I)] | 1821 |
| Nref | 2072 |
| Npar | 136 |
| R1 | 0.0322 |
| wR2 (2sigma < I) | 0.0920 |
| GOF | 1.077 |
| Max. and Av. σ/esd | 0.00, 0.00 |
| Min. and Max. ∆ρ [e/Å3] | −0.78, 0.77 |
| Bond Lengths/Å | Bond Angles/deg | ||
|---|---|---|---|
| Fe1-O1 | 2.097(1) | O5-Fe1-N1 | 75.54(5) |
| Fe1-O2 | 2.098(1) | O6-Fe1-N2 | 75.58(5) |
| Fe1-O6 | 2.107(1) | N2-Fe1-N1 | 108.54(5) |
| Fe1-N2 | 2.199(1) | O6-Fe1-O5 | 100.33(5) |
| Fe1-O5 | 2.114(1) | O1-Fe1-O6 | 89.47(5) |
| Fe1-N1 | 2.191(1) | O1-Fe1-N2 | 85.21(6) |
| Fe1-Fe1 | 3.8947(4) | O1-Fe1-N1 | 89.10(6) |
| O2-Fe1-N2 | 88.66(6) | ||
| O2-Fe1-N1 | 88.70(6) | ||
| O2-Fe1-O5 | 91.93(5) | ||
| O2-Fe1-O6 | 93.25(5) | ||
| O1-Fe1-O5 | 94.50(5) | ||
| D-H···A | D-H/(Å) | H⋯A/(Å) | D⋯A/(Å) | <D-H⋯A/(°) | Symmetry |
|---|---|---|---|---|---|
| O1W-H1W1⋯O3 | 0.85 | 2.07 | 2.706(2) | 131 | 3/2-x, −1/2+y, z |
| O1W-H2W1⋯O1 | 0.85 | 2.05 | 2.745(3) | 138 | 1-x, −1/2+y, 1/2-z |
| O2W-H1W2⋯O1 | 0.85 | 1.99 | 2.794(3) | 157 | x, 1/2-y, 1/2+z |
| C7-H7⋯O1W | 0.93 | 2.58 | 3.365(3) | 142 | 3/2-x, −y, 1/2+z |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatterjee, A.; Thompson, L.K.; Dey, S.K. Synthesis, Structure and Magnetic Study of a Di-Iron Complex Containing N-N Bridges. Magnetochemistry 2018, 4, 53. https://doi.org/10.3390/magnetochemistry4040053
Chatterjee A, Thompson LK, Dey SK. Synthesis, Structure and Magnetic Study of a Di-Iron Complex Containing N-N Bridges. Magnetochemistry. 2018; 4(4):53. https://doi.org/10.3390/magnetochemistry4040053
Chicago/Turabian StyleChatterjee, Abhishikta, Laurence K. Thompson, and Subrata K. Dey. 2018. "Synthesis, Structure and Magnetic Study of a Di-Iron Complex Containing N-N Bridges" Magnetochemistry 4, no. 4: 53. https://doi.org/10.3390/magnetochemistry4040053





