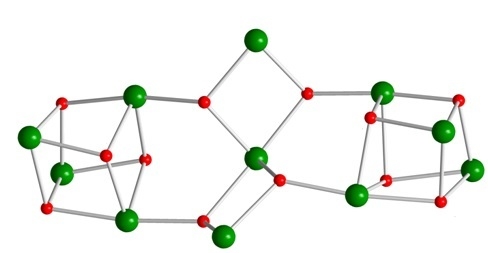
A Ni11 Coordination Cluster from the Use of the Di-2-Pyridyl Ketone/Acetate Ligand Combination: Synthetic, Structural and Magnetic Studies
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Physical Measurements
2.2. Synthesis of [Ni11(OH)6(O2CMe)12{(py)2C(OH)(O)}4(H2O)2]∙1.2MeCN∙3.2H2O (1∙1.2MeCN∙3.2H2O)
2.3. Single-Crystal X-ray Crystallography
3. Results and Discussion
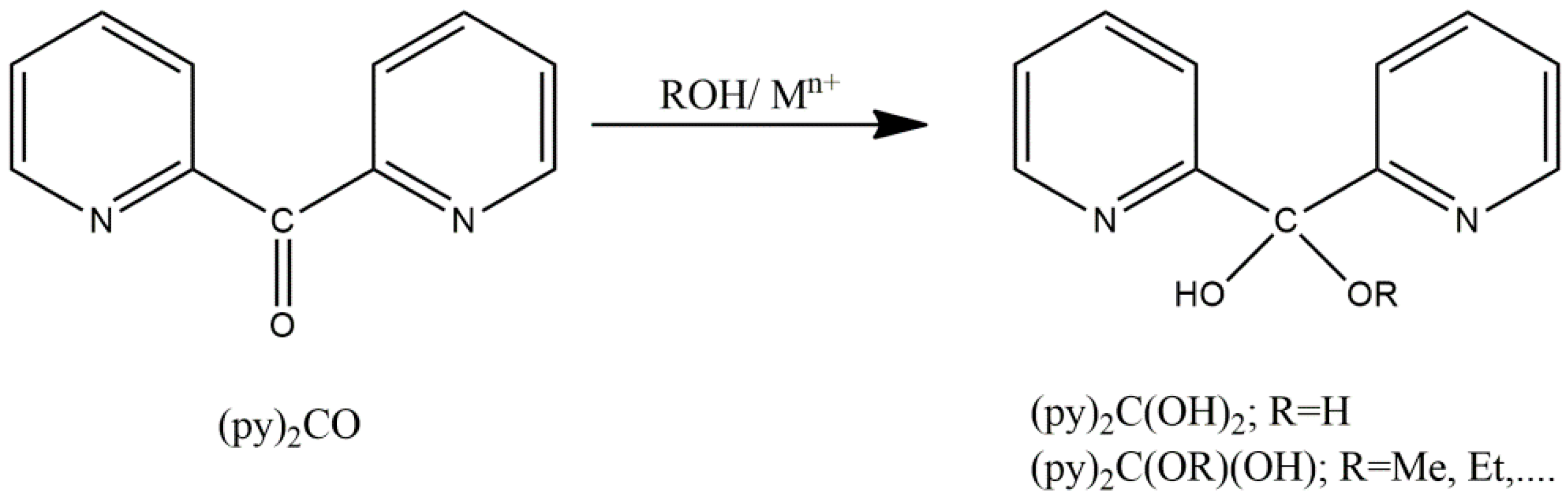
3.1. Synthetic Comments and IR Discussion in Brief
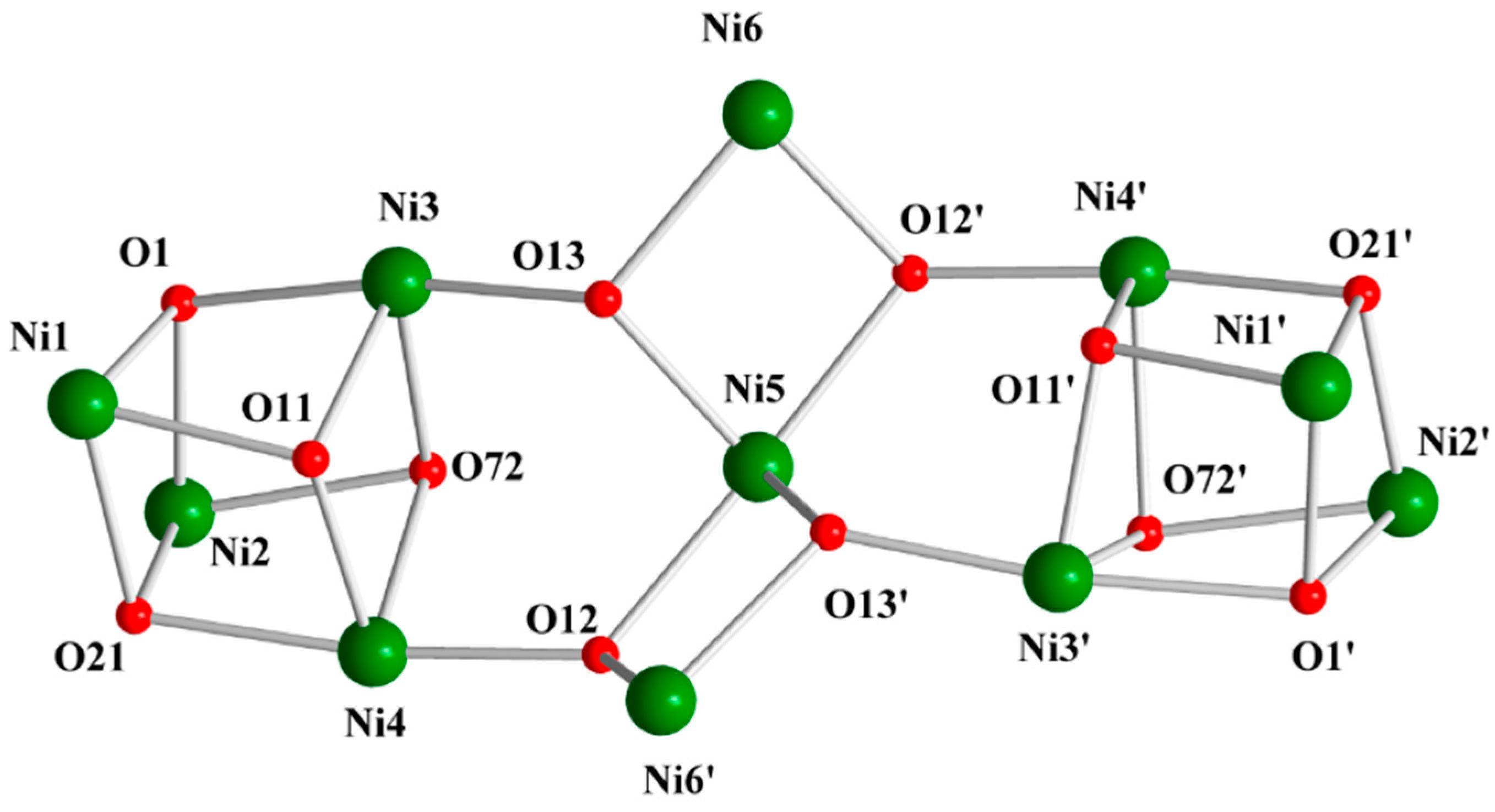
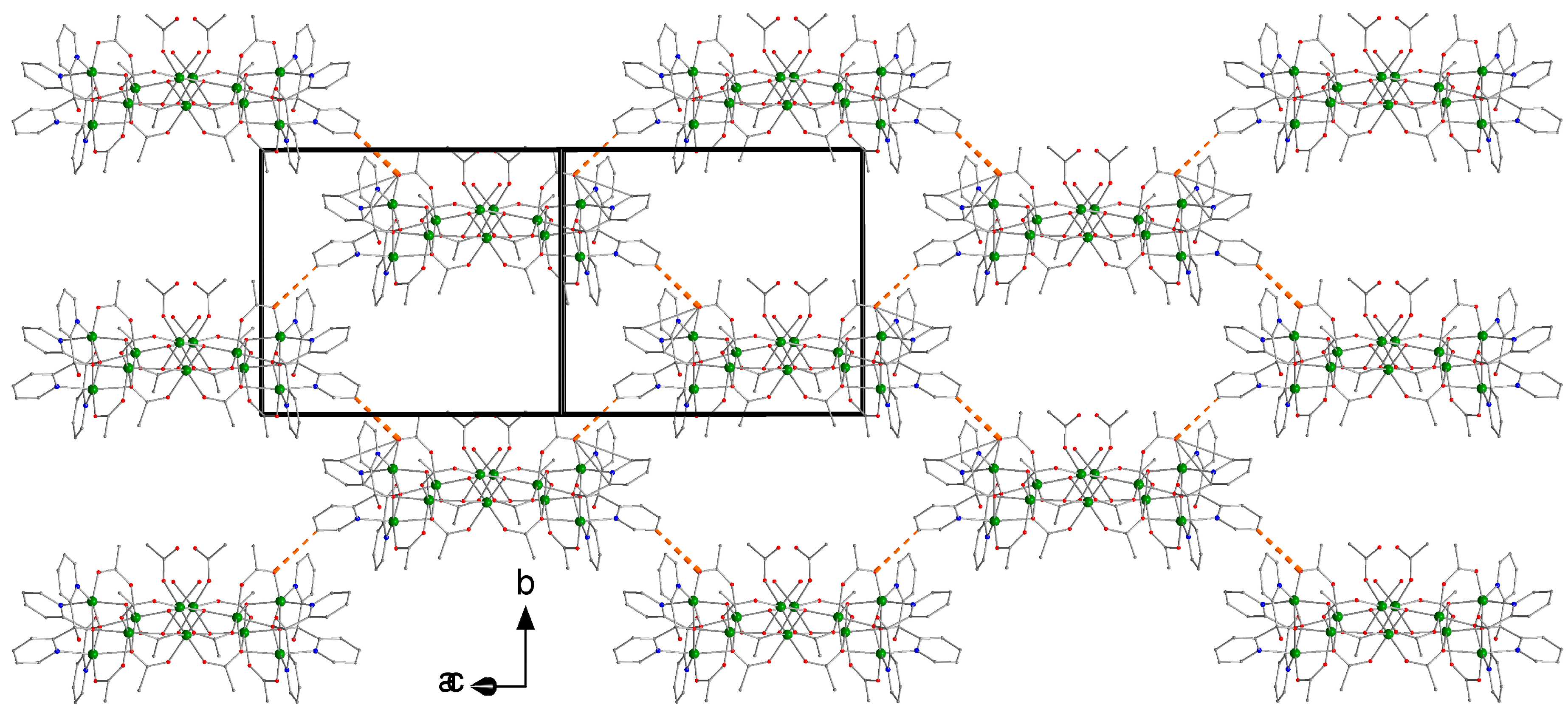
3.2. Description of Structure
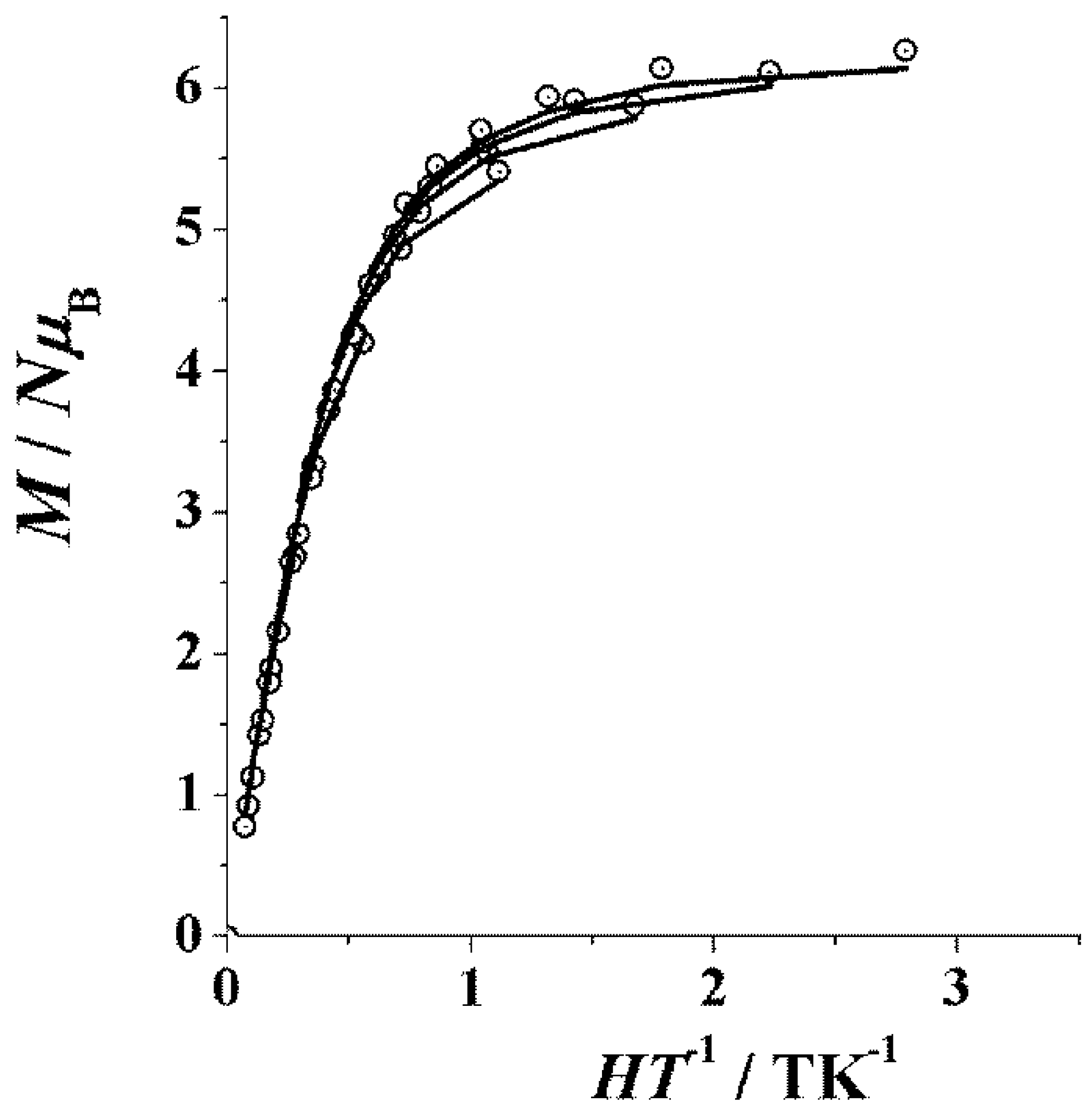
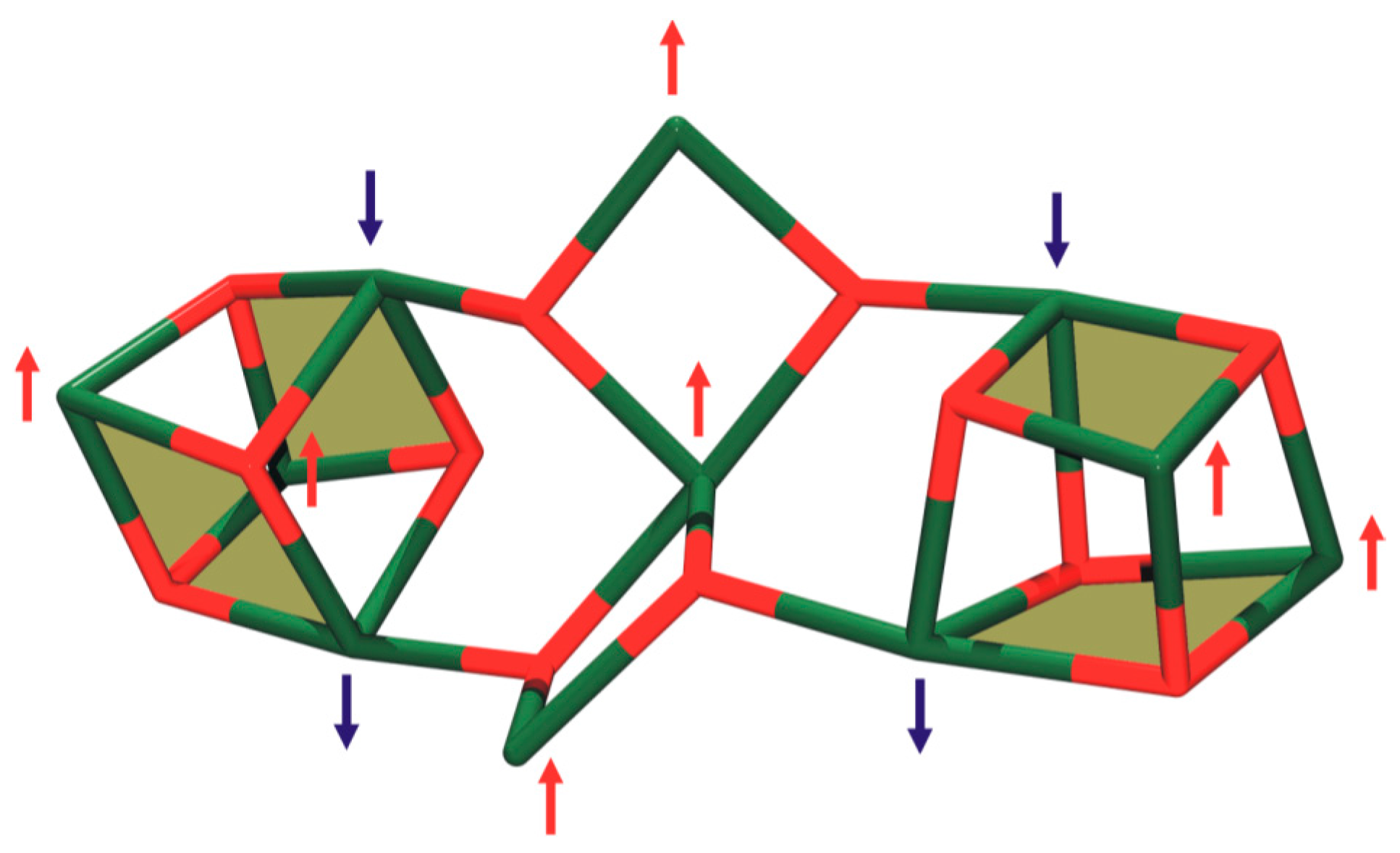
3.3. Magnetochemistry
4. Concluding Comments and Perspectives
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vinslava, A.; Tasiopoulos, A.J.; Wernsdorfer, W.; Abboud, K.A.; Christou, G. Molecules at the Quantum-Classical Nanoparticle Interface: Giant Mn70 Single-Molecule Magnets of ~4 nm Diameter. Inorg. Chem. 2016, 55, 3419–3430. [Google Scholar] [CrossRef] [PubMed]
- Brechin, E.K. Using tripodal alcohols to build high-spin molecules and single-molecule magnets. Chem. Commun. 2005, 5141–5153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, C.; Dong, H.; Shen, J.-R.; Dau, H.; Zhao, J. A synthetic Mn4Ca-cluster mimicking the oxygen-evolving center of photosynthesis. Science 2015, 348, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Glatzel, P.; Schroeder, H.; Pushkar, Y.; Boron, T., III; Mukherjee, S.; Christou, G.; Pecoraro, V.L.; Messinger, J.; Yachandra, V.K.; Bergmann, U.; et al. Electronic Structural Changes of Mn in the Oxygen-Evolving Complex of Photosystem II during the Catalytic Cycle. Inorg. Chem. 2013, 52, 5642–5644. [Google Scholar] [CrossRef] [PubMed]
- Ako, A.M.; Hewitt, I.J.; Mereacre, V.; Clérac, R.; Wernsdorfer, W.; Anson, C.E.; Powell, A.K. A Ferromagnetically Coupled Mn19 Aggregate with a Record S = 83/2 Ground Spin State. Angew. Chem. Int. Ed. 2006, 45, 4926–4929. [Google Scholar] [CrossRef] [PubMed]
- Bagai, R.; Christou, G. The Drosophila of single-molecule magnetism: [Mn12O12(O2CR)16(H2O)4]. Chem. Soc. Rev. 2009, 38, 1011–1026. [Google Scholar] [CrossRef] [PubMed]
- Milios, C.J.; Winpenny, R.E.P. Cluster-Based Single-Molecule Magnets. Struct. Bond. 2015, 164, 1–109. [Google Scholar]
- Evangelisti, M.; Luis, F.; De Jongh, L.J.; Affronte, M. Magnetothermal properties of molecule-based materials. J. Mater. Chem. 2006, 16, 2534–2549. [Google Scholar] [CrossRef]
- Shaw, R.; Laye, R.H.; Jones, L.F.; Low, D.M.; Talbot-Eeckelaers, C.; Wei, Q.; Milios, C.J.; Teat, S.; Helliwell, M.; Raftery, J.; et al. 1,2,3-Triazolate-Bridged Tetradecametallic Transition Metal Clusters [Mn14(L)6O6(OMe)18X6] (M = FeIII, CrIII and VIII/IV) and Related Compounds: Ground-State Spins Ranging from S = 0 to S = 25 and Spin-Enhanced Magnetocaloric Effect. Inorg. Chem. 2007, 46, 4968–4978. [Google Scholar] [CrossRef] [PubMed]
- Carretta, S.; Santini, P.; Amoretti, G.; Affronte, M.; Candini, A.; Ghirri, A.; Tidmarsh, I.S.; Laye, R.H.; Shaw, R.; McInnes, E.J.L. High-Temperature Slow Relaxation of the Magnetization in Ni10 Magnetic Molecules. Phys. Rev. Lett. 2006, 97, 207201-1–207201-4. [Google Scholar] [CrossRef] [PubMed]
- Stamatatos, T.C.; Escuer, A.; Abboud, K.A.; Raptopoulou, C.P.; Perlepes, S.P.; Christou, G. Unusual Structural Types in Nickel Cluster Chemistry from the Use of Pyridyl Oximes: Ni5, Ni12Na2 and Ni14 Clusters. Inorg. Chem. 2008, 47, 11825–11838. [Google Scholar] [CrossRef] [PubMed]
- Papatriantafyllopoulou, C.; Stamatatos, T.C.; Wernsdorfer, W.; Teat, S.J.; Tasiopoulos, A.J.; Escuer, A.; Perlepes, S.P. Combining Azide, Carboxylate, and 2-Pyridyloximate Ligands in Transition-Metal Chemistry: Ferromagnetic NiII5 Clusters with a Bowtie Skeleton. Inorg. Chem. 2010, 49, 10486–10496. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulou, K.I.; Raptopoulou, C.P.; Psycharis, V.; Terzis, A.; Tangoulis, V.; Stamatatos, T.C.; Perlepes, S.P. Solvent-Dependent Access to Two Different NiII4 Core Topologies from the First Use of Pyridine-2,6-dimethanol in Nickel(II) Cluster Chemistry. Aust. J. Chem. 2012, 65, 1608–1619. [Google Scholar] [CrossRef]
- Perlepe, P.S.; Athanasopoulou, A.A.; Alexopoulou, K.I.; Raptopoulou, C.P.; Psycharis, V.; Escuer, A.; Perlepes, S.P.; Stamatatos, T.C. Structural and magnetic variations in tetranuclear NiII clusters: the effect of the reaction solvent and ligand substitution on product identity. Dalton Trans. 2014, 43, 16605–16609. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulou, K.I.; Terzis, A.; Raptopoulou, C.P.; Psycharis, V.; Escuer, S.P.; Perlepes, S.P. NiII20 “Bowls” from the Use of Tridentate Schiff Bases. Inorg. Chem. 2015, 54, 5615–5617. [Google Scholar] [CrossRef] [PubMed]
- Papaefstathiou, G.S.; Perlepes, S.P. Families of Polynuclear Manganese, Cobalt, Nickel and Copper Complexes Stabilized by Various Forms of Di-2-pyridyl Ketone. Comments. Inorg. Chem. 2002, 23, 249–274. [Google Scholar] [CrossRef]
- Stamatatos, T.C.; Efthymiou, C.G.; Stoumpos, C.C.; Perlepes, S.P. Adventures in the Coordination Chemistry of Di-2-pyridyl Ketone and Related Ligands: From High-Spin Molecules and Single-Molecule Magnets to Coordination Polymers, and from Structural Aesthetics to an Exciting New Reactivity Chemistry of Coordinated Ligands. Eur. J. Inorg. Chem. 2009, 2009, 3361–3391. [Google Scholar]
- Katsenis, A.D.; Kessler, V.G.; Papaefstathiou, G.S. High-spin Ni(II) clusters: Triangles and planar tetranuclear complexes. Dalton Trans. 2011, 40, 4590–4598. [Google Scholar] [CrossRef] [PubMed]
- Jedner, S.B.; Schwöppe, H.; Nimir, H.; Rompel, A.; Brown, D.A.; Krebs, B. Ni(II) complexes as models for inhibited urease. Inorg. Chim. Acta 2002, 340, 181–186. [Google Scholar] [CrossRef]
- Efthymiou, C.G.; Raptopoulou, C.P.; Terzis, A.; Boča, R.; Korabic, M.; Mrozinski, J.; Perlepes, S.P.; Bakalbassis, E.G. A Systematic Exploration of Nickel(II)/Acetate/Di-2-pyridyl Ketone Chemistry: Neutral and Cationic Clusters, and a Novel Mononuclear Complex. Eur. J. Inorg. Chem. 2006, 2236–2252. [Google Scholar] [CrossRef]
- Tong, M.-L.; Zheng, S.-L.; Shi, J.-X.; Tong, Y.-X.; Lee, H.K.; Chen, X.-M. Synthesis, crystal structures and properties of six cubane-like transition metal complexes of di-2-pyridyl ketone in gem-diol form. J. Chem. Soc. Dalton Trans. 2002, 1727–1734. [Google Scholar] [CrossRef]
- Li, Y.-M.; Zhang, J.-J.; Fu, R.-B.; Xiang, S.-C.; Sheng, T.-L.; Yuan, D.-Q.; Huang, X.-H.; Wu, X.-T. Three new cubane like transition metal complexes of di-2-pyridyl ketone in gem-diol form: Syntheses, crystal structures and properties. Polyhedron 2006, 25, 1618–1624. [Google Scholar] [CrossRef]
- Papaefstathiou, G.S.; Escuer, E.; Vicente, R.; Font-Bardia, M.; Solans, X.; Perlepes, S.P. Reactivity in polynuclear transition metal chemistry as a means to obtain high-spin molecules: Substitution of μ4-OH− by η1, μ4-N3− increases nine times the ground-state S value of a nonanuclear nickel(II) cage. Chem. Commun. 2001, 2414–2415. [Google Scholar] [CrossRef]
- Ji, B.; Deng, D.; Wang, Z.; Liu, X.; Miao, S. Structure of an infinite helical water chain and discrete lattice water in metal-organic framework structures. Struct. Chem. 2008, 19, 619–623. [Google Scholar] [CrossRef]
- CrystalClear, version 1.40; Rigaku/MSC: The Woodlands, TX, USA, 2005.
- Sheldrick, G.M. SHELXS 97, Program for Structure Solution; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Stamatatos, T.C.; Raptopoulou, C.P.; Perlepes, S.P.; Boudalis, A.K. The first non-acetato members of the bis(anion)octacarboxylatotetrakis{di-2-pyridyl-methanediolate(-2)enneametal(II) family of complexes: Synthesis, X-ray structures and magnetism of [M9(N3)2(O2CCMe3)8{(py)2CO2}4] (M = Co, Ni). Polyhedron 2011, 30, 3026–3033. [Google Scholar] [CrossRef]
- Athanasopoulou, A.A.; Pilkington, M.; Raptopoulou, C.P.; Escuer, A.; Stamatatos, T.C. Structural aesthetics in molecular nanoscience: A unique Ni26 cluster with a ‘rabbit-face’ topology and a discrete Ni18 ‘molecular chain’. Chem. Commun. 2014, 50, 14942–14945. [Google Scholar] [CrossRef] [PubMed]
- Foguet-Albiol, D.; Abboud, K.A.; Christou, G. High-nuclearity homometallic iron and nickel clusters: Fe22 and Ni24 complexes from the use of N-methyldiethanolamine. Chem. Commun. 2005, 4282–4284. [Google Scholar] [CrossRef] [PubMed]
- Muche, S.; Levacheva, I.; Samsonova, O.; Plam, L.; Christou, G.; Bakowsky, U.; Holyńska, M. A Chiral, Low-Cytotoxic [Ni15]-Wheel Complex. Inorg. Chem. 2014, 53, 7642–7649. [Google Scholar] [CrossRef] [PubMed]
- Wix, P.; Kostakis, G.E.; Blatov, V.A.; Proserpio, D.M.; Perlepes, S.P.; Powell, A.K. A Database of Topological Representations of Polynuclear Nickel Compounds. Eur. J. Inorg. Chem. 2013, 2013, 520–526. [Google Scholar] [CrossRef]
- Dearden, A.L.; Parsons, S.; Winpenny, R.E.P. Synthesis, Structure and Preliminary Magnetic Studies of a Ni24 Wheel. Angw. Chem. Int. Ed. 2001, 40, 152–154. [Google Scholar] [CrossRef]
- Athanasopoulou, A.A.; Raptopoulou, C.P.; Escuer, A.; Stamatatos, T.C. Rare nuclearities in Ni(II) cluster chemistry: A Ni11 cage from the first use of N-salicylidene-2-amino-5-chlorobenzoic acid in metal cluster chemistry. RSC Adv. 2014, 4, 12680–12684. [Google Scholar] [CrossRef]
- Brechin, E.K.; Clegg, W.; Murrie, M.; Parsons, S.; Teat, S.J.; Winpenny, R.E.P. Nanoscale Cages of Manganese and Nickel with “Rock Salt” Cores. J. Am. Chem. Soc. 1998, 120, 7365–7366. [Google Scholar] [CrossRef]
- Brechin, E.K.; Parsons, S.; Winpenny, R.E.P. Uncapped and polar capped prisms of cobalt and nickel. J. Chem. Soc. Dalton Trans. 1996, 3745–3746. [Google Scholar] [CrossRef]
- Benelli, C.; Blake, A.J.; Brechin, E.K.; Coles, S.J.; Graham, A.; Harris, S.G.; Meier, S.; Parkin, A.; Parsons, S.; Seddon, A.M.; et al. A Family of Polynuclear Cobalt and Nickel Complexes Stabilized by 2-Pyridonate and Carboxylate Ligands. Chem. Eur. J. 2000, 6, 883–896. [Google Scholar]
- Nikiforova, M.E.; Kiskin, M.A.; Bogomyakov, A.S.; Aleksandrov, G.G.; Sidorov, A.A.; Mironov, V.S.; Eremenko, I.L. Synthesis, structure, and magnetic properties of new pyridonate-pivalate nickel(II) complex, [Ni11(OH)2(mhp)8(Piv)10(HCO2)2(H2O)2(Hmhp)2(HPiv)2] (mhp = 6-methyl-2-pyridonate), containing formate bridges. Inorg. Chem. Commun. 2011, 14, 362–365. [Google Scholar] [CrossRef]
- Halcrow, M.A.; Sun, J.-S.; Huffman, J.C.; Christou, G. Structural and Magnetic Properties of [Ni4(μ3-OMe)4(dbm)4(MeOH)4] and [Ni4(η1, μ3-N3)4(dbm)4(EtOH)4]. Magnetostructuctal Correlations for [Ni4X4]4+ Cubane Complexes. Inorg. Chem. 1995, 34, 4167–4177. [Google Scholar] [CrossRef]
- El Fallah, M.S.; Rentschler, E.; Caneschi, A.; Gatteschi, D. Synthesis, crystal structure and magnetic properties of the tetranuclear complex [Ni4(OCH3)4(dbm)4(CH3OH)4](C2H5)2O. Inorg. Chim. Acta 1996, 247, 231–235. [Google Scholar] [CrossRef]
- Escuer, A.; Font-Bardia, M.; Kumar, S.B.; Solans, X.; Vicente, R. Two new nickel(II) cubane compounds derived from pyridine-2-methoxide (Pym): {Ni4(Pym)4Cl4(CH3OH)4} and {Ni4(Pym)4(N3)4(CH3OH)4}. Crystal structures and magnetic properties. Polyhedron 1999, 18, 909–914. [Google Scholar] [CrossRef]
- Clemente-Juan, J.M.; Chansou, B.; Donnadieu, B.; Tuchagues, J.-P. Synthesis, Structure, and Magnetic Properties of the Low-Symmetry Tetranuclear Cubane-like Nickel Complex [Ni4(pypentO)(pym)(μ3-OH)2(μ3-Oac)2(NCS)2(OH)2]. Inorg. Chem. 2000, 39, 5515–5519. [Google Scholar] [CrossRef] [PubMed]
- Boskovic, C.; Rusanov, E.; Stoeckli-Evans, H.; Güdel, H.U. New tri- and tetranuclear transition metal spin clusters incorporating a versatile polydentate Schiff base ligand. Inorg. Chem. Commun. 2002, 5, 881–886. [Google Scholar] [CrossRef]
- Mukherjee, S.; Weyhermüller, T.; Bothe, E.; Wieghardt, K.; Chaudhuri, P. Single-Atom O-Bridged Urea in a Dinickel(II) Complex with NiII4, CuII2 and CuII4 Complexes of a Pentadentate Phenol-Containing Schiff Base with (O,N,O,N,O)-Donor Atoms. Eur. J. Inorg. Chem. 2003, 863–875. [Google Scholar] [CrossRef]
- Paine, T.K.; Rentschler, E.; Weyhermüller, T.; Chaudhuri, P. Polynuclear Nickel(II) Complexes: Preparation and Magnetic Properties of NiII4, NiII5 and NiII6 Species with Ligands containing OXO (X = S, Se, or N) Donor Atoms. Eur. J. Inorg. Chem. 2003, 3167–3178. [Google Scholar] [CrossRef]
- Aromi, G.; Batsanov, A.S.; Christian, P.; Helliwell, M.; Roubeau, O.; Timco, G.A.; Winpenny, R.E.P. Synthesis, structure and magnetic properties of hydroxyquinaldine-bridged cobalt and nickel cubanes. Dalton Trans. 2003, 4466–4471. [Google Scholar] [CrossRef]
- Moragues-Cánovas, M.; Helliwell, M.; Ricard, L.; Riviѐre, E.; Wernsdorfer, W.; Brechin, E.; Mallah, T. An Ni4 Single-Molecule Magnet: Synthesis, Structure and Low-Temperature Magnetic Behaviour. Eur. J. Inorg. Chem. 2004, 2014, 2219–2222. [Google Scholar] [CrossRef]
- Papaefstathiou, G.S.; Escuer, A.; Mautner, F.A.; Raptopoulou, G.; Terzis, A.; Perlepes, S.P.; Vicente, R. Use of the Di-2-pyridyl Ketone/Acetate/ Dicyanamide “Blend” in Manganese(II), Cobalt(II) and Nickel(II) Chemistry: Neutral Cubane Complexes. Eur. J. Inorg. Chem. 2005, 879–893. [Google Scholar] [CrossRef]
- Papatriantafyllopoulou, C.; Efthymiou, C.G.; Raptopoulou, C.P.; Vicente, R.; Manessi-Zoupa, E.; Psycharis, V.; Escuer, A.; Perlepes, S.P. Initial use of the di-2-pyridyl ketone/sulfate “blend” in 3d-metal cluster chemistry: Preparation, X-ray structures and physical studies of zinc(II) and nickel(II) cubanes. J. Mol. Struct. 2007, 829, 176–188. [Google Scholar] [CrossRef]
- Chilton, N.F.; Anderson, R.P.; Turner, L.D.; Soncini, A.; Murrax, K.S. PHI: A Powerful New Program for the Analysis of Anisotropic Monomeric and Exchange-Coupled Polynuclear d- and f-block Complexes. J. Comput. Chem. 2013, 34, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- Papaefstathiou, G.S.; Perlepes, S.P.; Escuer, A.; Vicente, R.; Font-Bardia, M.; Solans, X. Unique Single-Atom Binding of Pseudohalogeno Ligands to Four Metal Ions Induced by Their Trapping into High-Nuclearity Cages. Angew. Chem. Int. Ed. 2001, 40, 884–886. [Google Scholar] [CrossRef]
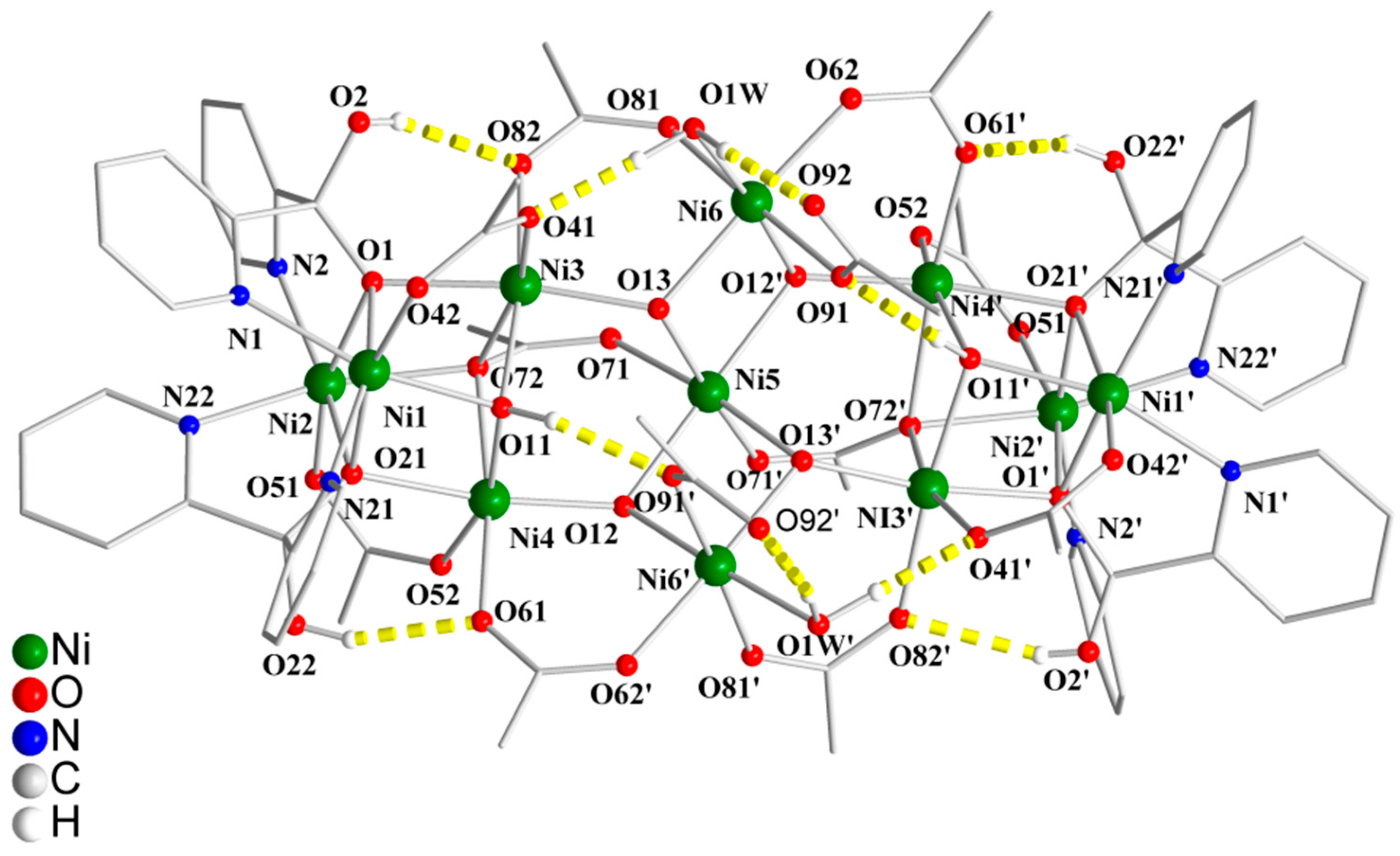
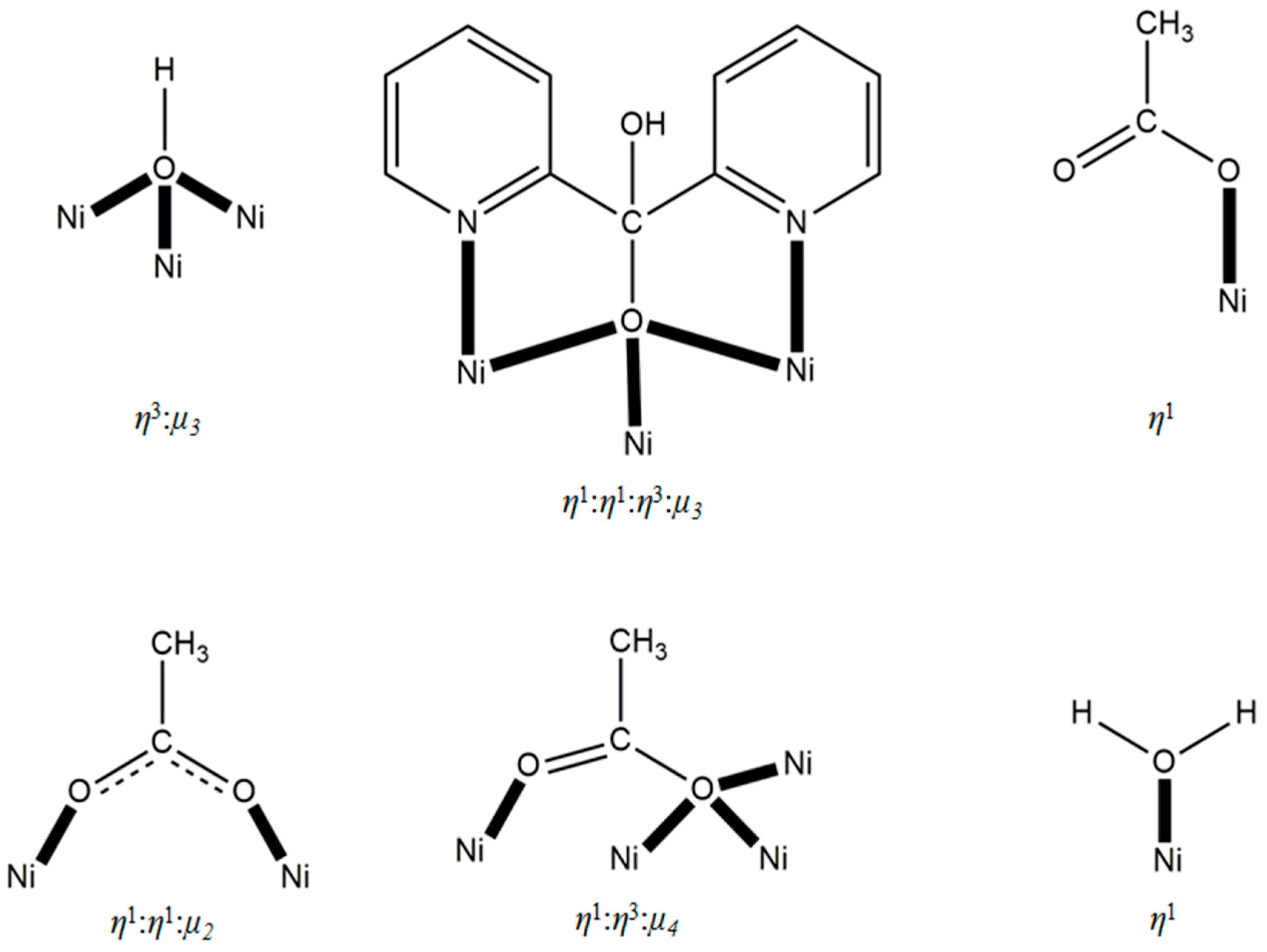




| Formula | C70.4H92N9.2Ni11O43.2 |
|---|---|
| Formula weight | 2404.14 |
| Crystal system | monoclinic |
| Space group | I2/a |
| Radiation | Cu Kα |
| T/K | 293 |
| a/Å | 24.8808(5) |
| b/Å | 15.2009(3) |
| c/Å | 27.2265(6) |
| β/° | 96.521(6) |
| V/Å3 | 10230.7(4) |
| Z | 4 |
| Dcalc/g·cm−3 | 1.561 |
| μ/mm−1 | 2.87 |
| Reflns measured | 60292 |
| Reflns unique (Rint) | 8852 |
| Reflns with I > 2σ (Ι) | 7067 |
| GOF on F2 | 1.05 |
| R1 a [Ι > 2σ (Ι)] | 0.0520 |
| wR2 b (all data) | 0.1611 |
| Interatomic Distances (Å) a | |||
| Ni1∙∙∙Ni2 | 3.024(1) | Ni1∙∙∙O1 | 2.045(3) |
| Ni1∙∙∙Ni3 | 2.928(1) | Ni1∙∙∙O21 | 2.106(3) |
| Ni1∙∙∙Ni4 | 3.285(1) | Ni1∙∙∙N1 | 2.151(4) |
| Ni2∙∙∙Ni3 | 3.440(1) | Ni2∙∙∙O21 | 1.997(3) |
| Ni2∙∙∙Ni4 | 3.002(1) | Ni2∙∙∙O72 | 2.229(3) |
| Ni3∙∙∙Ni4 | 3.154(1) | Ni2∙∙∙N22 | 2.143(3) |
| Ni5∙∙∙Ni6 | 3.083(1) | Ni3∙∙∙O11 | 2.014(3) |
| Ni1∙∙∙Ni5 | 5.763(1) | Ni3∙∙∙O72 | 2.214(3) |
| Ni2∙∙∙Ni5 | 5.496(1) | Ni3∙∙∙O82 | 2.017(3) |
| Ni3∙∙∙Ni5 | 3.457(1) | Ni4∙∙∙O11 | 2.018(3) |
| Ni4∙∙∙Ni5 | 3.634(1) | Ni4∙∙∙O52 | 2.066(3) |
| Ni1∙∙∙Ni6′ | 5.831(1) | Ni4∙∙∙O72 | 2.258(3) |
| Ni2∙∙∙Ni6′ | 6.396(1) | Ni5∙∙∙O12 | 2.099(3) |
| Ni3∙∙∙Ni6′ | 4.897(1) | Ni5∙∙∙O13 | 2.050(3) |
| Ni4∙∙∙Ni6′ | 3.477(1) | Ni5∙∙∙O71 | 2.074(3) |
| Ni1∙∙∙Ni1′ | 10.859(1) | Ni6∙∙∙O12′ | 2.053(3) |
| Ni1∙∙∙Ni2′ | 8.599(1) | Ni6∙∙∙O81 | 2.078(3) |
| Ni2∙∙∙Ni4′ | 8.855(1) | Ni6∙∙∙O1W | 2.090(3) |
| Bond Angles (°) a | |||
| O1–Ni1–N21 | 158.2(1) | Ni1–O1–Ni3 | 89.1(1) |
| O11–Ni1–N1 | 160.7(1) | Ni2–O1–Ni3 | 109.3(1) |
| O21–Ni1–O42 | 165.5(1) | Ni1–O11–Ni3 | 92.1(1) |
| O1–Ni2–O51 | 164.4(1) | Ni1–O11–Ni4 | 107.6(1) |
| O21–Ni2–N2 | 158.8(1) | Ni3–O11–Ni4 | 102.9(1) |
| O72–Ni2–N22 | 166.1(1) | Ni1–O21–Ni2 | 95.0(1) |
| O1–Ni3–O13 | 171.0(1) | Ni1–O21–Ni4 | 102.8(1) |
| O11–Ni3–O82 | 171.8(1) | Ni2–O21–Ni4 | 94.3(1) |
| O41–Ni3–O72 | 166.1(1) | Ni2–O72–Ni3 | 101.5(1) |
| O11–Ni4–O52 | 161.2(1) | Ni2–O72–Ni4 | 84.0(1) |
| O12–Ni4–O21 | 172.1(1) | Ni3–O72–Ni4 | 89.7(1) |
| O61–Ni4–O72 | 172.6(1) | Ni4–O12–Ni5 | 125.3(2) |
| O12–Ni5–O12′ | 175.6(2) | Ni4–O12–Ni6′ | 118.5(1) |
| O13–Ni5–O71′ | 172.6(1) | Ni5–O12–Ni6′ | 95.9(1) |
| O12'–Ni6–O1W | 174.4(1) | Ni3–O13–Ni5 | 117.1(2) |
| O13–Ni6–O62 | 175.8(1) | Ni3–O13–Ni6 | 121.4(2) |
| O81–Ni6–O91 | 178.0(1) | Ni5–O13–Ni6 | 97.8(1) |
| Ni1–O1–Ni2 | 94.0(1) | - | - |
| D–H∙∙∙A | D–H (Å) | H∙∙∙A (Å) | D∙∙∙A (Å) | D–H∙∙∙A (°) |
|---|---|---|---|---|
| Intramolecular | ||||
| O11–H(O11)∙∙∙O91′ | 0.90(5) | 1.93(5) | 2.793(4) | 159(4) |
| O2–H(O2)∙∙∙O82 | 0.71(5) | 2.08(5) | 2.759(5) | 161(6) |
| O22–H(O22)∙∙∙O61 | 0.84(6) | 1.98(6) | 2.763(4) | 157(6) |
| O1W–HA(O1W)∙∙∙O41 | 0.97(6) | 1.76(6) | 2.718(4) | 169(5) |
| O1W–HB(O1W)∙∙∙O92 | 0.86(5) | 1.80(5) | 2.637(6) | 165(5) |
| Intermolecular | ||||
| C8–H8∙∙∙O1W′′ | 0.91(5) | 2.49(5) | 3.212(7) | 136(4) |
| C30–H30∙∙∙O42′′′ | 1.10(6) | 2.45(6) | 3.495(6) | 158(4) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
G. Efthymiou, C.; Mylonas-Margaritis, I.; P. Raptopoulou, C.; Psycharis, V.; Escuer, A.; Papatriantafyllopoulou, C.; P. Perlepes, S. A Ni11 Coordination Cluster from the Use of the Di-2-Pyridyl Ketone/Acetate Ligand Combination: Synthetic, Structural and Magnetic Studies. Magnetochemistry 2016, 2, 30. https://doi.org/10.3390/magnetochemistry2030030
G. Efthymiou C, Mylonas-Margaritis I, P. Raptopoulou C, Psycharis V, Escuer A, Papatriantafyllopoulou C, P. Perlepes S. A Ni11 Coordination Cluster from the Use of the Di-2-Pyridyl Ketone/Acetate Ligand Combination: Synthetic, Structural and Magnetic Studies. Magnetochemistry. 2016; 2(3):30. https://doi.org/10.3390/magnetochemistry2030030
Chicago/Turabian StyleG. Efthymiou, Constantinos, Ioannis Mylonas-Margaritis, Catherine P. Raptopoulou, Vassilis Psycharis, Albert Escuer, Constantina Papatriantafyllopoulou, and Spyros P. Perlepes. 2016. "A Ni11 Coordination Cluster from the Use of the Di-2-Pyridyl Ketone/Acetate Ligand Combination: Synthetic, Structural and Magnetic Studies" Magnetochemistry 2, no. 3: 30. https://doi.org/10.3390/magnetochemistry2030030