Comparative Magnetic Studies in the Solid State and Solution of Two Isostructural 1D Coordination Polymers Containing CoII/NiII-Curcuminoid Moieties
Abstract
:1. Introduction
2. Results and Discussions
2.1. Experimental Results
2.1.1. Synthesis of [Co(9Accm)2(4,4´-bpy)]n (1)
2.1.2. Synthesis of [Ni(9Accm)2(4,4´-bpy)]n (2)
2.2. Structural Description
2.3. Studies in Solution
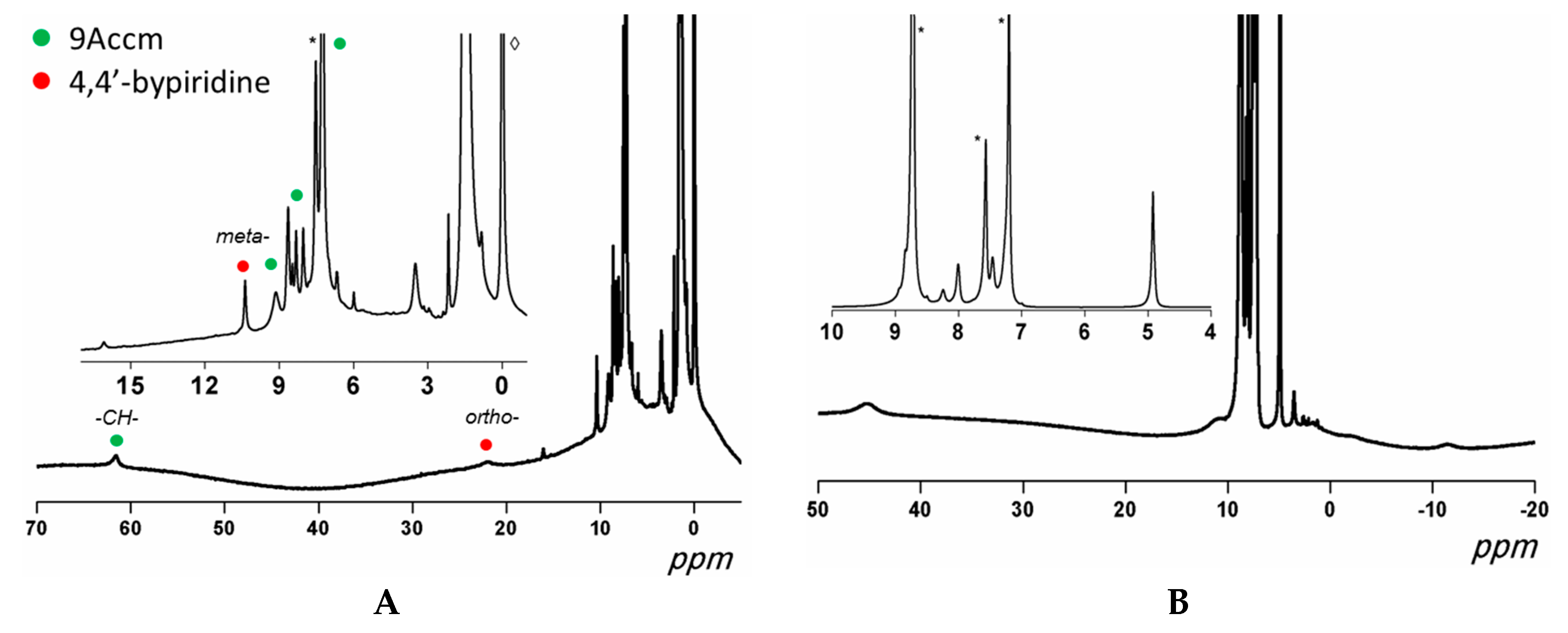
2.3.1. Paramagnetic 1H NMR
2.3.2. UV-Vis Absorption Spectra and Fluorescence
2.4. Studies in the Solid State
2.4.1. Static Magnetic Properties
2.4.2. Dynamic magnetic properties
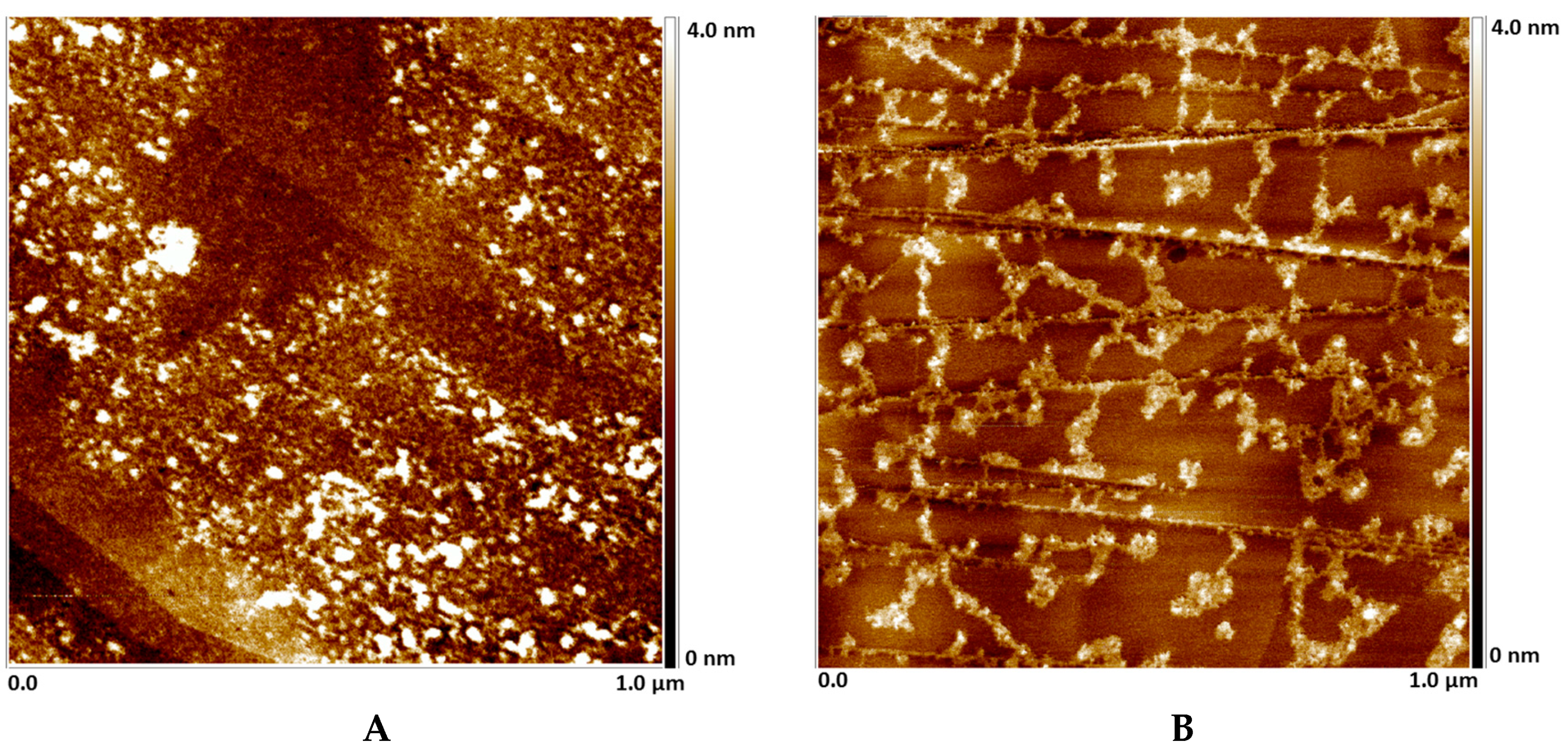
2.5. AFM deposition studies on HOPG
3. Materials and Methods
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pedersen, K.S.; Bendix, J.; Clérac, R. Single-Molecule magnet engineering: building-block approaches. Chem. Commun. 2014, 50, 4396–4415. [Google Scholar]
- Coulon, C.; Pianet, V.; Urdampilleta, M.; Clérac, R. Single-Chain Magnets and Related Systems. In Molecular Nanomagnetis and Related Phenomena; Springer GmbH: Berlin, Germany, 2015; Volume 164, pp. 143–184. [Google Scholar]
- Metzger, R.M. Unimolecular Electronics. Chem. Rev. 2015, 115, 5056–5115. [Google Scholar] [CrossRef]
- Schwarz, F.; Lörtscher, E. Break-junctions for investigating transport at the molecular scale. J. Phys.: Condens. Matter 2014, 26, 474201. [Google Scholar] [CrossRef]
- Clemente-Juan, J.M.; Coronado, E.; Gaita-Ariño, A. Magnetic polyoxometalates: From molecular magnetism to molecular spintronics and quantum computing. Chem. Soc. Rev. 2012, 41, 7464–7478. [Google Scholar] [CrossRef]
- Bogani, L.; Wernsdorfer, W. Molecular spintronics using single-molecule magnets. Nature Mat. 2008, 7, 179–186. [Google Scholar] [CrossRef]
- Atanasov, M.; Aravena, D.; Suturina, E.; Bill, E.; Maganas, D.; Neese, F. First principles approach to the electronic structure, magnetic anisotropy and spin relaxation in mononuclear 3d-transition metal single molecule magnets. Coord. Chem. Rev. 2015, 289–290, 177–214. [Google Scholar] [CrossRef]
- Dreiser, J. Molecular lanthanide single-ion magnets: From bulk to submonolayers. J. Phys.: Condens. Matter 2015, 27, 183203. [Google Scholar] [CrossRef]
- Harriman, K.L.; Murugesu, M. An Organolanthanide Building Block Approach to Single-Molecule Magnets. Acc. Chem. Res. 2016. [Google Scholar] [CrossRef]
- Gómez-Coca, S.; Cremades, E.; Aliaga-Alcalde, N.; Ruiz, E. Mononuclear Single-Molecule Magnets: Tailoring the Magnetic Anisotropy of First-Row Transition-Metal Complexes. J. Am. Chem. Soc. 2013, 135, 7010–7018. [Google Scholar] [CrossRef]
- Fataftah, M.S.; Zadrozny, J.M.; Rogers, D.M.; Freedman, D.E. A Mononuclear Transition Metal Single-Molecule Magnet in a Nuclear Spin-Free Ligand Environment. Inorg. Chem. 2014, 53, 10716–10721. [Google Scholar] [CrossRef]
- Habib, F.; Korobkov, I.; Murugesu, M. Exposing the intermolecular nature of the second relaxation pathway in a mononuclear cobalt (II) single-molecule magnet with positive anisotropy. Dalton Trans. 2015, 44, 6368–6373. [Google Scholar] [CrossRef]
- Gómez-Coca, S.; Urtizberea, A.; Cremades, E.; Alonso, P.J.; Camon, A.; Ruiz, E.; Luis, F. Origin of slow magnetic relaxation in Kramers ions with non-uniaxial anisotropy. Nature Commun. 2014, 5, 4300. [Google Scholar] [CrossRef]
- Jiang, G.; Song, Y.; Guo, X.; Zhang, D.; Zhu, D. Organic Functional Molecules toward Information Processing and High-Density Information Storage. Adv. Mat. 2008, 20, 2888–2898. [Google Scholar] [CrossRef]
- Aliaga-Alcalde, N.; Marqués-Gallego, P.; Kraaijkamp, M.; Herranz-Lancho, C.; den Dulk, H.; Gorner, H.; Roubeau, O.; Teat, S.J.; Weyhermüller, T.; Reedijk, J. Copper Curcuminoid Containing Anthracene Groups: Fluorescent Molecules with Cytotoxic Activity. Inorg. Chem. 2010, 49, 9655–9663. [Google Scholar] [CrossRef]
- Prins, F.; Barreiro, A.; Ruitenberg, J.W.; Seldenthuis, J.S.; Aliaga-Alcalde, N.; Vandersypen, L.M.K.; van der Zant, H.S.J. Room-Temperature Gating of Molecular Junctions Using Few-Layer Graphene Nanogap Electrodes. Nano Letters 2011, 11, 4607–4611. [Google Scholar] [CrossRef]
- Aliaga-Alcalde, N.; Rodríguez, L.; Ferbinteanu, M.; Hofer, P.; Weyhermüller, T. Crystal Structure, Fluorescence and Nanostructuration Studies of the First ZnII Anthracene-Based Curcuminoid. Inorg. Chem. 2012, 51, 864–873. [Google Scholar] [CrossRef]
- Menelaou, M.; Ouharrou, F.; Rodríguez, L.; Roubeau, O.; Teat, S.J.; Aliaga-Alcalde, N. DyIII- and YbIII-Curcuminoid Compounds: Original Fluorescent Single-Ion Magnet and Magnetic Near-IR Luminescent Species. Chem. Eur. J. 2012, 18, 11545–11549. [Google Scholar] [CrossRef]
- Aliaga-Alcalde, N.; Rodríguez, L. Solvatochromic studies of a novel Cd2+-anthracene-based curcuminoid and related complexes. Inorg. Chim. Acta 2012, 380, 187–193. [Google Scholar] [CrossRef]
- Menelaou, M.; Weyhermüller, T.; Soler, M.; Aliaga-Alcalde, N. Novel paramagnetic-luminescent building blocks containing manganese (II) and anthracene-based curcuminoids. Polyhedron 2013, 52, 398–405. [Google Scholar] [CrossRef]
- Tanh, J.; Harold, B.; Staudt, C.; Janiak, C. Metal-organic frameworks in mixed-matrix membranes for gas separation. Dalton Trans. 2012, 41, 14003–14027. [Google Scholar] [CrossRef]
- Díaz-Torres, R.; Menelaou, M.; Roubeau, O.; Sorrenti, A.; Brandariz-de-Pedro, G.; Sañudo, E.C.; Teat, S.J.; Fraxedas, J.; Ruiz, E.; Aliaga-Alcalde, N. Multiscale study of mononuclear CoII SMMs based on curcuminoid ligands. Chem. Sci. 2016, 7, 2793–2803. [Google Scholar] [CrossRef]
- Gangu, K.K.; Maddila, S.; Mukkamala, S.B.; Jonnalagadda, S.B. A review on contemporary Metal-Organic Framework materials. Inorg. Chim. Acta 2016, 446, 61–74. [Google Scholar] [CrossRef]
- Charde, M.; Shukla, A.; Bukhariya, V.; Mehta, J.; Chakole, R. A Review on: A significance of microwave assist technique in green chemistry. Int. J. Phytopharm. 2012, 2, 39–50. [Google Scholar] [CrossRef]
- Shoichiro, Y. Recent aspects of the stereochemistry of Schiff base-metal complexes. Coord. Chem. Rev. 1996, 1, 415–437. [Google Scholar] [CrossRef]
- Schwarzhans, K.E. NMR spectroscopy of paramagnetic complexes. Angew. Chem. Int. Ed. 1970, 9, 946–953. [Google Scholar] [CrossRef]
- Wagner, B.D.; McManus, G.J.; Moulton, B. Zaworotko, Exciplex fluorescence of {[Zn(bipy)1.5(NO3)2}]·CH3OH·0.5pyrene}n: A coordination polymer containing intercalated pyrene molecules (bipy = 4,4'-bipyridine). Chem. Commun. 2002, 2176–2177. [Google Scholar] [CrossRef]
- Birks, J.B. Excimers. Rep. Prog. Phys. 1975, 38, 903–974. [Google Scholar] [CrossRef]
- Khokhlova, S.S.; Burshtein, A.I. General theory of Weller Scheme I of exciplex formation. Chem. Phys. Letters 2011, 508, 324–328. [Google Scholar] [CrossRef]
- Eisenthal, K.B. Intermolecular and intramolecular excited state charge transfer. Laser Chem. 1983, 3, 145–162. [Google Scholar] [CrossRef]
- Jensen, P.; Batten, S.R.; Moubaraki, B.; Murray, K.S. Synthesis, crystal structures and magnetic properties of first row transition metal coordination polymers containing dicyanamide and 4,4´-bipyridine. Dalton Trans. 2002, 3712–3722. [Google Scholar] [CrossRef]
- Aguilà, D.; Barrios, L.A.; Roubeau, O.; Teat, S.J.; Aromí, G. Molecular assembly of two [Co(II)4] linear arrays. Chem. Commun. 2011, 47, 707–709. [Google Scholar] [CrossRef]
- Plenk, C.; Krause, J.; Rentschler, E. A Click-Functionalized Single-Molecule Magnet Based on Cobalt(II) and Its Analogous Manganese(II) and Zinc(II) Compounds. Eur. J. Inorg. Chem. 2015, 370–374. [Google Scholar] [CrossRef]
- Chilton, N.F.; Anderson, R.P.; Turner, L.D.; Soncini, A.; Murray, K.S. PHI: A powerful new program for the analysis of anisotropic monomeric and exchange-coupled polynuclear d- and f-block complexes. J. Comput. Chem. 2013, 34, 1164–1175. [Google Scholar] [CrossRef]
- Miklovič, J.; Valiqura, D.; Boča, R.; Titiš, J. A mononuclear Ni(II) complex: A field induced single-molecule magneti showing two slow relaxation processes. Inorg. Chem. 2015, 44, 12484–12487. [Google Scholar] [CrossRef]
- Miyasaka, H.; Mizushima, K.; Sugiura, K.; Yamashita, M. A series of Ni(II) pyridyloximato (pao-) compounds [Ni(pao)2(L)2] (L = unidentate ligand): As a coordination donor building block in the assembly with Mn(III) salen analogues. Synth. Metals 2003, 137, 1245–1246. [Google Scholar] [CrossRef]
- Miyasaka, H.; Saitoh, A.; Yanagida, S.; Kachi-Terajima, C.; Sugiura, K.-I.; Yamashita, M. Nickel(II) and iron(II) mononuclear complexes with 1-methylimidazole-2-aldoximate: New building units for molecule-assembled magnetic materials. Inorg. Chim. Acta 2005, 358, 3525–3535. [Google Scholar] [CrossRef]
- Paharová, J.; Cernák, J.; Boča, R.; Zák, Z. Preparation, structure and magnetic properties of two tetracyanonickellates: One-dimensional Ni(dien)(mea)Ni(CN)4 and ionic [Ni(aepn)2][Ni(CN)4]·H2O. Inorg. Chim. Acta 2003, 346, 25–31. [Google Scholar] [CrossRef]
- Chen, M.; Zhao, H.; Sañudo, E.C.; Liu, C.-S.; Du, M. Two isostructural coordination polymers showing diverse magnetic behaviors: weak coupling (NiII) and an ordered array of single-shain sagnets (CoII). Inorg. Chem. 2016, 55, 3715–3717. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef]




© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Torres, R.; Menelaou, M.; González-Campo, A.; Teat, S.J.; Sañudo, E.C.; Soler, M.; Aliaga-Alcalde, N. Comparative Magnetic Studies in the Solid State and Solution of Two Isostructural 1D Coordination Polymers Containing CoII/NiII-Curcuminoid Moieties. Magnetochemistry 2016, 2, 29. https://doi.org/10.3390/magnetochemistry2030029
Díaz-Torres R, Menelaou M, González-Campo A, Teat SJ, Sañudo EC, Soler M, Aliaga-Alcalde N. Comparative Magnetic Studies in the Solid State and Solution of Two Isostructural 1D Coordination Polymers Containing CoII/NiII-Curcuminoid Moieties. Magnetochemistry. 2016; 2(3):29. https://doi.org/10.3390/magnetochemistry2030029
Chicago/Turabian StyleDíaz-Torres, Raúl, Melita Menelaou, Arántzazu González-Campo, Simon J. Teat, E. Carolina Sañudo, Mónica Soler, and Núria Aliaga-Alcalde. 2016. "Comparative Magnetic Studies in the Solid State and Solution of Two Isostructural 1D Coordination Polymers Containing CoII/NiII-Curcuminoid Moieties" Magnetochemistry 2, no. 3: 29. https://doi.org/10.3390/magnetochemistry2030029