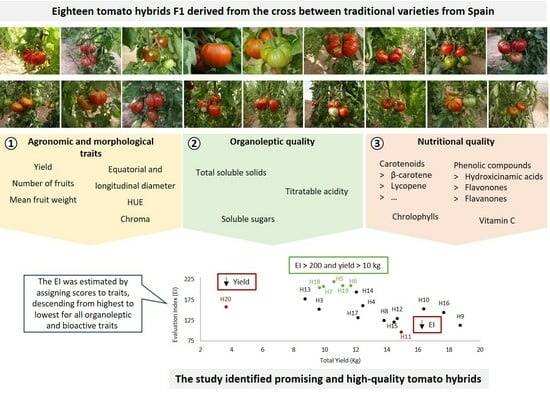
Fruit Agronomic and Quality Traits of Tomato F1 Hybrids Derived from Traditional Varieties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cultivation Conditions and Plant Material
2.2. Agronomic and Phenotypic Evaluation
2.3. Analysis of Fruit Quality and Nutritional Value Traits
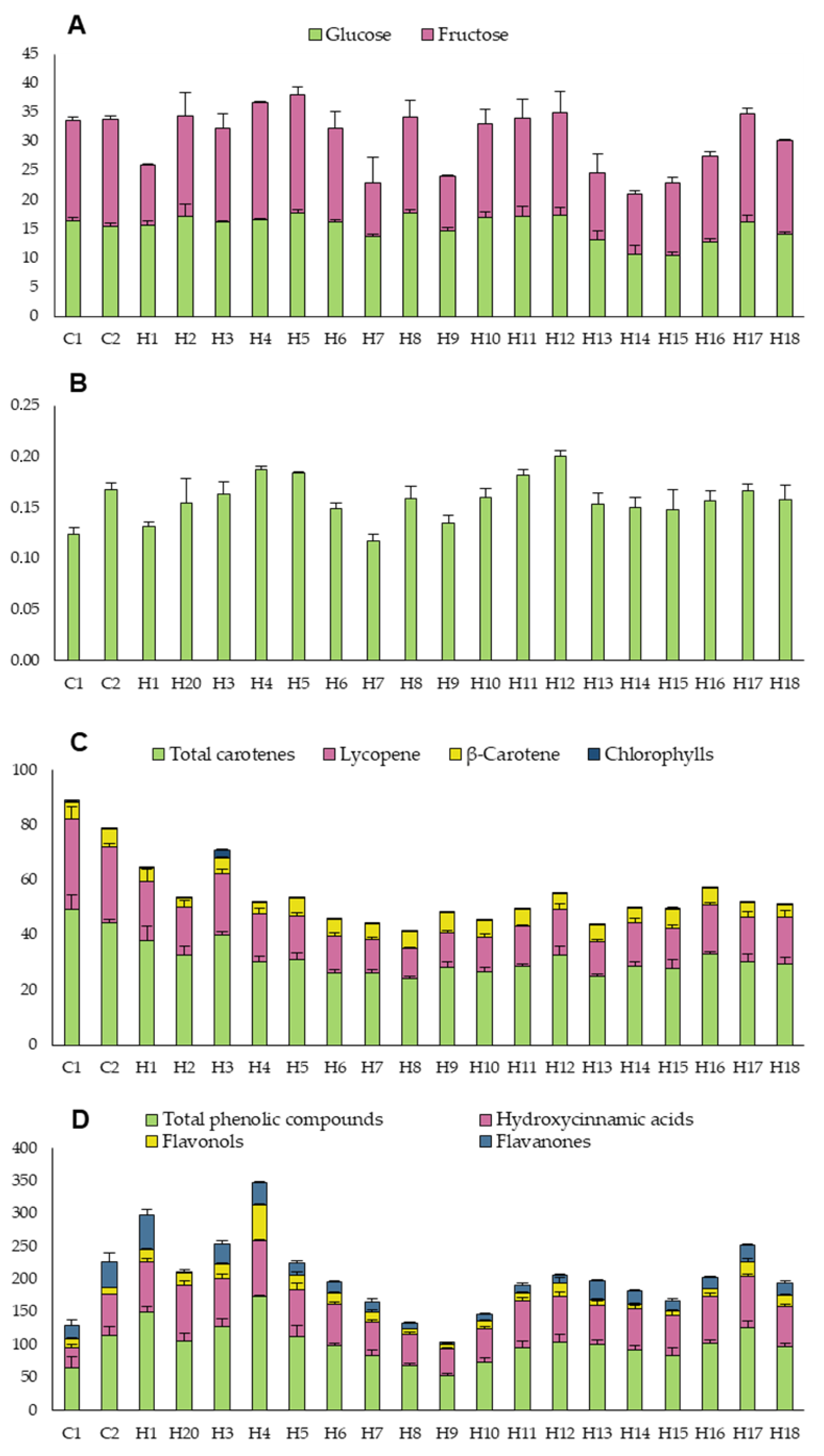
2.4. Determination of Soluble Sugars
2.5. Determination of Carotenoids and Chlorophylls
2.6. Determination of Vitamin C
2.7. Determination of Phenolic Compounds
2.8. Statistical Analysis
3. Results
3.1. Description of Agronomic, Physical, Organoleptic, and Bioactive Traits in Parental Lines and Hybrids
3.1.1. Agronomic and Physical Traits
3.1.2. Organoleptic and Bioactive Traits
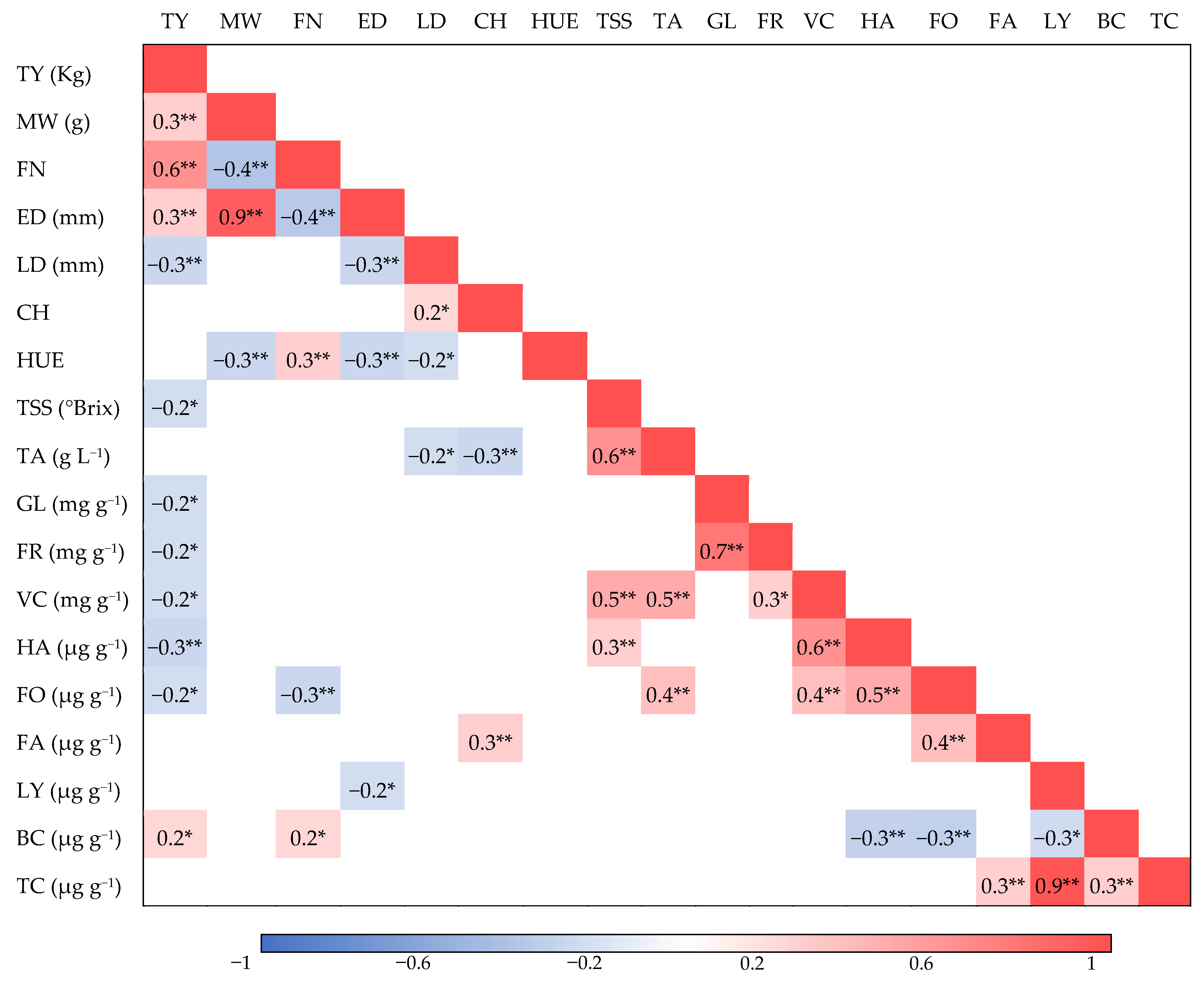
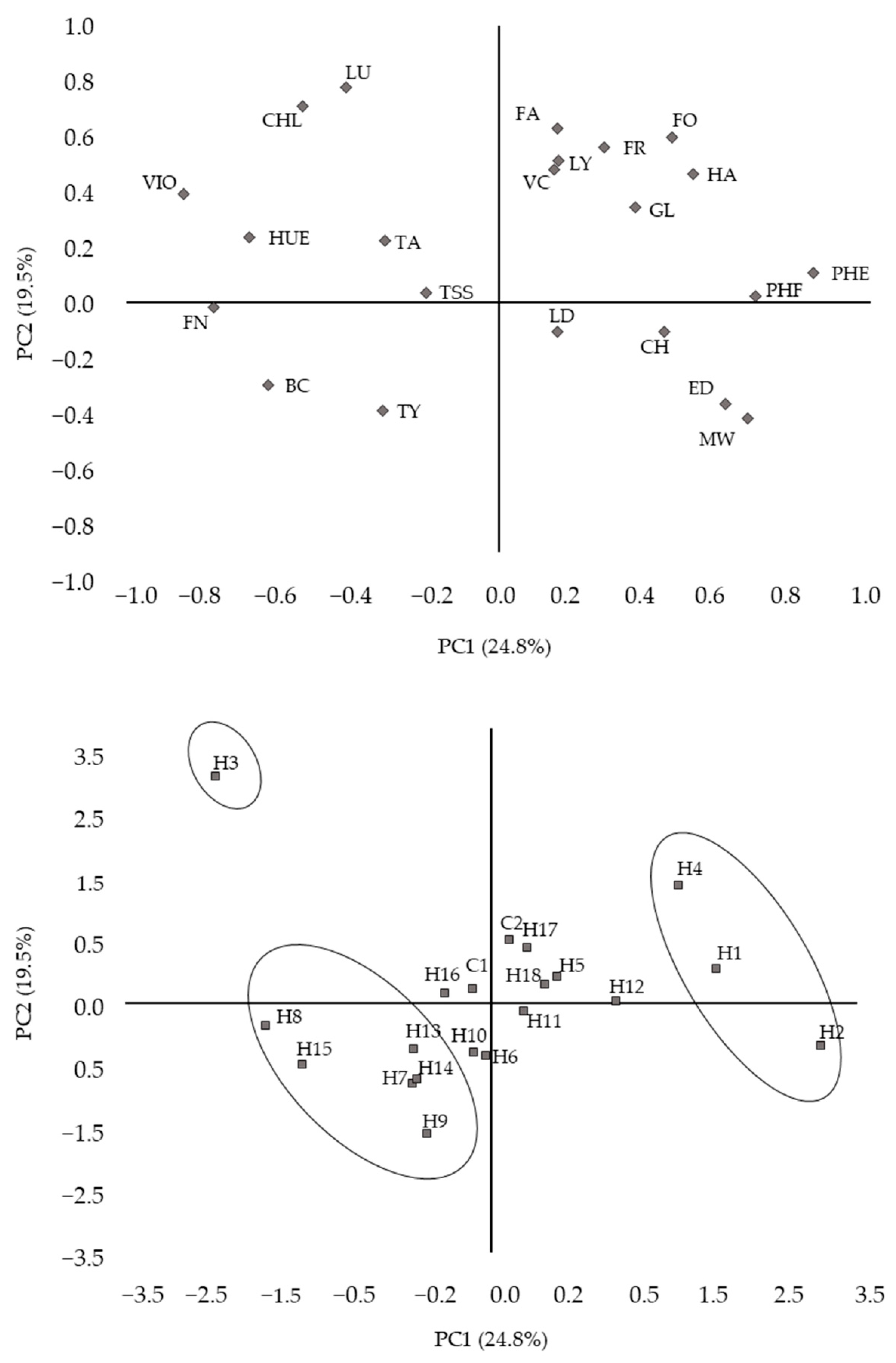
3.2. Correlation between Agronomic, Physical, Organoleptic, and Bioactive Traits and Similarities between Hybrids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. FAO Statistical Datababases FAOSTAT. Available online: https://www.fao.org/ (accessed on 20 January 2024).
- Morales-Soto, A.; García-Salas, P.; Rodríguez-Pérez, C.; Jiménez-Sánchez, C.; de la Luz Cádiz-Gurrea, M.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Antioxidant capacity of 44 cultivars of fruits and vegetables grown in Andalusia (Spain). Food Res. Int. 2014, 58, 35–46. [Google Scholar] [CrossRef]
- Kavitha, P.; Shivashankara, K.S.; Rao, V.K.; Sadashiva, A.T.; Ravishankar, K.V.; Sathish, G.J. Genotypic variability for antioxidant and quality parameters among tomato cultivars, hybrids, cherry tomatoes and wild species. J. Sci. Food Agric. 2014, 94, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Flores, P.; Hernández, V.; Hellín, P.; Fenoll, J.; Cava, J.; Mestre, T.; Martínez, V. Metabolite profile of the tomato dwarf cultivar Micro-Tom and comparative response to saline and nutritional stresses with regard to a commercial cultivar. J. Sci. Food Agric. 2016, 96, 1562–1570. [Google Scholar] [CrossRef] [PubMed]
- Flores, P.; Sánchez, E.; Fenoll, J.; Hellín, P. Genotypic variability of carotenoids in traditional tomato cultivars. Food Res. Int. 2017, 100, 510–516. [Google Scholar] [CrossRef]
- Felföldi, Z.; Ranga, F.; Roman, I.A.; Sestras, A.F.; Vodnar, D.C.; Prohens, J.; Sestras, R.E. Analysis of Physico-Chemical and Organoleptic Fruit Parameters Relevant for Tomato Quality. Agronomy 2022, 12, 1232. [Google Scholar] [CrossRef]
- Ali, M.Y.; Sina, A.A.I.; Khandker, S.S.; Neesa, L.; Tanvir, E.M.; Kabir, A.; Khalil, M.I.; Gan, S.H. Nutritional composition and bioactive compounds in tomatoes and their impact on human health and disease: A review. Foods 2021, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, M.; Duan, X.; AbuIzneid, T.; Rauf, A.; Khan, Z.; Mitra, S.; Emran, T.B.; Aljohani, A.S.M.; Alhumaydhi, F.A.; et al. Phytochemical and Nutritional Profiling of Tomatoes; Impact of Processing on Bioavailability—A Comprehensive Review. Food Rev. Int. 2023, 39, 5986–6010. [Google Scholar] [CrossRef]
- Adalid, A.M.; Roselló, S.; Nuez, F. Evaluation and selection of tomato accessions (Solanum lycopersicon) for content of lycopene, β-carotene and ascorbic acid. J. Food Comp. Anal. 2010, 23, 613–618. [Google Scholar] [CrossRef]
- Anđelini, M.; Major, N.; Išić, N.; Kovačević, T.K.; Ban, D.; Palčić, I.; Radunić, M.; Goreta Ban, S. Sugar and Organic Acid Content Is Dependent on Tomato (Solanum lycoperiscum L.) Peel Color. Horticulturae 2023, 9, 313. [Google Scholar] [CrossRef]
- Friedman, M. Anticarcinogenic, cardioprotective, and other health benefits of tomato compounds lycopene, α-tomatine, and tomatidine in pure form and in fresh and processed tomatoes. J. Agric. Food Chem. 2013, 61, 9534–9950. [Google Scholar] [CrossRef]
- Tommonaro, G.; de Prisco, R.; Abbamondi, G.R.; Marzocco, S.; Saturnino, C.; Poli, A.; Nicolaus, B. Evaluation of antioxidant properties, total phenolic content, and biological activities of new tomato hybrids of industrial interest. J. Med. Food. 2012, 15, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Olivieri, F.; Gerardi, C.; Liso, M.; Chiesa, M.; Chieppa, M.; Frusciante, L.; Barone, A.; Santino, A.; Rigano, M.M. Selection of tomato landraces with high fruit yield and nutritional quality under elevated temperatures. J. Sci. Food Agric. 2020, 100, 2791–2799. [Google Scholar] [CrossRef]
- Figàs, M.R.; Prohens, J.; Raigón, M.D.; Fita, A.; García-Martínez, M.D.; Casanova, C.; Borràs, D.; Plazas, M.; Andújar, I.; Soler, S. Characterization of composition traits related to organoleptic and functional quality for the differentiation, selection and enhancement of local varieties of tomato from different cultivar groups. Food Chem. 2015, 187, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Natalini, A.; Acciarri, N.; Cardi, T. Breeding for nutritional and organoleptic quality in vegetable crops: The case of tomato and cauliflower. Agriculture 2021, 11, 606. [Google Scholar] [CrossRef]
- Dumas, Y.; Dadomo, M.; Di Lucca, G.; Grolier, P. Effects of environmental factors and agricultural techniques on antioxidant content of tomatoes. J. Sci. Food Agric. 2003, 83, 369–382. [Google Scholar] [CrossRef]
- Messmer, M.; Willbois, K.P.; Baier, C.; Schafer, F.; Arncken, C.; Drexler, D.; Hildermann, I. Plant Breeding Techniques-an Assessment for Organic Farming, 2nd ed.; Research Institute of Organic Agriculture FiBL: Frick, Switzerland, 2015; p. 48. [Google Scholar]
- Ficiciyan, A.; Loos, J.; Sievers-Glotzbach, S.; Tscharntke, T. More than yield: Ecosystem services of traditional versus modern crop varieties revisited. Sustainability 2018, 10, 2834. [Google Scholar] [CrossRef]
- Ribes-Moya, A.M.; Raigón, M.D.; Moreno-Peris, E.; Fita, A.; Rodríguez-Burruezo, A. Response to organic cultivation of heirloom Capsicum peppers: Variation in the level of bioactive compounds and effect of ripening. PLoS ONE 2018, 13, e0207888. [Google Scholar] [CrossRef]
- Casals, J.; Martí, M.; Rull, A.; Pons, C. Sustainable transfer of tomato landraces to modern cropping systems: The effects of environmental conditions and management practices on long-shelf-life tomatoes. Agronomy 2021, 11, 533. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, S.; Huang, Z.; Zhang, S.; Liao, Q.; Zhang, C.; Lin, T.; Qin, M.; Peng, M.; Yang, C.; et al. Rewiring of the Fruit Metabolome in Tomato Breeding. Cell 2018, 172, 249–261.e12. [Google Scholar] [CrossRef]
- Oltman, A.E.; Jervis, S.M.; Drake, M.A. Consumer attitudes and preferences for fresh market tomatoes. J. Food Sci. 2014, 79, S2091–S2097. [Google Scholar] [CrossRef]
- Sinesio, F.; Cammareri, M.; Cottet, V.; Fontanet, L.; Jost, M.; Moneta, E.; Palombieri, S.; Peparaio, M.; del Castillo, R.R.; Civitelli, E.S.; et al. Sensory traits and consumer’s perceived quality of traditional and modern fresh market tomato varieties: A study in three european countries. Foods 2021, 10, 2521. [Google Scholar] [CrossRef] [PubMed]
- Arca, M.; Gouesnard, B.; Mary-Huard, T.; le Paslier, M.C.; Bauland, C.; Combes, V.; Madur, D.; Charcosset, A.; Nicolas, S.D. Genotyping of DNA pools identifies untapped landraces and genomic regions to develop next-generation varieties. J. Plant Biotech. 2023, 21, 1123–1139. [Google Scholar] [CrossRef] [PubMed]
- Caramante, M.; Rouphael, Y.; Corrado, G. Genetic diversity among and within tomato (Solanum lycopersicum L.) landraces grown in Southern Italy. Genet. Resour. Crop Evol. 2024, 71, 157–166. [Google Scholar] [CrossRef]
- Baldina, S.; Picarella, M.E.; Troise, A.D.; Pucci, A.; Ruggieri, V.; Ferracane, R.; Barone, A.; Fogliano, V.; Mazzucato, A. Metabolite profiling of Italian tomato landraces with different fruit types. Front. Plant Sci. 2016, 7, 191011. [Google Scholar] [CrossRef]
- Figàs, M.; Soler, S.; Díez, M.J.; Granell, A.; Monforte, A.; Prohens, J. Strategies for the enhancement of local tomato varieties: A study case with varieties from the Spanish region of València. In Proceedings of the INNOHORT Symposium, Avignon, France, 8–12 June 2015. [Google Scholar]
- Soler, S.; Figàs, M.R.; Casanova, C.; Borràs, D.; Granell, A.; Prohens, J. Higher yield and more uniform fruit set in selections of the ‘Valenciana’ local tomato landrace. In Proceedings of the 12th Solanaceae Conference, Burdeos, France, 25–29 October 2015. [Google Scholar]
- García-Martínez, S.; Grau, A.; Alonso, A.; Rubio, F.; Carbonell, P.; Ruiz, J.J. UMH 916, UMH 972, UMH 1093, UMH 1127, and UMH 1139: Four Freshmarket Breeding Lines Resistant to Viruses within the Muchamiel Tomato Type. Hort. Sci. 2015, 50, 927–929. [Google Scholar] [CrossRef]
- Casals, J.; Bosch, L.; Casañas, F.; Cebolla, J.; Nuez, F. Montgrí, a Cultivar within the Montserrat Tomato Type. Hort. Sci. 2010, 45, 1885–1886. [Google Scholar] [CrossRef]
- Casals, J. Filogènia i Variabilitat Genètica de les Varietats Tradicionals de Tomàquet (Solanum lycopersicum L.) Montserrat/Pera de Girona i Penjar. Estratègies per a la Millora de la Seva Qualitat Organolèptica. Ph.D. Thesis, Universitat Politècnica de Catalunya, Barcelona, Spain, 2014. [Google Scholar]
- Carli, P.; Barone, A.; Fogliano, V.; Frusciante, L.; Ercolano, M.R. Dissection of genetic and environmental factors involved in tomato organoleptic quality. BMC Plant Biol. 2011, 11, 58. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jiang, J.; Ren, A.; Xu, X.; Zhang, H.; Zhao, T.; Jiang, X.; Sun, Y.; Li, J.; Yang, H. Heterosis and combining ability analysis of fruit yield, early maturity, and quality in tomato. Agronomy 2021, 11, 807. [Google Scholar] [CrossRef]
- Fortuny, A.P.; Bueno, R.A.; Pereira Da Costa, J.H.; Zanor, M.I.; Rodríguez, G.R. Tomato fruit quality traits and metabolite content are affected by reciprocal crosses and heterosis. J. Exp. Bot. 2021, 72, 5407–5425. [Google Scholar] [CrossRef]
- Kumar, L.; Yadav, G.C. Evaluation of the Performance of Parental Lines and Their F1 Hybrids for Yield and Attributing Traits in Tomato (Solanum lycopersicum L.). Int. J. Plant Soil Sci. 2023, 35, 77–85. [Google Scholar] [CrossRef]
- Sánchez, A.; Hernández, V.; Molina, E.; Fenoll, J.; Flores, P.; Hellín, P. Characterization and phenotypic evaluation of fruit quality traits related to functional and organoleptic quality of Spanish tomato landraces. Agri. Food 2023, 11, 30–41. [Google Scholar] [CrossRef]
- Flores, A.; Pérez, A.; Abadía, E.; Sánchez, J.; Garrido, I.; Molina, M.V.; Rodríguez-Burruezo, A.; Raigón, M.D.; Ribes-Moya, A.M.; Fenoll, J.; et al. Evaluación de la productividad de variedades tradicionales de tomate de alta calidad sensorial y nutricional. Acta Hortic. 2018, 80, 18–21. [Google Scholar]
- Böhm, V. Use of column temperature to optimize carotenoid isomer separation by C30 high performance liquid chromatography. J. Sep. Sci. 2001, 24, 955–959. [Google Scholar] [CrossRef]
- Hernandez, V.; Hellín, P.; Fenoll, J.; Flores, P. Increased temperature produces changes in the bioactive composition of tomato, depending on its developmental stage. J. Agric. Food Chem. 2015, 63, 2378–2382. [Google Scholar] [CrossRef]
- Fenoll, J.; Martínez, A.; Hellín, P.; Flores, P. Simultaneous determination of ascorbic and dehydroascorbic acids in vegetables and fruits by liquid chromatography with tandem-mass spectrometry. Food Chem. 2011, 127, 340–344. [Google Scholar] [CrossRef]
- Vallverdu-Queralt, A.; Jauregui, O.; Medina-Remon, A.; Andres-Lacueva, C.; Lamuela-Raventos, R.M. Improved characterization of tomato polyphenols using liquid chromatography/electrospray ionization linear ion trap quadrupole orbitrap mass spectrometry and liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 2986–2992. [Google Scholar] [CrossRef]
- Flores, P.; Hernández, V.; Fenoll, J.; Hellín, P. Pre-harvest application of ozonated water on broccoli crops: Effect on head quality. J. Food Compos. Anal. 2019, 83, 10360. [Google Scholar] [CrossRef]
- Nour, V.; Ionica, M.E.; Trandatfir, I. Bioactive Compounds, Antioxidant Activity and Color of Hydroponic Tomato Fruits at Different Stages of Ripening. Not. Bot. Horti Agrobot. 2015, 43, 404–412. [Google Scholar] [CrossRef]
- Kang, S.I.; Hwang, I.; Goswani, G.; Jung, H.J.; Nath, U.K.; Yoo, H.J.; Lee, J.M.; Nous, I.S. Molecular insights reveal Psy1, SGR, and SlMYB12 genes are associated with diverse fruit color pigments in tomato (Solanum lycopersicum L.). Molecules 2017, 22, 2180. [Google Scholar] [CrossRef]
- Ballester, A.R.; Molthoff, J.; de Vos, R.; Hekkert, B.T.; Orzaez, D.; Fernández-Moreno, J.P.; Tripodi, P.; Grandillo, S.; Martin, C.; Heldens, J.; et al. Biochemical and molecular analysis of pink tomatoes: Deregulated expression of the gene encoding transcription factor SLMYB12 leads to pink tomato fruit color. Plant Physiol. 2010, 152, 71–84. [Google Scholar] [CrossRef]
- Hu, Z.L.; Deng, L.; Yan, B.; Pan, Y.; Luo, M.; Chen, X.Q. Silencing of the LeSGR1 gene in tomato inhibits chlorophyll degradation and exhibits a stay-green phenotype. Biol. Plant 2011, 55, 27–34. [Google Scholar] [CrossRef]
- Casals, J.; Rivera, A.; Sabaté, J.; Romero del Castillo, R.; Simó, J. Cherry and Fresh Market Tomatoes: Differences in Chemical, Morphological, and Sensory Traits and Their Implications for Consumer Acceptance. Agronomy 2019, 9, 9. [Google Scholar] [CrossRef]
- Glogovac, S.; Takač, A.; Belović, M.; Gvozdanović-Varga, J.; Nagl, N.; Červenski, J.; Danojević, D.; Trkulja, D.; Prodanović, S.; Živanović, T. Characterization of tomato genetic resources in the function of breeding. Ratar. Povrt. 2022, 59, 1–8. [Google Scholar] [CrossRef]
- Frusciante, L.; Carli, P.; Ercolano, M.R.; Pernice, R.; Di Matteo, A.; Fogliano, V.; Pellegrini, N. Antioxidant nutritional quality of tomato. Mol. Nutr. Food Res. 2007, 51, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Collins, E.J.; Bowyer, C.; Tsouza, A.; Chopra, M. Tomatoes: An Extensive Review of the Associated Health Impacts of Tomatoes and Factors That Can Affect Their Cultivation. Biology 2022, 11, 239. [Google Scholar] [CrossRef]
- Jayappriyan, K.R.; Rajkumar, R.; Venkatakrishnan, V.; Nagaraj, S.; Rengasamy, R. In Vitro Anticancer Activity of Natural β-Carotene from Dunaliella Salina EU5891199 in PC-3. Cells Biomed. Prev. Nutr. 2013, 3, 99–105. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Stinco, C.M.; Mapelli Brahm, P. Skin. Carotenoids in Public Health and Nutricosmetics: The Emerging Roles and Applications of the UV Radiation-Absorbing Colourless Carotenoids Phytoene and Phytofluene. Nutrients 2019, 11, 1093. [Google Scholar] [CrossRef] [PubMed]
- Martí, R.; Leiva-Brondo, M.; Lahoz, I.; Campillo, C.; Cebolla-Cornejo, J.; Roselló, S. Polyphenol and L-ascorbic acid content in tomato as influenced by high lycopene genotypes and organic farming at different environments. Food Chem. 2018, 239, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Rosa-Martínez, E.; García-Martínez, M.D.; Adalid-Martínez, A.M.; Pereira-Dias, L.; Casanova, C.; Soler, E.; Figàs, M.R.; Raigón, M.D.; Plazas, M.; Soler, S.; et al. Fruit composition profile of pepper, tomato and eggplant varieties grown under uniform conditions. Food Res. Int. 2021, 147, 110531. [Google Scholar] [CrossRef]
- Martínez-Valverde, I.; Periago, M.J.; Provan, G.; Chesson, A. Phenolic compounds, lycopene and antioxidant activity in commercial varieties of tomato (Lycopersicum esculentum). J. Sci. Food Agric. 2002, 82, 323–330. [Google Scholar] [CrossRef]
- Martí, R.; Roselló, S.; Cebolla-Cornejo, J. Tomato as a Source of Carotenoids and Polyphenols Targeted to Cancer Prevention. Cancers 2016, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Hallmann, E. The influence of organic and conventional cultivation systems on the nutritional value and content of bioactive compounds in selected tomato types. J. Sci. Food Agric. 2012, 92, 2840–2848. [Google Scholar] [CrossRef] [PubMed]
- Hernández, V.; Hellín, P.; Fenoll, J.; Flores, P. Interaction of nitrogen and shading on tomato yield and quality. Sci. Hortic. 2019, 255, 255–259. [Google Scholar] [CrossRef]
- Hellín, P.; Hernández, V.; Sánchez, E.; Garrido, I.; Cava, J.; Pérez, A.; Gomariz, J.; Molina, M.V.; Fernández, I.; Molina, E.; et al. Evaluation of genotype, environment, and genotype-by-environment interaction for phenolic compounds in tomato landraces. Acta Hortic. 2023, 1384, 497–504. [Google Scholar] [CrossRef]
- Chassy, A.W.; Bui, L.; Renaud, E.N.C.; Van Horn, M.; Mitchell, A.E. Three-Year Comparison of the Content of Antioxidant Microconstituents and Several Quality Characteristics in Organic and Conventionally Managed Tomatoes and Bell Peppers. J. Agric. Food Chem. 2006, 54, 8244–8252. [Google Scholar] [CrossRef]
- Klee, H.J.; Tieman, D.M. Genetic challenges of flavor improvement in tomato. Trends Genet. 2013, 29, 257–262. [Google Scholar] [CrossRef]

| Genotypes | Type | Colour | Size * |
|---|---|---|---|
| Parents | |||
| BGMU01010600 (P1) | Corazón de toro | Pink | L |
| BGMU01010922 (P2) | de la Sierra | Red-Black | XL |
| BGMU01010639 (P3) | Flor de Baladre | Pink | XL |
| BGMU01010640 (P4) | Flor de Baladre | Pink | XL |
| BGMU01010609 (P5) | Kumato | Red-Black | M |
| BGMU01010661 (P6) | Mesa Murciano | Pink | XL |
| BGMU01010643 (P7) | Mesa Murciano | Red | XL |
| BGMU01010675 (P8) | Mesa Murciano | Red | L |
| BGMU01010646 (P9) | Muchamiel | Red | L |
| BGMU01010665 (P10) | Muchamiel | Red | L |
| BGMU01010672 (P11) | Muchamiel | Red | XL |
| BGMU01010683 (P12) | de la Pera | Red | M |
| BGMU01010602 (P13) | Pimiento | Red | L |
| BGMU01010633 (P14) | Pimiento | Red | L |
| Hybrids | |||
| Pasadena (C1) | Tomate gordo | Red | L |
| Mongo (C2) | Marmande | Red | L |
| P3 × P2 (H1) | Flor de baladre × de la Sierra | Red | XL |
| P3 × P4 (H2) | Flor de baladre × Flor de baladre | Pink | XL |
| P5 × P2 (H3) | Kumato × de la Sierra | Red-Black | M |
| P6 × P2 (H4) | Mesa murciano × de la Sierra | Red | XL |
| P7 × P6 (H5) | Mesa murciano × Mesa murciano | Red | XL |
| P7 × P11 (H6) | Mesa murciano × Muchamiel | Red | XL |
| P8 × P2 (H7) | Mesa murciano × de la Sierra | Red | L |
| P8 × P5 (H8) | Mesa murciano × Kumato | Red | M |
| P8 × P6 (H9) | Mesa murciano × Mesa murciano | Red | L |
| P8 × P7 (H10) | Mesa murciano × Mesa murciano | Red | L |
| P9 × P6 (H11) | Muchamiel × Mesa murciano | Red | L |
| P9 × P7 (H12) | Muchamiel × Mesa murciano | Red | L |
| P9 × P10 (H13) | Muchamiel × Muchamiel | Red | L |
| P9 × P11 (H14) | Muchamiel × Muchamiel | Red | L |
| P12 × P10 (H15) | de la Pera × Muchamiel | Red | M |
| P12 × P13 (H16) | de la Pera × Pimiento | Red | M |
| P13 × P1 (H17) | Pimiento × Corazón de toro | Red | L |
| P13 × P14 (H18) | Pimiento × Pimiento | Red | L |
| Parental Lines | Hybrids | Control | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | CV | Range | Mean ± SD | CV | Range | Mean ± SD | CV | Range | |
| TY (kg) | 9.65 ± 1.33 | 25 | 5.97–15.07 | 12.35 ± 0.67 | 29 | 3.66–18.72 | 17.35 ± 1.35 | 3 | 16.99–17.70 |
| MW (g) | 243.47 ± 23.37 | 44 | 76.89–365.14 | 254.84 ± 11.32 | 36 | 140.47–436.69 | 232.68 ± 19.53 | 7 | 207.31–258.05 |
| FN | 48.60 ± 5.82 | 54 | 23.67–104.33 | 59.63 ± 1.85 | 51 | 19–137 | 87.00 ± 10.39 | 4 | 84.67–89.33 |
| ED (mm) | 80.33 ± 2.64 | 21 | 51.56–100.90 | 83.44 ± 1.47 | 17 | 61.22–108.31 | 81.17 ± 2.80 | 1 | 80.72–81.62 |
| LD (mm) | 68.51 ± 1.13 | 26 | 50.71–113.16 | 62.95 ± 0.86 | 17 | 50.34–90.66 | 57.00 ± 1.32 | 7 | 54.26–59.75 |
| CH | 34.49 ± 0.42 | 13 | 24.42–41.63 | 35.78 ± 0.24 | 10 | 26.25–40.53 | 37.13 ± 0.10 | 13 | 33.63–40.62 |
| HUE | 49.44 ± 0.25 | 16 | 37.28–64.09 | 49.38 ± 0.40 | 8 | 36.81–56.31 | 49.62 ± 2.07 | 0 | 49.47–49.77 |
| TSS (°Brix) | 6.34 ± 0.11 | 9 | 5.20–7.20 | 6.38 ± 0.08 | 12 | 5.3–8.1 | 5.84 ± 0.18 | 9 | 5.50–6.20 |
| TA (g L−1) | 4.02 ± 0.15 | 22 | 2.90–6.20 | 4.25 ± 0.02 | 16 | 2.7–5.2 | 4.22 ± 0.26 | 12 | 3.90–4.60 |
| GL (mg g−1) | 16.64 ± 0.40 | 16 | 13.60–24.60 | 15.24 ± 0.37 | 15 | 10.4–17.8 | 15.91 ± 0.23 | 5 | 15.40–16.40 |
| FR (mg g−1) | 17.16 ± 1.46 | 20 | 10.10–25.00 | 14.99 ± 0.81 | 24 | 9.1–20.4 | 17.82 ± 0.35 | 4 | 17.30–18.40 |
| VC (mg g−1) | 0.16 ± 0.00 | 15 | 0.10–0.20 | 0.16 ± 0.00 | 13 | 0.1–0.2 | 0.15 ± 0.00 | 21 | 0.10–0.20 |
| TPC (µg g−1) | 116.28 ± 5.56 | 33 | 8.10–130.40 | 102.79 ± 3.54 | 28 | 14.4–92.8 | 89.70 ± 5.81 | 38 | 36.50–57.90 |
| TC (µg g−1) | 34.92 ± 0.18 | 24 | 21.60–51.10 | 29.98 ± 0.60 | 14 | 24.00–40.20 | 46.94 ± 2.27 | 7 | 44.60–49.30 |
| Hybrids | TY (kg) | MW (g) | FN | LD (mm) | ED (mm) | CH | HUE | TSS (°Brix) | TA (g L−1) |
|---|---|---|---|---|---|---|---|---|---|
| C1 | 17.7 ± 0.6 | 211 ± 12 | 85 ± 6 | 60 ± 2 | 82 ± 4 | 34 ± 1 | 49 ± 3 | 6.2 ± 0.4 | 4.6 ± 0.3 |
| C2 | 17.0 ± 3.1 | 192 ± 11 | 89 ± 17 | 54 ± 1 | 81 ± 4 | 41 ± 1 | 50 ± 1 | 5.5 ± 0.1 | 3.9 ± 0.3 |
| H1 | 9.6 ± 1.6 | 349 ± 33 | 27 ± 2 | 60 ± 2 | 93 ± 2 | 39 ± 2 | 50 ± 2 | 5.5 ± 0.3 | 3.0 ± 0.3 |
| H2 | 12.4 ± 2.2 | 395 ± 27 | 31 ± 4 | 63 ± 1 | 108 ± 4 | 29 ± 4 | 37 ± 1 | 6.6 ± 0.5 | 4.2 ± 0.2 |
| H3 | 10.6 ± 0.6 | 123 ± 5 | 86 ± 3 | 50 ± 2 | 67 ± 2 | 26 ± 0 | 56 ± 2 | 6.8 ± 0.3 | 5.2 ± 0.2 |
| H4 | 11.7 ± 1.7 | 301 ± 33 | 39 ± 5 | 58 ± 5 | 88 ± 9 | 35 ± 2 | 49 ± 1 | 5.5 ± 0.1 | 4.1 ± 0.1 |
| H5 | 9.9 ± 0.4 | 307 ± 20 | 33 ± 3 | 57 ± 0 | 91 ± 1 | 36 ± 1 | 51 ± 2 | 6.9 ± 0.4 | 4.7 ± 0.3 |
| H6 | 13.8 ± 1.1 | 319 ± 34 | 45 ± 7 | 59 ± 2 | 99 ± 5 | 34 ± 1 | 50 ± 2 | 6.0 ± 0.2 | 4.1 ± 0.1 |
| H7 | 18.7 ± 1.7 | 238 ± 10 | 79 ± 10 | 58 ± 2 | 87 ± 6 | 33 ± 1 | 51 ± 1 | 5.9 ± 0.1 | 5.0 ± 0.3 |
| H8 | 16.4 ± 3.1 | 118 ± 4 | 137 ± 22 | 51 ± 3 | 70 ± 3 | 32 ± 0 | 52 ± 1 | 6.2 ± 0.3 | 5.0 ± 0.8 |
| H9 | 14.9 ± 1.2 | 247 ± 20 | 62 ± 9 | 62 ± 1 | 93 ± 3 | 31 ± 1 | 51 ± 2 | 5.3 ± 0.2 | 3.6 ± 0.5 |
| H10 | 14.7 ± 1.9 | 256 ± 6 | 57 ± 6 | 60 ± 0 | 90 ± 2 | 34 ± 2 | 51 ± 1 | 6.6 ± 0.2 | 4.0 ± 0.4 |
| H11 | 8.7 ± 2.3 | 275 ± 33 | 31 ± 7 | 58 ± 4 | 86 ± 8 | 35 ± 4 | 49 ± 1 | 7.1 ± 0.4 | 4.7 ± 0.7 |
| H12 | 12.0 ± 2.2 | 274 ± 3 | 44 ± 9 | 56 ± 1 | 95 ± 2 | 38 ± 1 | 49 ± 1 | 6.2 ± 0.2 | 4.1 ± 0.0 |
| H13 | 14.5 ± 0.6 | 230 ± 17 | 64 ± 6 | 61 ± 2 | 80 ± 2 | 39 ± 1 | 48 ± 1 | 5.3 ± 0.2 | 2.7 ± 0.3 |
| H14 | 17.7 ± 2.0 | 278 ± 18 | 65 ± 12 | 62 ± 2 | 96 ± 2 | 39 ± 1 | 53 ± 3 | 7.3 ± 0.2 | 5.2 ± 0.8 |
| H15 | 12.1 ± 2.8 | 115 ± 11 | 105 ± 21 | 68 ± 3 | 62 ± 4 | 37 ± 1 | 48 ± 1 | 6.8 ± 0.4 | 3.5 ± 0.2 |
| H16 | 11.2 ± 4.7 | 119 ± 25 | 83 ± 30 | 76 ± 2 | 68 ± 3 | 38 ± 1 | 46 ± 1 | 8.1 ± 0.6 | 4.6 ± 0.3 |
| H17 | 9.7 ± 0.6 | 154 ± 19 | 66 ± 11 | 83 ± 3 | 61 ± 3 | 37 ± 1 | 47 ± 1 | 5.9 ± 0.3 | 4.2 ± 0.6 |
| H18 | 3.7 ± 0.2 | 195 ± 14 | 19 ± 2 | 91 ± 3 | 70 ± 2 | 31 ± 4 | 50 ± 1 | 6.4 ± 0.3 | 4.4 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez, A.S.; Flores, P.; Hernández, V.; Sánchez, E.; Molina, E.; López, N.; Rodríguez-Burruezo, A.; Fenoll, J.; Hellín, P. Fruit Agronomic and Quality Traits of Tomato F1 Hybrids Derived from Traditional Varieties. Horticulturae 2024, 10, 440. https://doi.org/10.3390/horticulturae10050440
Sánchez AS, Flores P, Hernández V, Sánchez E, Molina E, López N, Rodríguez-Burruezo A, Fenoll J, Hellín P. Fruit Agronomic and Quality Traits of Tomato F1 Hybrids Derived from Traditional Varieties. Horticulturae. 2024; 10(5):440. https://doi.org/10.3390/horticulturae10050440
Chicago/Turabian StyleSánchez, Alicia Sánchez, Pilar Flores, Virginia Hernández, Elena Sánchez, Elia Molina, Nuria López, Adrián Rodríguez-Burruezo, José Fenoll, and Pilar Hellín. 2024. "Fruit Agronomic and Quality Traits of Tomato F1 Hybrids Derived from Traditional Varieties" Horticulturae 10, no. 5: 440. https://doi.org/10.3390/horticulturae10050440